-
PDF
- Split View
-
Views
-
Cite
Cite
David Brown, Kazuyuki Nakagome, Joachim Cordes, Ronald Brenner, Gerhard Gründer, Richard S E Keefe, Robert Riesenberg, David P Walling, Kristen Daniels, Lara Wang, Jennifer McGinniss, Michael Sand, Evaluation of the Efficacy, Safety, and Tolerability of BI 409306, a Novel Phosphodiesterase 9 Inhibitor, in Cognitive Impairment in Schizophrenia: A Randomized, Double-Blind, Placebo-Controlled, Phase II Trial, Schizophrenia Bulletin, Volume 45, Issue 2, March 2019, Pages 350–359, https://doi.org/10.1093/schbul/sby049
Close - Share Icon Share
Abstract
Patients with cognitive impairment associated with schizophrenia may benefit from treatments targeting dysfunctional glutamatergic neurotransmission. BI 409306, a potent and selective phosphodiesterase 9 inhibitor, was assessed in patients with schizophrenia using a learn-and-confirm adaptive trial design.
This double-blind, parallel-group trial randomized patients 2:1:1:1:1 to once-daily placebo or BI 409306 (10, 25, 50, or 100 mg) for 12 weeks. Stage 1 (learn) assessed change from baseline in Cambridge Neuropsychological Test Automated Battery (CANTAB) scores (week 12) to identify ≥1 meaningful endpoints for stage 2 (confirm). If no domains showed efficacy, change from baseline in Measurements and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (MCCB) composite scores (week 12) was the primary endpoint. The key secondary endpoint was change from baseline in Schizophrenia Cognition Rating Scale (SCoRS) total score. Safety was monitored.
Five hundred eighteen patients were randomized. In stage 1, CANTAB did not differentiate between BI 409306 and placebo (n = 120), so the primary endpoint of change from baseline in MCCB composite score was analyzed in 450 patients in stage 2. There was no significant difference between BI 409306 (1.2–2.8) and placebo (2.5) in MCCB composite score change. BI 409306 did not significantly improve change from baseline in SCoRS total score (−3.1 to −2.0) vs placebo (−2.5). Adverse events were dose-dependent, increasing from 33.3% (10 mg) to 53.5% (100 mg), vs 36.4% for placebo.
The primary endpoint of cognitive function improvement was not met. BI 409306 was well-tolerated, with an acceptable safety profile.
Introduction
In schizophrenia, cognitive impairment is a major determinant of functional outcomes,1–3 with approximately 20%–60% of functional outcome variance attributed to cognitive performance.4 Currently, approved antipsychotics target symptoms, but these therapies have not demonstrated efficacy in cognitive impairment associated with schizophrenia (CIAS). Therefore, there is an unmet need for treatment options that can improve cognition and functional outcomes in schizophrenia.
Cognitive dysfunction in schizophrenia has been associated with dysfunctional N-methyl-D-aspartate (NMDA) receptor signaling and impaired functioning of the γ-aminobutyric acid (GABA)-mediated and glutamatergic pathways in the prefrontal cortex and limbic areas of the brain.5,6 Glutamatergic neurotransmission, which is associated with functions of memory formation and learning, is mediated by postsynaptic NMDA receptors.7 Activation of these receptors increases intracellular levels of cyclic guanosine monophosphate (cGMP) and subsequent activation of protein kinases involved in long-term potentiation and synaptic plasticity, required for learning and memory formation.8 Phosphodiesterase 9A (PDE9A) hydrolyses cGMP9 and is highly expressed in the neocortex and hippocampus, where it is likely to be a significant determinant of intracellular basal cGMP levels.10 Inhibition of PDE9A improves intracellular cGMP levels and may offer the potential to treat patients with CIAS by increasing cGMP levels and improving glutamatergic neurotransmission and synaptic plasticity.11 BI 409306, a potent and selective PDE9 inhibitor, may also improve cognition by increasing cGMP levels within the brain based on preclinical findings in rodents12 (supplementary figure S1). BI 409306 induced a dose-dependent increase in cGMP levels within rat cerebrospinal fluid and improved memory performance in an object recognition task in rats, in addition to reversing memory deficits in the mouse T-maze task, induced with MK-801, an NMDA receptor antagonist.13,14
The objective of this trial was to evaluate the efficacy, safety, and tolerability of BI 409306 10–100 mg compared with placebo in patients with schizophrenia receiving stable antipsychotic treatment. The choice of dose was based on preclinical data demonstrating that the IC50 of BI 409306 for PDE9 was 52 nM, calculated using cytosolic extracts of SF9 insect cells over-expressing full-length human PDE9.13 Clinical trials assessing efficacy in schizophrenia often use a battery of tests to assess cognition. Such batteries include the Cambridge Neuropsychological Test Automated Battery (CANTAB) for schizophrenia and the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (MCCB). However, these batteries may not be sensitive enough to detect treatment effects in clinical trials, as there are still no currently approved medications that demonstrate efficacy in the treatment of cognitive deficits in schizophrenia. For example, CANTAB tests individual domains, but it is not feasible to adequately power a study to adjust for the multiplicity of testing when using the entire battery. Selected CANTAB tests can be prespecified for a study, but there is often a lack of evidence to support the selection of specific tests early in development. The MCCB uses a composite score, but there is the risk that not all domains (or even most domains) are sufficiently improved to show overall statistical significance, or that certain domains will add noise to the data. The trial was therefore designed to show superiority of BI 409306 over placebo in cognition using a novel learn-and-confirm trial design. This design enabled meaningful CANTAB endpoint(s) to be identified from a range of relevant CANTAB cognitive tests from a small number of patients in stage 1, as it was unclear which domains would be the most responsive to BI 409306, for use as the primary endpoint(s) in stage 2, where a larger cohort was assessed. However, if no CANTAB endpoints demonstrated efficacy, the MCCB was to be selected as the primary endpoint in stage 2.
Methods
Trial Design
This was a phase II, multicenter (55 centers), multinational (6 countries), double-blind, placebo-controlled, parallel-group trial (Clinicaltrials.gov: NCT02281773) (supplementary figure S2). Patients were randomized 2:1:1:1:1 to receive once-daily placebo or BI 409306 (10, 25, 50, or 100 mg) for 12 weeks, followed by a 4-week follow-up period using an Interactive Response Technology (IRT) called ClinPhone Randomization and Trial Supply Management (PAREXEL). IRT, accessed via phone or web, was used to randomize eligible patients, perform subsequent drug assignment, manage initial/re-supply ordering of drug supplies, and handle emergency un-blinding. The randomization schedule was generated using validated software and was verified by a statistician who was not involved in the trial. The trial included 7 visits: screening (visit 1), randomization and baseline (visit 2), week 3 (visit 3), week 6 (visit 4), week 9 (visit 5), week 12 (visit 6), and a follow-up visit at week 16 (visit 7).
The trial was analyzed in 2 stages. In stage 1 (learn), unblinded data from a subset (n = 120) of completed patients were used to identify a robust effect size (>0.5), if any, on cognitive endpoint(s) within the CANTAB for schizophrenia (cognitive assessment software, Cambridge Cognition [2017], all rights reserved, www.cantab.com). The results of this interim analysis were used to inform the selection of the primary endpoint to be used in stage 2 (confirm) in the remaining sample of patients (n = 396) not assessed from the learn phase. If no CANTAB test reached the threshold of 0.5, then the primary endpoint was prespecified as the MCCB.
The trial was approved by an institutional review board/independent ethics committee and competent authority and was conducted in accordance with the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Good Clinical Practice (GCP) guidelines,15 the Japanese GCP regulations, and the Declaration of Helsinki.16 All patients provided written, informed consent.
Treatments
BI 409306 was administered orally as a single tablet of either 10, 25, or 50 mg or as two 50 mg tablets. Placebo treatment was administered as size- and color-matched tablets to maintain blinding.
Patients
Eligible patients were 18–55 years of age with a diagnosis of schizophrenia as per the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.17 Eligibility depended on the following clinical features: clinically stable, nonacute, and residual phase of illness for ≥8 weeks; score ≤4 for the hallucinatory behavior, delusions, conceptual disorganization, and depressive symptoms items from the Positive and Negative Syndrome Scale (PANSS)18; and a minimal level of extrapyramidal symptoms (Simpson-Angus Scale total score <619). Patients were maintained on stable doses of current antipsychotics and concomitant psychotropic medications before randomization (for ≥8 weeks for atypical antipsychotics and psychotropic medications other than anticholinergics, antiepileptics, and lithium; or for ≥6 months for typical antipsychotics and anticholinergics, antiepileptics, and lithium). Anticholinergics, antiepileptics, and lithium were discontinued for ≥6 months before randomization if they had been discontinued before the trial.
Patients were excluded if they had received treatment with clozapine, more than 2 antipsychotics, long-acting hypnotics and anxiolytics, or strong or moderate CYP3A4 inhibitors; if they had cognitive impairment severity that might compromise the validity of the cognitive outcome measures as judged by the investigator; if they exhibited suicidal behavior within 2 years or suicidal ideation of types 4 or 5 in the Columbia Suicidal Severity Rating Scale (C-SSRS)20 within 3 months of trial initiation; or if they had a current diagnosis of another major psychiatric disorder assessed using the Mini International Neuropsychiatric Interview.21 Additional exclusion criteria are described in the supplementary material.
Trial Endpoints
Efficacy Endpoints.
In stage 1, the endpoint of change from baseline in cognitive function at week 12 was assessed using CANTAB. Seven CANTAB domains, including speed of processing, verbal learning, working memory, attention/vigilance, visual learning, social cognition, and reasoning and problem solving were assessed using 8 CANTAB measurements (supplementary table S1).22 For the CANTAB tests of Reaction Time, Spatial Working Memory, and Paired Associates Learning, improvement was indicated by a negative change in test scores. For the Verbal Recognition Memory, Rapid Visual Information Processing, Emotion Recognition, and One Touch Stockings of Cambridge tests, improvement was indicated by a positive change in test scores. The scoring of the Attention Switching Task is complex, measuring top-down cognitive control processes involving the prefrontal cortex. As no CANTAB domain(s) achieved an effect size of 0.5 or greater during the stage 1 interim analysis, change from baseline in the composite score of the MCCB at week 12 (supplementary table S1) was used as the primary efficacy endpoint for stage 2. The MCCB produced a composite score from 10 tests assessing 7 domains, with a positive change in scores indicating improvement (supplementary table S1). Both batteries were conducted at visits 1, 2, 4, and 6. Patients were randomized to complete either CANTAB or MCCB first at visit 1, with the order reversed and alternating at subsequent visits. The key secondary efficacy endpoint was change from baseline in the effect of cognitive deficits on day-to-day functioning, as measured by Schizophrenia Cognition Rating Scale (SCoRS) total score at week 12. SCoRS produced a total score from 20 items assessing cognitive deficits and their effect on daily function, each rated from low (1) to high (4) impairment.23 Additional secondary endpoints included the Clinical Global Impressions-Severity (CGI-S) scale score and the Patient Global Impressions-Improvement (PGI-I) scale score at week 12. The CGI-S and PGI-I were questionnaires completed by the clinician and patient, respectively, to assess the severity of a patient’s psychopathology (CGI-S) and their overall status (PGI-I).
Safety Endpoints.
The primary safety endpoints were the occurrences of adverse events (AEs) and serious AEs (SAEs); specified AEs of special interest (AESIs) indicative of drug-induced liver injury; clinically significant worsening of disease state, assessed using PANSS and judged in a descriptive manner by the investigator; and suicidality assessed using C-SSRS. Safety assessments included physical examinations, vital signs, electrocardiograms (ECGs), and clinical laboratory assessments.
The secondary safety endpoint was change in psychopathology symptoms as assessed using PANSS. PANSS contains 30 items, including 7 positive symptom items, 7 negative symptom items, and 16 general psychopathology symptom items. Each item is rated from 1 (absent) to 7 (extreme) and used to produce a total score, with higher scores indicating worsening health state.18
Other Endpoints.
The CANTAB domain of speed of processing, verbal learning, working memory, attention/vigilance, social cognition, and reasoning and problem solving were further assessed using additional CANTAB measurements (supplementary table S1). Further details of other endpoints are included in the supplementary material.
Statistical Analysis
Trial Populations.
The treated set (TS) included all patients who were randomized and received ≥1 dose of trial medication and was used for safety analyses. A full analysis set (FAS) was used for the primary analyses in stages 1 and 2 and contained all randomized patients who received ≥1 dose of trial medication and had a baseline and ≥1 postbaseline assessment for either CANTAB or MCCB measurements, respectively.
Sample Size.
Sample size assumptions were based on a meta-analysis of schizophrenia case-control studies in which effect sizes were calculated between patients with schizophrenia and controls without schizophrenia using CANTAB. Sample size assumptions were based on an effect size of 0.45 (Cambridge Cognition). For stage 1, the sample size was to be 30% of the sample size required for stage 2, equating to 20 per active treatment group and 40 for placebo. In stage 2, a sample size of 66 per active treatment group and 132 for placebo provided 84% power to detect an effect size of 0.45.
Stage 1—Learn.
In stage 1, 120 patients (20 per BI 409306 treatment arm and 40 from placebo) were randomly selected and their CANTAB data unblinded to an independent statistician after 70% of patients had completed the 12-week treatment period. The trial team, including those from the learn phase, remained blinded to patient-level information until after stage 2. The adjusted mean change from baseline in CANTAB domains was analyzed using an analysis of covariance (ANCOVA) model, which included a continuous fixed covariate of baseline CANTAB measurement, to identify 1 or more domain(s) with a treatment effect >0.5. Missing data were imputed using last observation carried forward. As no CANTAB domains had an effect size >0.5 in stage 1, in accordance with pre-specified criteria, the composite score of MCCB was selected as the primary endpoint for stage 2. An a priori hypothesis testing order for stage 2 was prespecified.
Stage 2—Confirm.
As MCCB was selected as the primary endpoint, the FAS was used for stage 2 analyses. The MCCB composite score was analyzed using SAS PROC MIXED software with a restricted maximum likelihood based mixed-model for repeated measures (MMRM), which included baseline as a fixed covariate; planned treatment, analysis visit, first test done (MCCB or CANTAB), region, and planned treatment by analysis visit as fixed effects; and baseline and baseline-by-analysis visit interaction as continuous fixed covariates. SCoRS and CGI-S were analyzed using an ANCOVA model adjusted for the fixed categorical covariates of treatment and the fixed continuous covariate of baseline score. Dose response was determined using linear and quadratic models. PGI-I, PANSS scores, and safety analyses were descriptive in nature. A post hoc Fisher’s exact test was performed to analyze the presence of exacerbations in the placebo group vs all BI 409306 groups.
Results
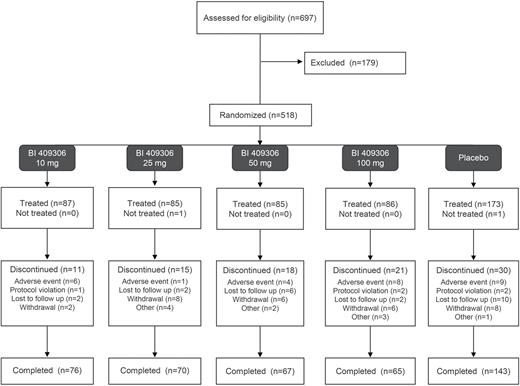
Trial Population and Patient Disposition
Of the 697 patients who were screened for eligibility, 518 patients were randomized and 516 patients received treatment. In stage 1, the FAS included 120 patients and all patients were analyzed. In stage 2, the FAS included 482 patients, with 450 analyzed for the primary endpoint. The majority of patients (n = 421, 81.6%) completed the trial medication. The most common reason for premature discontinuation of trial medication was withdrawal by the patient (n = 30, 5.8%) (figure 1). The majority of patients (n = 360, 69.8%) were male, with a mean (standard deviation [SD]) age of 42.3 [9.5] years; table 1). The baseline CANTAB domain scores were similar across the groups. The mean (SD) baseline MCCB, PANSS total, and SCoRS total scores were 30.1 (13.2), 58.6 (12.5), and 36.5 (8.9), respectively. All but 1 patient (n = 515, 99.8%) in the BI 409306 25 mg group were receiving antipsychotic medication related to schizophrenia (supplementary table S2). Antidepressant medications were received by 59 (11.4%) and 101 (19.6%) patients for schizophrenia or other reasons, respectively.
| . | BI 409306, 10 mg (N = 87) . | BI 409306, 25 mg (N = 85) . | BI 409306, 50 mg (N = 85) . | BI 409306, 100 mg (N = 86) . | Placebo (N = 173) . | Total (N = 516) . |
|---|---|---|---|---|---|---|
| Male, n (%) | 53 (60.9) | 56 (65.9) | 65 (76.5) | 58 (67.4) | 128 (74.0) | 360 (69.8) |
| Age, mean (SD), y | 44.1 (8.9) | 43.2 (9.4) | 41.4 (9.5) | 42.3 (9.5) | 41.5 (9.7) | 42.3 (9.5) |
| Race, n (%) | ||||||
| Asian | 19 (21.8) | 11 (12.9) | 16 (18.8) | 19 (22.1) | 33 (19.1) | 98 (19.0) |
| Black or African American | 41 (47.1) | 41 (48.2) | 40 (47.1) | 38 (44.2) | 84 (48.6) | 244 (47.3) |
| White | 27 (31.0) | 32 (37.6) | 28 (32.9) | 26 (30.2) | 54 (31.2) | 167 (32.4) |
| Current antipsychotic treatments, n (%) | ||||||
| 1 | 67 (77.0) | 68 (80.0) | 56 (65.9) | 61 (70.9) | 118 (68.2) | 370 (71.7) |
| 2 | 9 (10.3) | 7 (8.2) | 16 (18.8) | 10 (11.6) | 22 (12.7) | 64 (12.4) |
| 3 | 1 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) |
| CANTAB, mean (SD) | ||||||
| Reaction Time | 371.4 (85.0) | 370.1 (74.8) | 356.1 (68.5) | 372.2 (111.5) | 375.1 (92.7) | 370.0 (88.6) |
| Verbal Recognition Memory | 6.5 (2.2) | 7.4 (3.0) | 7.0 (2.8) | 6.6 (2.3) | 6.7 (2.3) | 6.8 (2.5) |
| Spatial Working Memory | 21.5 (11.2) | 21.8 (11.0) | 18.2 (11.2) | 20.1 (11.6) | 21.3 (12.5) | 20.7 (11.7) |
| Rapid Visual Information Processing | 0.87 (0.06) | 0.87 (0.06) | 0.88 (0.06) | 0.87 (0.06) | 0.88 (0.06) | 0.88 (0.06) |
| Paired Associates Learning | 36.2 (27.1) | 33.4 (26.5)* | 30.0 (27.2) | 31.1 (26.0) | 30.5 (25.1)† | 32.0 (26.2)# |
| Emotion Recognition Task | 51.2 (14.0) | 52.5 (16.6) | 55.5 (14.1) | 53.7 (13.7) | 53.4 (15.0) | 53.3 (14.7) |
| One Touch Stockings of Cambridge | 7.6 (3.2) | 7.7 (3.2) | 8.5 (2.9) | 8.0 (2.9)‡ | 8.2 (3.0)# | 8.0 (3.0)# |
| Attention Switching Task | 70.5 (72.3) | 90.9 (91.8) | 74.2 (68.8) | 79.0 (81.7) | 64.9 (67.7) | 74.0 (75.7) |
| MCCB composite score, mean (SD) | 30.0 (13.6) | 29.4 (13.3) | 31.9 (13.6)* | 29.8 (13.3) | 29.8 (12.6) | 30.1 (13.2)^ |
| PANSS total score, mean (SD) | 59.2 (12.7) | 58.8 (12.5) | 59.5 (11.7) | 59.3 (13.0) | 57.5 (12.7) | 58.6 (12.5) |
| SCoRS total score, mean (SD) | 36.0 (8.7) | 35.8 (8.6) | 37.9 (9.8) | 37.7 (8.6) | 35.9 (8.7) | 36.5 (8.9) |
| . | BI 409306, 10 mg (N = 87) . | BI 409306, 25 mg (N = 85) . | BI 409306, 50 mg (N = 85) . | BI 409306, 100 mg (N = 86) . | Placebo (N = 173) . | Total (N = 516) . |
|---|---|---|---|---|---|---|
| Male, n (%) | 53 (60.9) | 56 (65.9) | 65 (76.5) | 58 (67.4) | 128 (74.0) | 360 (69.8) |
| Age, mean (SD), y | 44.1 (8.9) | 43.2 (9.4) | 41.4 (9.5) | 42.3 (9.5) | 41.5 (9.7) | 42.3 (9.5) |
| Race, n (%) | ||||||
| Asian | 19 (21.8) | 11 (12.9) | 16 (18.8) | 19 (22.1) | 33 (19.1) | 98 (19.0) |
| Black or African American | 41 (47.1) | 41 (48.2) | 40 (47.1) | 38 (44.2) | 84 (48.6) | 244 (47.3) |
| White | 27 (31.0) | 32 (37.6) | 28 (32.9) | 26 (30.2) | 54 (31.2) | 167 (32.4) |
| Current antipsychotic treatments, n (%) | ||||||
| 1 | 67 (77.0) | 68 (80.0) | 56 (65.9) | 61 (70.9) | 118 (68.2) | 370 (71.7) |
| 2 | 9 (10.3) | 7 (8.2) | 16 (18.8) | 10 (11.6) | 22 (12.7) | 64 (12.4) |
| 3 | 1 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) |
| CANTAB, mean (SD) | ||||||
| Reaction Time | 371.4 (85.0) | 370.1 (74.8) | 356.1 (68.5) | 372.2 (111.5) | 375.1 (92.7) | 370.0 (88.6) |
| Verbal Recognition Memory | 6.5 (2.2) | 7.4 (3.0) | 7.0 (2.8) | 6.6 (2.3) | 6.7 (2.3) | 6.8 (2.5) |
| Spatial Working Memory | 21.5 (11.2) | 21.8 (11.0) | 18.2 (11.2) | 20.1 (11.6) | 21.3 (12.5) | 20.7 (11.7) |
| Rapid Visual Information Processing | 0.87 (0.06) | 0.87 (0.06) | 0.88 (0.06) | 0.87 (0.06) | 0.88 (0.06) | 0.88 (0.06) |
| Paired Associates Learning | 36.2 (27.1) | 33.4 (26.5)* | 30.0 (27.2) | 31.1 (26.0) | 30.5 (25.1)† | 32.0 (26.2)# |
| Emotion Recognition Task | 51.2 (14.0) | 52.5 (16.6) | 55.5 (14.1) | 53.7 (13.7) | 53.4 (15.0) | 53.3 (14.7) |
| One Touch Stockings of Cambridge | 7.6 (3.2) | 7.7 (3.2) | 8.5 (2.9) | 8.0 (2.9)‡ | 8.2 (3.0)# | 8.0 (3.0)# |
| Attention Switching Task | 70.5 (72.3) | 90.9 (91.8) | 74.2 (68.8) | 79.0 (81.7) | 64.9 (67.7) | 74.0 (75.7) |
| MCCB composite score, mean (SD) | 30.0 (13.6) | 29.4 (13.3) | 31.9 (13.6)* | 29.8 (13.3) | 29.8 (12.6) | 30.1 (13.2)^ |
| PANSS total score, mean (SD) | 59.2 (12.7) | 58.8 (12.5) | 59.5 (11.7) | 59.3 (13.0) | 57.5 (12.7) | 58.6 (12.5) |
| SCoRS total score, mean (SD) | 36.0 (8.7) | 35.8 (8.6) | 37.9 (9.8) | 37.7 (8.6) | 35.9 (8.7) | 36.5 (8.9) |
Note: Assessed in *84, †172, #514, ‡85, and ^515 patients only. CANTAB, Cambridge Neuropsychological Test Automated Battery; MCCB, Measurements and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery; PANSS, Positive and Negative Syndrome Scale; SCoRS, Schizophrenia Cognition Rating Scale; SD, standard deviation; TS, treated set.
| . | BI 409306, 10 mg (N = 87) . | BI 409306, 25 mg (N = 85) . | BI 409306, 50 mg (N = 85) . | BI 409306, 100 mg (N = 86) . | Placebo (N = 173) . | Total (N = 516) . |
|---|---|---|---|---|---|---|
| Male, n (%) | 53 (60.9) | 56 (65.9) | 65 (76.5) | 58 (67.4) | 128 (74.0) | 360 (69.8) |
| Age, mean (SD), y | 44.1 (8.9) | 43.2 (9.4) | 41.4 (9.5) | 42.3 (9.5) | 41.5 (9.7) | 42.3 (9.5) |
| Race, n (%) | ||||||
| Asian | 19 (21.8) | 11 (12.9) | 16 (18.8) | 19 (22.1) | 33 (19.1) | 98 (19.0) |
| Black or African American | 41 (47.1) | 41 (48.2) | 40 (47.1) | 38 (44.2) | 84 (48.6) | 244 (47.3) |
| White | 27 (31.0) | 32 (37.6) | 28 (32.9) | 26 (30.2) | 54 (31.2) | 167 (32.4) |
| Current antipsychotic treatments, n (%) | ||||||
| 1 | 67 (77.0) | 68 (80.0) | 56 (65.9) | 61 (70.9) | 118 (68.2) | 370 (71.7) |
| 2 | 9 (10.3) | 7 (8.2) | 16 (18.8) | 10 (11.6) | 22 (12.7) | 64 (12.4) |
| 3 | 1 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) |
| CANTAB, mean (SD) | ||||||
| Reaction Time | 371.4 (85.0) | 370.1 (74.8) | 356.1 (68.5) | 372.2 (111.5) | 375.1 (92.7) | 370.0 (88.6) |
| Verbal Recognition Memory | 6.5 (2.2) | 7.4 (3.0) | 7.0 (2.8) | 6.6 (2.3) | 6.7 (2.3) | 6.8 (2.5) |
| Spatial Working Memory | 21.5 (11.2) | 21.8 (11.0) | 18.2 (11.2) | 20.1 (11.6) | 21.3 (12.5) | 20.7 (11.7) |
| Rapid Visual Information Processing | 0.87 (0.06) | 0.87 (0.06) | 0.88 (0.06) | 0.87 (0.06) | 0.88 (0.06) | 0.88 (0.06) |
| Paired Associates Learning | 36.2 (27.1) | 33.4 (26.5)* | 30.0 (27.2) | 31.1 (26.0) | 30.5 (25.1)† | 32.0 (26.2)# |
| Emotion Recognition Task | 51.2 (14.0) | 52.5 (16.6) | 55.5 (14.1) | 53.7 (13.7) | 53.4 (15.0) | 53.3 (14.7) |
| One Touch Stockings of Cambridge | 7.6 (3.2) | 7.7 (3.2) | 8.5 (2.9) | 8.0 (2.9)‡ | 8.2 (3.0)# | 8.0 (3.0)# |
| Attention Switching Task | 70.5 (72.3) | 90.9 (91.8) | 74.2 (68.8) | 79.0 (81.7) | 64.9 (67.7) | 74.0 (75.7) |
| MCCB composite score, mean (SD) | 30.0 (13.6) | 29.4 (13.3) | 31.9 (13.6)* | 29.8 (13.3) | 29.8 (12.6) | 30.1 (13.2)^ |
| PANSS total score, mean (SD) | 59.2 (12.7) | 58.8 (12.5) | 59.5 (11.7) | 59.3 (13.0) | 57.5 (12.7) | 58.6 (12.5) |
| SCoRS total score, mean (SD) | 36.0 (8.7) | 35.8 (8.6) | 37.9 (9.8) | 37.7 (8.6) | 35.9 (8.7) | 36.5 (8.9) |
| . | BI 409306, 10 mg (N = 87) . | BI 409306, 25 mg (N = 85) . | BI 409306, 50 mg (N = 85) . | BI 409306, 100 mg (N = 86) . | Placebo (N = 173) . | Total (N = 516) . |
|---|---|---|---|---|---|---|
| Male, n (%) | 53 (60.9) | 56 (65.9) | 65 (76.5) | 58 (67.4) | 128 (74.0) | 360 (69.8) |
| Age, mean (SD), y | 44.1 (8.9) | 43.2 (9.4) | 41.4 (9.5) | 42.3 (9.5) | 41.5 (9.7) | 42.3 (9.5) |
| Race, n (%) | ||||||
| Asian | 19 (21.8) | 11 (12.9) | 16 (18.8) | 19 (22.1) | 33 (19.1) | 98 (19.0) |
| Black or African American | 41 (47.1) | 41 (48.2) | 40 (47.1) | 38 (44.2) | 84 (48.6) | 244 (47.3) |
| White | 27 (31.0) | 32 (37.6) | 28 (32.9) | 26 (30.2) | 54 (31.2) | 167 (32.4) |
| Current antipsychotic treatments, n (%) | ||||||
| 1 | 67 (77.0) | 68 (80.0) | 56 (65.9) | 61 (70.9) | 118 (68.2) | 370 (71.7) |
| 2 | 9 (10.3) | 7 (8.2) | 16 (18.8) | 10 (11.6) | 22 (12.7) | 64 (12.4) |
| 3 | 1 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) |
| CANTAB, mean (SD) | ||||||
| Reaction Time | 371.4 (85.0) | 370.1 (74.8) | 356.1 (68.5) | 372.2 (111.5) | 375.1 (92.7) | 370.0 (88.6) |
| Verbal Recognition Memory | 6.5 (2.2) | 7.4 (3.0) | 7.0 (2.8) | 6.6 (2.3) | 6.7 (2.3) | 6.8 (2.5) |
| Spatial Working Memory | 21.5 (11.2) | 21.8 (11.0) | 18.2 (11.2) | 20.1 (11.6) | 21.3 (12.5) | 20.7 (11.7) |
| Rapid Visual Information Processing | 0.87 (0.06) | 0.87 (0.06) | 0.88 (0.06) | 0.87 (0.06) | 0.88 (0.06) | 0.88 (0.06) |
| Paired Associates Learning | 36.2 (27.1) | 33.4 (26.5)* | 30.0 (27.2) | 31.1 (26.0) | 30.5 (25.1)† | 32.0 (26.2)# |
| Emotion Recognition Task | 51.2 (14.0) | 52.5 (16.6) | 55.5 (14.1) | 53.7 (13.7) | 53.4 (15.0) | 53.3 (14.7) |
| One Touch Stockings of Cambridge | 7.6 (3.2) | 7.7 (3.2) | 8.5 (2.9) | 8.0 (2.9)‡ | 8.2 (3.0)# | 8.0 (3.0)# |
| Attention Switching Task | 70.5 (72.3) | 90.9 (91.8) | 74.2 (68.8) | 79.0 (81.7) | 64.9 (67.7) | 74.0 (75.7) |
| MCCB composite score, mean (SD) | 30.0 (13.6) | 29.4 (13.3) | 31.9 (13.6)* | 29.8 (13.3) | 29.8 (12.6) | 30.1 (13.2)^ |
| PANSS total score, mean (SD) | 59.2 (12.7) | 58.8 (12.5) | 59.5 (11.7) | 59.3 (13.0) | 57.5 (12.7) | 58.6 (12.5) |
| SCoRS total score, mean (SD) | 36.0 (8.7) | 35.8 (8.6) | 37.9 (9.8) | 37.7 (8.6) | 35.9 (8.7) | 36.5 (8.9) |
Note: Assessed in *84, †172, #514, ‡85, and ^515 patients only. CANTAB, Cambridge Neuropsychological Test Automated Battery; MCCB, Measurements and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery; PANSS, Positive and Negative Syndrome Scale; SCoRS, Schizophrenia Cognition Rating Scale; SD, standard deviation; TS, treated set.
Efficacy Analyses
CANTAB Domains in Stage 1.
At the end of stage 1, none of the CANTAB tests differentiated between BI 409306 and placebo, with an effect size (demonstrating improvement) of 0.5 or greater (figures 2A–H, supplementary table S3); effect sizes ranged from −0.554 for change from baseline in CANTAB One Touch Stockings of Cambridge, problems solved on first choice (demonstrating worsening) to 0.432 for change from baseline in Rapid Visual Information Processing, A prime (demonstrating improvement). The change from baseline in the composite score of MCCB after 12 weeks of treatment was therefore specified as the primary endpoint for stage 2.
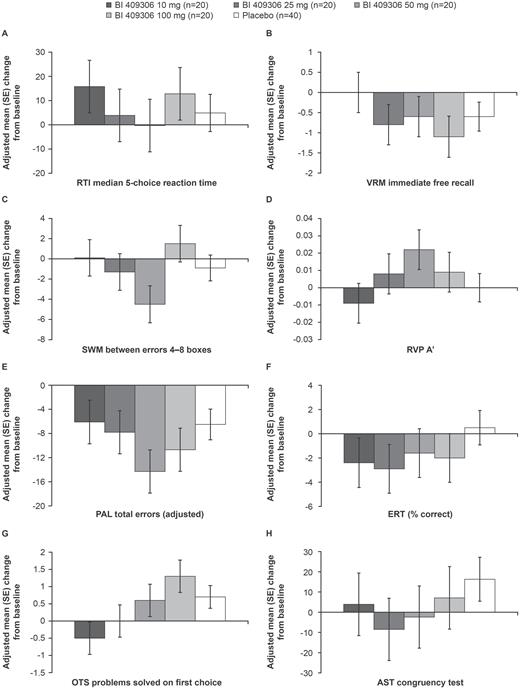
Change from baseline in CANTAB elements (Stage 1); (A) RTI median 5-choice reaction time; (B) VRM immediate free recall; (C) SWM between errors 4–8 boxes; (D) RVP A’; (E) PAL total errors; (adjusted); (F) ERT (% correct); (G) OTS problems solved on first choice; (H) AST congruency test. AST, attention switching task; CANTAB, Cambridge Neuropsychological Test Automated Battery; ERT, emotion recognition task; OTS, One Touch Stockings of Cambridge; PAL, Paired Associates Learning; RTI, Reaction Time; RVP, Rapid Visual Information Processing; SE, standard error; SWM, Spatial Working Memory.
MCCB Composite Endpoint in Stage 2.
The adjusted mean (standard error [SE]) change from baseline in MCCB composite score at week 12 was 1.2 (0.71) to 2.8 (0.75) across BI 409306 treatment groups and 2.5 (0.57) for placebo. There were no BI 409306 treatment groups that were significantly different vs placebo (figure 3, supplementary table S4). The change from baseline in MCCB composite scores over time (baseline, week 6, and week 12) is shown in supplementary figure S3.
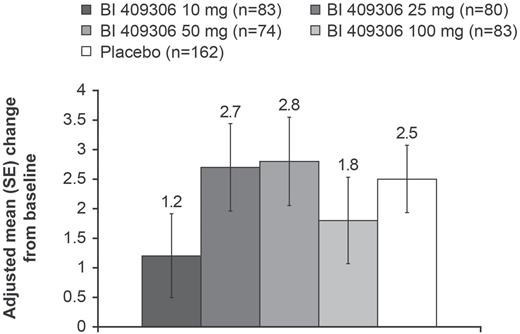
Change from baseline in MCCB composite score at week 12 (FAS). FAS, full analysis set; MCCB, Measurements and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery; SE, standard error.
There was no BI 409306 dose–response relationship for change from baseline at week 12 in the MCCB composite score, with linear and quadratic dose–response estimates of 0.00 (P = .7377) and 0.02 (P = .5769), respectively.
SCoRS Total Score.
The adjusted mean (SE) change from baseline in SCoRS total score at week 12 ranged from −2.0 (0.56) to −3.1 (0.56) across the treatment groups. There were no significant differences between the BI 409306 treatment groups and placebo (supplementary table S5).
Secondary Efficacy Endpoints.
For the adjusted mean (SE) change from baseline at week 12 in CGI-S scores, there were no significant differences between the BI 409306 treatment groups (−0.1 [0.05]) and placebo (−0.1 [0.03]) (supplementary table S5). The mean (SD) PGI-I scores at week 12 were similar for all BI 409306 treatment groups and placebo, ranging from 2.883 (1.124) to 3.192 (1.101) (supplementary table S5).
The results from the other endpoints are included in the supplementary materials.
Safety Analyses
Overall, AEs were reported in 204 (39.5%) patients (supplementary table S6). The proportions of AEs were similar in the BI 409306 10 mg and 25 mg and placebo arms (33.3%–36.5%) with higher proportions of AEs reported with BI 409306 50 mg and 100 mg (41.2% and 53.5%, respectively). Overall, 47 (9.1%) patients reported eye disorders (supplementary table S6). The proportion of patients reporting AEs leading to trial withdrawal was low (5.4%), with the highest proportion in the BI 409306 100 mg group (9.3%). One patient receiving BI 409306 50 mg and 1 patient receiving placebo discontinued owing to worsening of disease, as judged by the investigator. SAEs were reported in 10 of 516 (1.9%) patients, which were all in the placebo group and included 8 patients experiencing psychiatric disorders. Of these 8 patients, 5 experienced mild to moderate worsening of schizophrenia, 2 of whom required hospitalization; 3 patients experienced suicidal ideation, 1 of whom was hospitalized with worsening of schizophrenia. An additional patient was hospitalized for the AE of psychotic disorder.
Furthermore, there were 3 patients who experienced worsening of schizophrenia after the residual effect period (7 days after last dose). For the SAE category of psychiatric disorders, a post hoc Fisher’s exact test showed that there were significantly more exacerbations in the placebo group (n = 8) compared with no exacerbations in the pooled BI 409306 treatment groups (P = .0001). There were no deaths during the study and no patients reported an AESI.
For the endpoint of worsening of disease state as assessed by PANSS, the mean change from baseline in PANSS negative symptom factor scores at week 12 was small across BI 409306 treatment groups and generally similar to the placebo group (placebo, −0.48; 10 mg, −0.46; 25 mg, −0.17; 50 mg, −0.51; 100 mg, 0.15). The change from baseline in PANSS positive symptom factor score was also similar across BI 409306 treatment groups (ranging from −0.01 to −0.77) and compared with placebo (−0.42). There was no clinically meaningful change in psychopathology symptoms as assessed by PANSS. The mean change from baseline in PANSS general psychopathology scale ranged from −0.38 to −1.36 across BI 409306 treatment groups vs −0.76 for placebo (supplementary table S5). There were no patients with suicidal behavior (data not shown).
Results for other endpoints are included as supplementary material.
Discussion
This trial aimed to test superiority of BI 409306 over placebo on cognition in patients with schizophrenia, using a novel learn-and-confirm trial design. In stage 1 (learn), there were no CANTAB measures that differentiated between BI 409306 and placebo, so the MCCB composite score was prespecified as the primary endpoint in stage 2 (confirm). The results from the final analysis in stage 2 suggest that BI 409306 did not improve cognition in patients with CIAS in the residual phase and receiving stable treatment.
The small to absent effect sizes in this trial are consistent with those of previous studies investigating the effects of procognitive compounds in CIAS. Compounds that target the nicotinic α7 receptor have been shown to improve cognition in some trials, while having limited effects in others.24–29 The α7 agonists RG3487,29 encenicline,26 CDP-choline that was combined with galantamine (a positive allosteric modulator to improve signal transduction and preserve α7 receptor sensitivity),24 and the partial α7 agonist 3-(2,4-dimethoxybenzylidene) anabaseine (DMXB-A)25 did not significantly improve cognitive function in patients with CIAS compared with placebo, determined using the MCCB. In addition, a phase II trial conducted across 64 sites in the United States, Russia, Ukraine, Hungary, Romania, and Serbia showed that cognition, determined by the Cogstate Schizophrenia Battery and Subject Global Impression-Cognition, was not improved following treatment with the nicotinic α7 agonist, TC-5619.28 It is unlikely that the absence of a significant treatment effect in this trial was due to placebo producing similar changes in MCCB scores compared with BI 409306, as the MCCB scores for placebo were consistent with pretreatment scores reported by 2616 patients with schizophrenia from 15 trials conducted between February 2007 and July 2016.30 In addition, the mean change from baseline in MCCB composite score at week 12 (supplementary table S4) was similar to those previously reported from 12 trials assessing MCCB scores in patients receiving placebo for 24 weeks.
Overall, BI 409306 was well tolerated and SAEs were only reported in the placebo arm. The safety results from this trial are consistent with those from a previous trial that showed that BI 409306 10–100 mg administered as a tablet was well tolerated in healthy male patients who had been genotyped as extensive or poor metabolizers of cytochrome P450.31 Patients in the present trial receiving BI 409306 were also significantly less likely to have exacerbations compared with those receiving placebo (P = .0001). The present trial suggested that there was a dose-dependent increase in eye disorders, with vision blurred, photophobia, and visual brightness being the most commonly reported eye AEs. This observation is consistent with previous studies with BI 409306, which demonstrated that eye AEs (photopsia, photophobia, chromatopsia, and blurred vision) were the most frequently reported AE.31,32 All of these AEs have been nonserious and mild to moderate in intensity, and have generally been reported as transient, resolving upon treatment discontinuation. While the frequency of these AEs generally increased with dose, at higher doses, there was no obvious relationship between dose and maximum duration and intensity. The onset and duration of these AEs seems related to the maximum concentration of BI 409306 in the plasma (Cmax); overall, onset was approximately 1–2 hours post administration and duration was approximately 30–120 minutes.31,32
This trial used the novel learn-and-confirm trial design, which enabled the most suitable endpoint to be selected from many relevant cognitive tests, based on an interim analysis at the end of stage 1. The trial was randomized, well powered, included a large sample size, with few missing data and used large cognitive batteries to capture the many cognitive deficits in schizophrenia. Therefore, the data reported here are considered to be of high quality and integrity. The baseline MCCB composite scores parallel those previously reported, suggesting the trial population was a true representation of patients with schizophrenia.30
Despite good trial conduct and supportive phase I31,32 and nonclinical data,13,14 the trial did not demonstrate efficacy of BI 409306 once daily for 12 weeks in patients with CIAS who were clinically stable and receiving stable antipsychotic treatment. The effects of BI 409306 were not investigated in differing schizophrenia populations, eg, patients with different degrees of CIAS, first-episode psychosis, or patients at high risk, all of whom may have been responsive. A further potential limitation was the under-representation of female patients and the over-representation of African American males.
A previous study has suggested that environmental enrichment using cognitive remediation may improve cognitive performance and enhance the effects of pharmacotherapy, suggesting it is plausible that a positive response to BI 409306 treatment might be observed in a cognitively enriched environment, although this requires further study.33 Based on the results shown here, it is possible that the nonclinical model targeting PDE9 in CIAS,13,14 on which this trial is based, might not accurately predict cognitive impairment in patients with schizophrenia. Future studies may benefit from recruiting patients earlier in the disease course and administration of BI 409306 in conjunction with cognitive remediation therapy.
Conclusion
The results from this trial suggest that BI 409306 10–100 mg was well tolerated, but the primary endpoint of demonstrating efficacy in cognitive function in patients with CIAS on stable antipsychotic medications was not met. Based on its favorable safety profile, future clinical trials in differing schizophrenia populations will be of interest.
Funding
The work presented here, including the conduct of the trial, data analysis and interpretation, was funded by Boehringer Ingelheim International GmbH. The sponsor was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Acknowledgments
We acknowledge Kiri Granger, PhD, and Charlotte Housden, PhD, of Cambridge Cognition (CAMCOG) for their assistance with design and analysis of CANTAB data and the editorial support (in the form of initial preparation of the outline based on input from all authors, collation and incorporation of author feedback to develop subsequent drafts, assembling tables and figures, copyediting, and referencing) provided by Rachael Baylie, PhD, and Natasha Thomas, PhD, of Fishawack Communications, which was funded by Boehringer Ingelheim International GmbH. The authors met the criteria for authorship as recommended by the International Committee of Medical Journal Editors. Three of the authors (K.D., L.W., and M.S.) are employees of Boehringer Ingelheim Pharmaceuticals, Inc. and J.M. was an employee of Boehringer Ingelheim Pharmaceuticals, Inc. at time of study. Authors received no direct compensation related to the development of this manuscript. R.B. provides clinical trial support for Acadia Pharmaceuticals, Allergan, Alkermes, AstraZeneca, Boehringer-Ingelheim, Forum Pharmaceuticals, Intra-Cellular Therapies, Janssen Pharmaceutical Companies, Lundbeck, Neurocrine Biosciences, Takeda Development Center Americas, Tonix Pharmaceuticals, Reckitt-Benckister Pharmaceuticals, Otsuka Pharmaceutical Development and Commercialization, and Pfizer and provides support for Otsuka Pharmaceutical Development and Commercialization and Alkermes speaker bureaus. R.S.E.K. is a paid consultant to Boehringer Ingelheim and several other pharmaceutical companies and is the owner of NeuroCog Trials, which provided paid services for this trial. R.S.E.K. receives royalties for the Brief Assessment of Cognition in Schizophrenia (BACS) symbol coding, which is a part of the MATRICS Battery. From 2011 until present, J.C. was involved in studies which were sponsored by Boehringer Ingelheim Pharma GmbH, Otsuka Pharmaceutical Europe Ltd., EnVivo Pharmaceuticals, Bundesministerium für Gesundheit, Bundesministerium für Bildung und Forschung (BMBF) and Deutsche Forschungsgemeinschaft (DFG). G.G. has served as a consultant for Allergan, Boehringer Ingelheim, Eli Lilly, Janssen-Cilag, Lundbeck, Ono Pharmaceuticals, Otsuka, Recordati, Roche, Servier, and Takeda; has served on the speakers’ bureau of Eli Lilly, Janssen Cilag, Lundbeck, Neuraxpharma, Otsuka, Roche, Servier, and Trommsdorff; has received grant support from Boehringer Ingelheim and Roche; and is co-founder of Mind and Brain Institute GmbH and Brainfoods GmbH. D.W. receives grant and research support from Novartis, J&J PRD, Sunovion, Janssen, Pfizer, AbbVie, Alkermes, Allergan, Takeda, Otsuka, Zogenix, Omeros, CoMentis, IntraCellular, Lupin, Avanir, Lundbeck, and Roche, and serves as a consultant for Otsuka, Janssen and Acadia. K.N. has received lecture fees from Kyowa Hakko Kirin, Otsuka Pharmaceutical, Sumitomo Dainippon Pharma, Eli Lilly Japan, Shionogi, Meiji Seika Pharma, Pfizer Japan, Quintiles Transnational Japan, MSD, GlaxoSmithKline, Janssen Pharmaceutical, Mochida Pharmaceutical, and Yoshitomiyakuhin, and participated in an advisory board for Otsuka Pharmaceutical, Nippon Boehringer Ingelheim, Takeda Pharmaceutical, Toyama Chemical, Sumitomo Dainippon Pharma, Meiji Seika Pharma, Pfizer Japan, Janssen Pharmaceutical, and Mochida Pharmaceutical, and received research funding from Mitsubishi Tanabe Pharma, Asahi Kasei Pharma, Astellas Pharma, Mochida Pharmaceutical, Meiji Seika Pharma, Otsuka Pharmaceutical, Nippon Boehringer Ingelheim, and Sumitomo Dainippon Pharma. D.B. and R.R. have no disclosure details to declare.
References
World Medical Association.
American Psychiatric Association.
Author notes
at time of study




