-
PDF
- Split View
-
Views
-
Cite
Cite
Marine Mondino, Renaud Jardri, Marie-Françoise Suaud-Chagny, Mohamed Saoud, Emmanuel Poulet, Jérôme Brunelin, Effects of Fronto-Temporal Transcranial Direct Current Stimulation on Auditory Verbal Hallucinations and Resting-State Functional Connectivity of the Left Temporo-Parietal Junction in Patients With Schizophrenia, Schizophrenia Bulletin, Volume 42, Issue 2, March 2016, Pages 318–326, https://doi.org/10.1093/schbul/sbv114
Close - Share Icon Share
Abstract
Auditory verbal hallucinations (AVH) in patients with schizophrenia are associated with abnormal hyperactivity in the left temporo-parietal junction (TPJ) and abnormal connectivity between frontal and temporal areas. Recent findings suggest that fronto-temporal transcranial Direct Current stimulation (tDCS) with the cathode placed over the left TPJ and the anode over the left prefrontal cortex can alleviate treatment-resistant AVH in patients with schizophrenia. However, brain correlates of the AVH reduction are unclear. Here, we investigated the effect of tDCS on the resting-state functional connectivity (rs-FC) of the left TPJ. Twenty-three patients with schizophrenia and treatment-resistant AVH were randomly allocated to receive 10 sessions of active (2 mA, 20min) or sham tDCS (2 sessions/d for 5 d). We compared the rs-FC of the left TPJ between patients before and after they received active or sham tDCS. Relative to sham tDCS, active tDCS significantly reduced AVH as well as the negative symptoms. Active tDCS also reduced rs-FC of the left TPJ with the left anterior insula and the right inferior frontal gyrus and increased rs-FC of the left TPJ with the left angular gyrus, the left dorsolateral prefrontal cortex and the precuneus. The reduction of AVH severity was correlated with the reduction of the rs-FC between the left TPJ and the left anterior insula. These findings suggest that the reduction of AVH induced by tDCS is associated with a modulation of the rs-FC within an AVH-related brain network, including brain areas involved in inner speech production and monitoring.
Introduction
Auditory verbal hallucinations (AVH) are core symptoms of schizophrenia. AVH can be defined as perceptions of speech in the absence of external stimuli, frequently associated with significant functional disability. The neurobiological bases of AVH are complex and remain unclear. Nevertheless, recent advances in neuroimaging contributed to identify neural substrates of AVH. A recent coordinate-based meta-analysis of functional imaging studies reported that during the occurrence of AVH, patients with schizophrenia exhibited significant over-activation in distributed brain areas including left temporo-parietal areas (middle and superior temporal gyri and Wernicke’s area), left inferior frontal areas (Broca’s area, frontal operculum, anterior insula, precentral gyrus), as well as in their right homologues. 1 These findings suggested aberrant activations within speech perception and production brain areas during the occurrence of AVH.
Studies assessing structural and functional connectivity in vivo in patients with schizophrenia experiencing AVH (AVH+) suggest that, rather than localized functional impairments, AVH may result from a dysconnectivity between some temporo-parietal areas and distributed brain areas involved in the production and the monitoring of speech. For instance, AVH+ patients display a decreased fractional anisotropy in the left arcuate fasciculus (ie, an associative fiber tract connecting the frontal, parietal, and temporal cortices), 2 , 3 in line with the hypothesis of an impaired connectivity between perception and production speech areas. Resting-state Functional Connectivity (rs-FC) has also been investigated in AVH+ patients. Using functional magnetic resonance imaging (fMRI), rs-FC measurements refer to the correlations of Blood Oxygen Level-Dependent (BOLD) activity time-course between different brain regions. Again, numerous studies have highlighted abnormal rs-FC between some temporo-parietal, frontal and subcortical regions in AVH+ patients, reporting either an increased, 4 , 5 a reduced 6 , 7 or both pattern of rs-FC 8–11 within the fronto-temporal network (for reviews see ref. 12 , 13 ).
Essentially, based on the observations of a fronto-temporal dysconnectivity in AVH, it was hypothesized that modulating the abnormal activity of a brain area involved in this network with noninvasive brain stimulation techniques may alleviate AVH. 14 Numerous studies support the clinical efficacy of low-frequency repetitive Transcranial Magnetic Stimulation (rTMS) to reduce the severity of treatment-resistant AVH. 15 In such studies, rTMS used in order to diminish cortical excitability was applied over the left temporo-parietal junction (TPJ), a region encompassing the posterior part of the superior temporal gyrus (Wernicke’s area) and parts of the supramarginal gyrus and the angular gyrus in the inferior parietal lobule. More recently, transcranial Direct Current Stimulation (tDCS) was examined as a new tool to reduce treatment-resistant AVH. tDCS is a well-tolerated noninvasive neuromodulation technique, 16 which consists in delivering a low-intensity current over the scalp between 2 electrodes. Measurements of the impact of tDCS on sensory cortices excitability revealed a polarity-dependent effect of the stimulation: the anode induces an increase in cortical excitability while the cathode reduces it. 17 tDCS with the cathode placed over the left TPJ and the anode placed over the left dorsolateral prefrontal cortex (DLPFC) was reported to reduce AVH in patients with schizophrenia in numerous case studies, 14 , 18–26 2 open trials, 27 , 28 and in a randomized controlled trial including 30 patients. 29 In most of these studies, tDCS sessions were delivered twice a day for 5 consecutive days (10 sessions). 14 , 18 , 20–29 One study has investigated the effects of 15 once-daily tDCS sessions over 3 consecutive weeks and failed to report a significant effect on AVH. 30 However, brain correlates of the AVH reduction was not systematically investigated. Only a case study reported that cathodal tDCS applied over the left superior temporal gyrus coupled with anodal tDCS over the right supraorbital area was able to decrease cerebral blood flow in AVH-related brain areas (ie, Wernicke’s area, Broca’s area and left Heschl’s gyrus) in association with AVH reduction. 19
The present randomized sham-controlled study was designed to assess whether tDCS alleviating AVH significantly affects the rs-FC of the left TPJ in a sample of patients with schizophrenia. We hypothesized that tDCS modulates rs-FC between the left TPJ and the fronto-temporal network involved in AVH, in particular the interplay between speech perception and production areas. This hypothesis was tested at the clinical level by investigating changes in AVH scores and at the neurobiological level by comparing the seed-based rs-FC patterns of the left TPJ before and after 10 sessions of tDCS delivered twice-daily, depending on the treatment allocation group (ie, active or sham tDCS).
Methods
Participants
Twenty-three patients with schizophrenia according to DSM-IV-TR criteria were included in the experiment. The sample partially overlapped with a previous published sample 29 (clinical data from 7 patients of the sham group and 8 of the active group were already used). The absence of any other Axis I psychiatric disorder was assessed by the Mini International Neuropsychiatric Interview semi-standardized evaluation (MINI). 31 All participants exhibited daily AVH despite antipsychotic medications at an adequate stable dosage for at least 3 months. Their antipsychotic medication remained unchanged throughout the study period. Patients were randomly allocated to receive active tDCS ( n = 11) or sham tDCS ( n = 12). At inclusion, the 2 groups were not statistically different for age, sex, handedness, antipsychotic dosage, general psychopathology, and AVH severity ( table 1 ). Antipsychotic medication dosage was reported as olanzapine equivalent. 32 Three patients in the active group and 1 patient in the sham group were treated with clozapine. Two patients per group have previously been enrolled in an rTMS study at least 6 months before inclusion in this study. None of the patients have received electroconvulsivotherapy or tDCS in the past. Global symptoms of schizophrenia and AVH were assessed before the first tDCS session and following the final tDCS session by a blind investigator using respectively the Positive and Negative Syndrome Scale (PANSS) 33 and the Auditory Hallucinations Rating Scale (AHRS). 34 The study was approved by a local ethical committee (CPP Sud-Est 6, France) and registered ( clinicaltrials.gov NCT00870909). All the participants provided written informed consent after complete description of the study.
Baseline Demographical and Clinical Characteristics of the 23 Patients With Schizophrenia and Treatment-Resistant Auditory Verbal Hallucinations Receiving Either Active or Sham Transcranial Direct Current Stimulation
| . | Active Group ( N = 11) . | Sham Group ( N = 12) . | P value . | ||||
|---|---|---|---|---|---|---|---|
| . | N . | Mean . | SD . | N . | Mean . | SD . | |
| Gender (male/female) | 8/3 | 7/5 | .7 | ||||
| Handedness (right-/left-handed) | 10/1 | 10/2 | 1 | ||||
| Age (y) | 36.7 | 9.7 | 37.3 | 9.7 | .9 | ||
| Illness duration (y) | 11.8 | 3.3 | 12.2 | 1.9 | .9 | ||
| PANSS score | |||||||
| Total | 67.6 | 14.7 | 74.3 | 9.6 | .8 | ||
| Positive | 18.5 | 4.4 | 19.8 | 4.2 | .5 | ||
| Negative | 18.1 | 5.5 | 19.4 | 4.6 | .7 | ||
| General | 31.0 | 8.2 | 35.1 | 6.0 | .2 | ||
| AHRS score | 27.2 | 4.1 | 27.8 | 8.0 | .5 | ||
| Olanzapine equivalent dose (mg/d) | 23.0 | 11.4 | 25.4 | 12.3 | .5 | ||
| . | Active Group ( N = 11) . | Sham Group ( N = 12) . | P value . | ||||
|---|---|---|---|---|---|---|---|
| . | N . | Mean . | SD . | N . | Mean . | SD . | |
| Gender (male/female) | 8/3 | 7/5 | .7 | ||||
| Handedness (right-/left-handed) | 10/1 | 10/2 | 1 | ||||
| Age (y) | 36.7 | 9.7 | 37.3 | 9.7 | .9 | ||
| Illness duration (y) | 11.8 | 3.3 | 12.2 | 1.9 | .9 | ||
| PANSS score | |||||||
| Total | 67.6 | 14.7 | 74.3 | 9.6 | .8 | ||
| Positive | 18.5 | 4.4 | 19.8 | 4.2 | .5 | ||
| Negative | 18.1 | 5.5 | 19.4 | 4.6 | .7 | ||
| General | 31.0 | 8.2 | 35.1 | 6.0 | .2 | ||
| AHRS score | 27.2 | 4.1 | 27.8 | 8.0 | .5 | ||
| Olanzapine equivalent dose (mg/d) | 23.0 | 11.4 | 25.4 | 12.3 | .5 | ||
Note : PANSS, Positive and Negative Syndrome Scale; AHRS, Auditory Hallucination Rating Scale. Mann–Whitney U tests and chi-square tests were conducted to assess group differences for continuous and discrete variables, respectively.
Baseline Demographical and Clinical Characteristics of the 23 Patients With Schizophrenia and Treatment-Resistant Auditory Verbal Hallucinations Receiving Either Active or Sham Transcranial Direct Current Stimulation
| . | Active Group ( N = 11) . | Sham Group ( N = 12) . | P value . | ||||
|---|---|---|---|---|---|---|---|
| . | N . | Mean . | SD . | N . | Mean . | SD . | |
| Gender (male/female) | 8/3 | 7/5 | .7 | ||||
| Handedness (right-/left-handed) | 10/1 | 10/2 | 1 | ||||
| Age (y) | 36.7 | 9.7 | 37.3 | 9.7 | .9 | ||
| Illness duration (y) | 11.8 | 3.3 | 12.2 | 1.9 | .9 | ||
| PANSS score | |||||||
| Total | 67.6 | 14.7 | 74.3 | 9.6 | .8 | ||
| Positive | 18.5 | 4.4 | 19.8 | 4.2 | .5 | ||
| Negative | 18.1 | 5.5 | 19.4 | 4.6 | .7 | ||
| General | 31.0 | 8.2 | 35.1 | 6.0 | .2 | ||
| AHRS score | 27.2 | 4.1 | 27.8 | 8.0 | .5 | ||
| Olanzapine equivalent dose (mg/d) | 23.0 | 11.4 | 25.4 | 12.3 | .5 | ||
| . | Active Group ( N = 11) . | Sham Group ( N = 12) . | P value . | ||||
|---|---|---|---|---|---|---|---|
| . | N . | Mean . | SD . | N . | Mean . | SD . | |
| Gender (male/female) | 8/3 | 7/5 | .7 | ||||
| Handedness (right-/left-handed) | 10/1 | 10/2 | 1 | ||||
| Age (y) | 36.7 | 9.7 | 37.3 | 9.7 | .9 | ||
| Illness duration (y) | 11.8 | 3.3 | 12.2 | 1.9 | .9 | ||
| PANSS score | |||||||
| Total | 67.6 | 14.7 | 74.3 | 9.6 | .8 | ||
| Positive | 18.5 | 4.4 | 19.8 | 4.2 | .5 | ||
| Negative | 18.1 | 5.5 | 19.4 | 4.6 | .7 | ||
| General | 31.0 | 8.2 | 35.1 | 6.0 | .2 | ||
| AHRS score | 27.2 | 4.1 | 27.8 | 8.0 | .5 | ||
| Olanzapine equivalent dose (mg/d) | 23.0 | 11.4 | 25.4 | 12.3 | .5 | ||
Note : PANSS, Positive and Negative Syndrome Scale; AHRS, Auditory Hallucination Rating Scale. Mann–Whitney U tests and chi-square tests were conducted to assess group differences for continuous and discrete variables, respectively.
Clinical Data Analysis
Because of nonnormal distribution of the data, nonparametric statistics (Mann–Whitney U tests) were used to compare demographic and clinical characteristics between the 2 groups at baseline. Gender and handedness proportions differences were assessed using a chi-square test. The interaction between the group and time factors was investigated using a nonparametric adjusted rank-transform test. 35 In the 2 groups (active and sham), individual changes in AHRS scores after tDCS were investigated using Wilcoxon’s matched pairs T tests. Improvement in AHRS scores following tDCS was compared between groups (active and sham) using Mann–Whitney U tests. Significance was set at P < .05. Effects of tDCS on other symptoms measured by PANSS subscores and on AHRS in the “right-handed” subgroup were provided in Supplementary Data .
tDCS Procedure
We referred to a double-blind randomized parallel-arms design. Experimenters and patients were both blind to the tDCS-condition assignment.
tDCS was carried out with an Eldith DC stimulator (NeuroConn, GmbH) using 2 saline-soaked sponge electrodes (7×5cm) applied over the subject’s scalp. Electrodes were placed according to the 10–20 international system for electrode placement in electroencephalography. In accordance with previous tDCS studies, 27 , 29 the anode was positioned midway between F3 and Fp1 (left DLPFC) and the cathode midway between T3 and P3 (left TPJ). Active tDCS consisted in delivering a constant current of 2 mA for 20 minutes (ramp-in and ramp-out periods, 30 s). For the sham tDCS, which was activated by a code number, the 2 mA current was delivered only during the first 30 seconds of the 20-minute stimulation period mimicking somatosensory artefact of active tDCS. 36 Each participant received a total of 10 sessions conducted twice daily on 5 consecutive days (from Monday to Friday). Each day, the first session was delivered at 11.00 AM and the second at 1.30 PM. Participants were instructed to be as quiet as possible during sessions.
fMRI Data Acquisition and Preprocessing
Images were acquired at the “CERMEP-Imagerie du vivant” imaging centre of Lyon (France) on a 1.5 T Siemens Magnetom Sonata Maestro Class system with a standard 8-channel head coil. A 3-dimensional (3D) T1-weighted anatomical scan was first acquired with the following parameters: 176 transverse slices; repetition time = 1970ms; echo time = 3.93ms; field of view = 256mm 2 ; voxel size = 1mm. 3
All participants underwent 2 resting-state fMRI scans. The first fMRI scan was acquired at baseline on the Friday before starting tDCS sessions and the second fMRI scan was acquired 1 hour after the end of the 10th tDCS session, at 3.00 PM on Friday. One hundred twenty volumes of T2*-weighted BOLD-fMRI images were acquired using an EPI sequence (29 transverse slices, field of view = 220mm 2 , voxel size = 4mm 3 , repetition time = 2500ms, echo time = 50ms, and total acquisition = 5min). During acquisition, the participants remained in a state of wakeful rest; they were instructed to stay awake and to lie still with their eyes opened, fixating on a white cross presented at the centre of the visual field. All patients wore headphones to attenuate the scanner noise. The 2 first volumes of each functional scan were discarded for scan equilibration.
The anatomical and functional data were preprocessed and analyzed using the BrainVoyager software (v2.8.2, BrainInnovation, The Netherlands, http://www.brainvoyager.com ). Functional data were preprocessed using a slice scan time correction, 3D head motion correction, smoothing using a 3D Gaussian filter of 5.0mm spatial full width at half maximum value and a temporal high-pass filtering with 2 sines/cosines. The 3D anatomical image was normalized in the Talairach stereotaxic space. 37
Seed-Based rs-FC Analysis
For each subject, correlations between the extracted time-course of an a priori seed region with other brain voxels were computed. A spherical seed region of interest (diameter = 25mm), corresponding to the left TPJ (Talairach coordinates: X = −48; Y = −33; Z = 21) was defined according to Vercammen et al 38 ( figure 1A ). Vercammen and colleagues’ MNI coordinates for the left TPJ were converted to Talairach space using the icbm2tal algorithm ( http://www.brainmap.org/icbm2tal/ ).
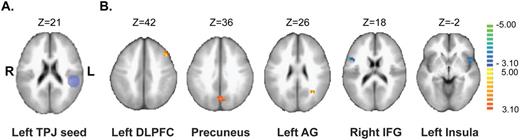
Effects of transcranial Direct Current stimulation (tDCS) on seed-based resting-state functional connectivity (rs-FC) of the left temporo-parietal junction in patients with schizophrenia suffering from auditory hallucinations ( N = 23). (A) Projection of the left temporo-parietal junction seed over the ICBM brain template. (B) Comparison of the seed-based rs-FC changes after tDCS according to the patient group (the contrast (after active tDCS > before active tDCS) > (after sham tDCS > before sham tDCS)) is presented in volume space. Slice-views synthesize contrast maps obtained after volumetric normalization (Talairach), overlaid over the ICBM-152 brain template. Analysis of the left Temporal-Parietal Junction seed-based rs-FC revealed a significant increase in rs-FC after active tDCS when compared with sham mainly with the left dorsolateral prefrontal cortex (DLPFC), the precuneus and the left angular gyrus (AG) and a significant decrease in rs-FC within inferior frontal regions including the right inferior frontal gyrus (IFG) and the left anterior insula.
A single-subject general linear model (GLM) with z -normalized predictors was first computed to obtain individual rs-FC maps, as described in Amad et al. 39 Eight covariates of no-interest were also introduced in the GLM: white matter signal, cerebro-spinal fluid signal, and head-motion parameters (x/y/z corrections applied to translation and rotation). For second-level analyses, statistical maps containing resulting beta-values from the individual GLM were computed with the multi-subject random effects (RFX) GLM function. Separated beta-maps were created for the after-tDCS > before-tDCS contrast for each subject and compared across groups (active and sham) using t-test. The resulting map was thresholded at a voxel-level threshold of P < .005, corrected for multiple comparisons at the cluster level using iterative Monte Carlo simulations (1000 iterations) to objectively discard small and/or isolated voxels. As a result of Monte–Carlo simulations, the minimum cluster size was set at 11 voxels.
Correlation Analyses
The relationship between individual changes in AVH severity (changes in AHRS scores after tDCS) and individual changes in TPJ functional connectivity in the regions that exhibited group differences (changes in beta values after tDCS) was assessed using Spearman rank correlation tests. To correct for multiple comparisons, significance was set at P < .01.
Results
Characteristics of Participants at Baseline
No significant differences were found between groups in age, gender, handedness, illness duration, medication dosage, PANSS scores, and AHRS scores at baseline ( table 1 ).
Effect of tDCS on AVH
A significant interaction was reported between the group and the time factors ( F = 22.1; P < .001). In the active group, AHRS score decreased from 27.2 (SD ± 4.1) to 19.1 (± 7.1) after the 10 sessions of tDCS, corresponding to a significant 28 % (± 26) reduction in AHRS scores ( T = 1; Z = 2.84; P < .01). In the sham group, AHRS scores decreased from 27.8 (± 8.0) to 24.9 (± 10.5), corresponding to a non-significant 10% (± 30) reduction ( T = 14; Z = 1.69; P = .09). Between-group comparison revealed a significant difference in AHRS changes after tDCS between active and sham group ( U = 21; Z = −2.77; P < .01).
Effect of tDCS on Left TPJ Seed-Based rs-FC
Between group contrast comparison (before-active-tDCS > after-active-tDCS) > (before-sham-tDCS > after-sham-tDCS) revealed significant differences in rs-FC of the left TPJ as shown in table 2 . After active tDCS and compared to sham, patients exhibited significant reduced rs-FC between the left TPJ and inferior frontal regions including the left anterior insula (BA 13) and the right inferior frontal gyrus (BA 44/45). Increased rs-FC was reported between the left TPJ and the left DLPFC (BA 8/9), the left angular gyrus (BA 39) and the precuneus (BA 7/31) ( figure 1 ).
Brain Regions Showing Significant Changes ( P value < .005) in Resting-State Functional Connectivity With the Left Temporo-Parietal Junction After 10 Sessions of Active Transcranial Direct Current Stimulation ( n = 11) as Compared With Baseline and With the Sham Group ( n = 12)
| Left Temporo-Parietal Junction Connectivity . | ||||||
|---|---|---|---|---|---|---|
| . | . | . | Talairach Coordinates . | . | ||
| Brain Area . | Cluster Size . | BA . | x . | y . | z . | tmax . |
| Decrease in FC | ||||||
| Right inferior frontal gyrus | 351 | 44/45 | 53 | 10 | 18 | −4.20 |
| Left anterior insula | 513 | 13 | −49 | 10 | −3 | −4.20 |
| Increase in FC | ||||||
| Left middle frontal gyrus (DLPFC) | 324 | 8/9 | −43 | 19 | 42 | 4.89 |
| Left angular gyrus | 324 | 39 | −31 | −56 | 27 | 4.91 |
| Precuneus | 621 | 7/31 | −1 | −68 | 33 | 4.28 |
| Left Temporo-Parietal Junction Connectivity . | ||||||
|---|---|---|---|---|---|---|
| . | . | . | Talairach Coordinates . | . | ||
| Brain Area . | Cluster Size . | BA . | x . | y . | z . | tmax . |
| Decrease in FC | ||||||
| Right inferior frontal gyrus | 351 | 44/45 | 53 | 10 | 18 | −4.20 |
| Left anterior insula | 513 | 13 | −49 | 10 | −3 | −4.20 |
| Increase in FC | ||||||
| Left middle frontal gyrus (DLPFC) | 324 | 8/9 | −43 | 19 | 42 | 4.89 |
| Left angular gyrus | 324 | 39 | −31 | −56 | 27 | 4.91 |
| Precuneus | 621 | 7/31 | −1 | −68 | 33 | 4.28 |
Note : BA, Brodmann areas; DLPFC, Dorsolateral prefrontal cortex; FC, Functional Connectivity. P value < .005.
Brain Regions Showing Significant Changes ( P value < .005) in Resting-State Functional Connectivity With the Left Temporo-Parietal Junction After 10 Sessions of Active Transcranial Direct Current Stimulation ( n = 11) as Compared With Baseline and With the Sham Group ( n = 12)
| Left Temporo-Parietal Junction Connectivity . | ||||||
|---|---|---|---|---|---|---|
| . | . | . | Talairach Coordinates . | . | ||
| Brain Area . | Cluster Size . | BA . | x . | y . | z . | tmax . |
| Decrease in FC | ||||||
| Right inferior frontal gyrus | 351 | 44/45 | 53 | 10 | 18 | −4.20 |
| Left anterior insula | 513 | 13 | −49 | 10 | −3 | −4.20 |
| Increase in FC | ||||||
| Left middle frontal gyrus (DLPFC) | 324 | 8/9 | −43 | 19 | 42 | 4.89 |
| Left angular gyrus | 324 | 39 | −31 | −56 | 27 | 4.91 |
| Precuneus | 621 | 7/31 | −1 | −68 | 33 | 4.28 |
| Left Temporo-Parietal Junction Connectivity . | ||||||
|---|---|---|---|---|---|---|
| . | . | . | Talairach Coordinates . | . | ||
| Brain Area . | Cluster Size . | BA . | x . | y . | z . | tmax . |
| Decrease in FC | ||||||
| Right inferior frontal gyrus | 351 | 44/45 | 53 | 10 | 18 | −4.20 |
| Left anterior insula | 513 | 13 | −49 | 10 | −3 | −4.20 |
| Increase in FC | ||||||
| Left middle frontal gyrus (DLPFC) | 324 | 8/9 | −43 | 19 | 42 | 4.89 |
| Left angular gyrus | 324 | 39 | −31 | −56 | 27 | 4.91 |
| Precuneus | 621 | 7/31 | −1 | −68 | 33 | 4.28 |
Note : BA, Brodmann areas; DLPFC, Dorsolateral prefrontal cortex; FC, Functional Connectivity. P value < .005.
Correlation Analyses
A significant positive correlation was found between the reduction of AVH and the decrease in rs-FC between the left TPJ and the left anterior insula (ρ = 0.50, P = .01; figure 2 ). No association was evidenced between AVH reduction and changes in rs-FC between the left TPJ and other brain areas (right inferior frontal gyrus, left angular gyrus, left DLPFC, precuneus).
![Correlation between individual changes in auditory hallucinations severity (changes in Auditory Hallucinations Rating Scale (AHRS) scores after transcranial Direct Current stimulation [tDCS]) and changes in the left Temporal-Parietal Junction seed-based resting-state functional connectivity (rs-FC) with the left anterior insula (changes in beta values after tDCS). The correlation was assessed using Spearman rank correlation tests.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/schizophreniabulletin/42/2/10.1093_schbul_sbv114/3/m_schbul_sbv114_f0002.jpeg?Expires=1749125947&Signature=FAntwq6DCb7M1nzkwedar~luQjyRBtuEc7e3f1-N37YxYrg~shy~cCeQKO0JZaEm3PQCDldycu3DtvjBhEr0tYd9g6-Fp~jfBcT4insWT8MtJRz2WoZh~GVvSLWBUAAKGDku54H09Rsl4ZO-WE8n9jtWpeEbSaCeznrvE~AczXLbGvvU1q2rErrziuUdzZQ7TAFYqKm9cv23m5pn0vIS8rZKD1QCzwx7CzWyxwGYyl4pCUMPYu2L~igJRZSej3skOAqDIKeYB0x4mHFkTsyZflASWSYa6u29QQuWabrtjO0jk~JFPlb2TcZZ~1G9uBjljIMwqxq3iPjDM2Z6v-HgbQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Correlation between individual changes in auditory hallucinations severity (changes in Auditory Hallucinations Rating Scale (AHRS) scores after transcranial Direct Current stimulation [tDCS]) and changes in the left Temporal-Parietal Junction seed-based resting-state functional connectivity (rs-FC) with the left anterior insula (changes in beta values after tDCS). The correlation was assessed using Spearman rank correlation tests.
Discussion
Our study aimed at investigating the neurobiological effect of tDCS in patients with schizophrenia and treatment-resistant AVH. As reported in a previous study, 29 we observed a reduction of AVH severity as well as the negative symptoms after active tDCS, as compared to sham. Active tDCS significantly reduced the left TPJ rs-FC with bilateral inferior frontal areas (left anterior insula and right inferior frontal gyrus) and significantly increased the left TPJ rs-FC with the left DLPFC, the left angular gyrus and the precuneus. Reduction in rs-FC between the left TPJ and the left anterior insula was correlated with the AVH reduction. Our results suggested that the effect of tDCS was not restricted to the FC between the brain areas located under the electrodes sites. Indeed, tDCS did not only modulate the left TPJ rs-FC with the left DLPFC but also with a large distributed network. These findings are in line with recent findings in healthy individuals showing that tDCS produced bihemispheric changes in the topology of rs-FC. 40–42
Decrease rs-FC Between the Left TPJ and Inferior Frontal Areas
After tDCS, we reported a reduction of AVH accompanied by a decreased rs-FC between the left TPJ and the inferior frontal areas (left anterior insula and right inferior frontal gyrus). These areas have been reported as aberrantly activated during the AVH occurrence. 1 Moreover, the link between the left anterior insula and AVH has been recently highlighted in a meta-analysis reporting a significant negative correlation between the severity of AVH and gray matter volume in the left insular cortex in patients with AVH. 43 Also, the right inferior frontal gyrus seems to be predominantly activated during AVH. 44 This predominant engagement may be related to the typical low semantic complexity and negative emotional content of AVH. Indeed AVH in psychotic patients often consisting of single words or truncated sentences with a predominantly negative emotional content that may be the product of right hemisphere language areas. 44
Furthermore, several studies investigating rs-FC in hallucinating patients with schizophrenia reported an aberrant rs-FC between the bilateral inferior frontal and temporo-parietal areas in AVH+ patients. For instance, rs-FC between the Wernicke’s region and the left inferior frontal gyrus was increased in AVH+ patients compared with patients without AVH 5 and was positively correlated with AVH reality. 4 In addition, Wernicke’s region rs-FC within the speech related network 45 and with the left Heschl’s gyrus 10 was positively correlated with AVH severity. Altogether, these reports support the hypothesis of an excessive rs-FC within a bilateral fronto-temporal network in AVH. Current findings of an rs-FC reduction between these brain regions that was correlated with the reduction in AVH severity effectively support such a hypothesis.
On the contrary, few reports found a decreased rs-FC between inferior frontal and temporo-parietal areas of both hemispheres in AVH+ patients. 7 , 11 For instance, Vercammen et al have reported a decrease in rs-FC between the left TPJ and the right inferior frontal gyrus in AVH+ patients as compared to healthy controls. 7 In another study comparing rs-FC between AVH+ patients and healthy controls, Sommer et al have reported a decreased rs-FC between the left superior temporal gyrus and the left frontal operculum extending to the anterior insula areas as well as the parietal opercular areas. 11 Several methodological issues may explain discrepancies between results. Indeed, most of the studies have compared patients suffering from AVH and healthy subjects and could have been biased by an impossibility to distinguish between impairments associated with schizophrenia and abnormalities more directly linked to AVH. 7 , 8 , 11 , 45 In this global context, we suggest that the decreased rs-FC reported in these specific studies may reflect a general decrease in connectivity related to schizophrenia, by opposition to the increase in fronto-temporal rs-FC, specifically related to AVH. Moreover, disparity of these findings may be explained by the use of different seed regions for rs-FC analyses 13 such as the inferior frontal gyrus, 4 , 5 , 8 , 11 the middle temporal gyrus, 8 the primary auditory cortex, 6 , 10 the superior temporal gyrus, 11 Wernicke’s area, 5 the angular gyrus, 8 or the TPJ. 7
Crucially, the inferior frontal areas (left anterior insula and right inferior frontal gyrus) are involved in overt and covert speech production. 46 Moreover, the left anterior insula has been advanced to play a key role in discriminating between self-generated and external information. 47 The hypothesis of an excessive rs-FC within the speech-related pathway stays fully coherent with other influential pathophysiological models of AVH based on a potential failure in language processing and especially in the process of recognizing self-generated inner speech. 48 , 49
Increase rs-FC Between the Left TPJ and the Left DLPFC, the Left Angular Gyrus and the Precuneus
The observed increase in rs-FC between the left TPJ and the left DLPFC is fully in line with our hypothesis that fronto-temporal tDCS restores the connectivity between the 2 targeted areas leading to reduced AVH. Patients with schizophrenia were reported to display a reduced functional connectivity between the left superior temporal gyrus and the left DLPFC during language tasks and this dysconnectivity was previously correlated with the severity of AVH. 50 Increasing rs-FC between the left DLPFC and the left TPJ may reflect an increase in top-down processing involved in the monitoring of speech, leading to less misattributions of speech and less AVH. It has been suggested that noninvasive brain stimulation to the left TPJ could affect inner speech and source monitoring networks. 48 , 51 This hypothesis was recently supported by a study reporting that fronto-temporal tDCS reduces the misattributions of inner speech in patients with schizophrenia and AVH. 52 Improvement in inner speech recognition may be linked to the observed reduction in rs-FC between the left TPJ and areas involved in monitoring of speech.
The increased rs-FC between the left TPJ and the left angular gyrus after active tDCS as compared to sham also appear important to discuss. The left angular gyrus is involved in a number of processes related to language, 46 spatial cognition, memory retrieval, attention, and out-of-body experiences, and constitutes one of the nodes of the default mode network (DMN). 53 It has been reported that patients with schizophrenia suffering from frequent AVH display a decreased FC between the left angular gyrus and the left temporo-parietal areas involved in speech processing, such as the left inferior temporal cortex and the left inferior parietal cortex. 8 Of note, it has recently been reported that in healthy subjects, rTMS applied over the right TPJ increases FC between the right TPJ and the right DLPFC and the right angular gyrus. 54 Thus, noninvasive brain stimulation may act on AVH by modulating the TPJ connectivity with the DLPFC and the angular gyrus, providing an increased top-down control on speech-related temporo-parietal areas.
We also reported an increased rs-FC between the left TPJ and the precuneus. The precuneus is also part of the DMN, which has recently been suggested to play a role in the generation of AVH. 55 , 56 Of note, the posterior cingulate/precuneus complex was associated with memory processes and self-referential thoughts. 57 Moreover, symptoms severity was correlated with precuneus instability within the DMN in AVH+ patients with schizophrenia. 56 Enhancing rs-FC between the left TPJ and the precuneus may improve memory and self-referential processes leading to a decrease in perception of inner speech as alien.
Several potential limitations have to be acknowledged. First, the sample size used in this experiment is limited and only constitutes a first account for the understanding of the neurophysiological effects of tDCS in patients with AVH. Moreover, even if power limit constitutes a crucial issue in brain imaging studies, 58 it has been pointed out that the real problem was not small sample size by itself, but more the choice for a significance threshold at P < .05, which regularly leads to false positive results. 59 , 60 Since the P values presented in our imaging findings are largely below 0.05, it appears unlikely that recruiting more participants would have change the results. Secondly, we chose to focus the rs-FC analysis on the left TPJ because it is a common brain target in noninvasive brain stimulation studies for reducing AVH in patients with schizophrenia. 15 , 34 , 48 Even if validated, such method keeps a limited sensitivity to discover tDCS-induced changes in circuits that are not directly connected to the defined seed regions. However, seed-based rs-FC also provides higher specificity regarding the functional interpretation of the changes observed after tDCS because it allows the assessment of induced changes in brain areas directly targeted by tDCS. In addition, even if the use of a large left TPJ seed (25mm) is consistent with the size of the brain area targeted with tDCS, it may have limited the comparison of our results with findings from the literature using different seed regions of various sizes in the temporal and parietal lobes such as the superior temporal gyrus or Wernicke’s area. Also, recruiting both left- and right-handed individuals in the sample clearly reflects a clinical reality but may have impacted temporal-parietal rs-FC. Even if the link between handedness and hemispheric dominance is not fully understood and shows large inter-individual variations, this may have reduced the chance to reach significance. However, because all the participants benefited from left tDCS and showed a significant decrease of AVH, a potential laterality effect associated with handedness was reduced ( Supplementary Data ).
Moreover, we could not rule out that the second fMRI session, 1 hour after the end of the last session, may reflect an acute effect of the 10th tDCS session rather than a cumulative effect of the 10 sessions. However, by assessing brain rs-FC 1 hour after the last tDCS session, we hypothesized that we measured cumulative effects of the 10 tDCS sessions. Indeed, in a recent neurophysiological study, Ulam et al reported a cumulative effect of 10 repeated sessions of tDCS on cortical activity as measured by EEG. 61 Another limitation was the absence of blinding efficacy assessments. However, the hypothesis of an effect solely caused by an inadequate sham condition appears unlikely because tDCS studies investigating blinding in healthy subjects and patients with major depressive disorder supported the good reliability of the sham condition. 36 , 62 , 63 Finally, it has been argued that tDCS effects may depend on the state of the brain at the stimulation onset. 64 In our study, to control for state-dependency, participants were required to be as quiet as possible during tDCS sessions and tDCS sessions were delivered at the same time-of-day for all participants. Future studies should control other parameters that may influence tDCS outcomes by changing the state of the brain during, before and after the tDCS session such as coffee intake, satiation-levels, quality and amount of sleep or exercise.
Despite these limitations, this study is the first to report that tDCS alleviating AVH severity in patients with schizophrenia is associated with a modulation of rs-FC between the left TPJ and the AVH-related brain network. Namely, tDCS decreased rs-FC between left TPJ and areas involved in inner speech production (the left anterior insula, the right inferior frontal gyrus). In addition, tDCS increased rs-FC between the left TPJ and areas involved in monitoring of inner speech (the left DLPFC, the left angular gyrus and the precuneus). These effects may explain the AVH reduction observed in patients with schizophrenia after tDCS. Moreover, these changes in FC may contribute to the improvement of source monitoring performances previously reported after tDCS.
Acknowledgments
The authors thank the CERMEP—imagerie du vivant (Lyon, France) for their help in acquiring fMRI data, the “Conseil Scientifique de la Recherche” of the Centre Hospitalier le Vinatier for their financial support (CSR C08), and C. Damasceno, the study nurse for her help with the project. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References




