-
PDF
- Split View
-
Views
-
Cite
Cite
Aoife Carolan, Caroline Hynes-Ryan, Sri Mahavir Agarwal, Rita Bourke, Walter Cullen, Fiona Gaughran, Margaret K Hahn, Amir Krivoy, John Lally, Stefan Leucht, John Lyne, Robert A McCutcheon, Michael J Norton, Karen O’Connor, Benjamin I Perry, Toby Pillinger, David Shiers, Dan Siskind, Andrew Thompson, Donal O’Shea, Dolores Keating, Brian O’Donoghue, Metformin for the Prevention of Antipsychotic-Induced Weight Gain: Guideline Development and Consensus Validation, Schizophrenia Bulletin, 2024;, sbae205, https://doi.org/10.1093/schbul/sbae205
Close - Share Icon Share
Abstract
Overweight and obesity are highly prevalent in people with severe mental illness (SMI). Antipsychotic-induced weight gain (AIWG) is one of the most commonly reported and distressing side effects of treatment and people living with SMI place a high value on the avoidance of this side effect. Metformin is the most effective pharmacological intervention studied for the prevention of AIWG yet clear guidelines are lacking and evidence has not translated into practice. The aim of this research was to develop a guideline for the use of metformin for the prevention of AIWG.
The appraisal of guidelines for research and evaluation II instrument (AGREE II) was followed for guideline development. Literature was reviewed to address key health questions. The certainty of evidence was evaluated using GRADE methodology and an evidence-to-decision framework informed the strength of the recommendations. A consensus meeting was held where the algorithm and strength of recommendations were agreed. An independent external review was conducted involving experts in the field, including patient and public partners.
Metformin is the only pharmacological agent that has demonstrated efficacy for preventing AIWG. Co-commencement with antipsychotic medicines can reduce the extent of weight gain by 4.03 kg (95% CI −5.78 kg to −2.28 kg) compared to controls. A guideline for the use of metformin for the prevention of AIWG was developed with specific recommendations for co-commencement of metformin at initiation with an antipsychotic or commencement if certain criteria are present. Core recommendations were graded as strong by consensus agreement.
This is the first published evidence-based guideline using the AGREE II framework and GRADE methods for the use of metformin to prevent AIWG incorporating recommendations for co-commencement. Implementation and evaluation of the guideline will be supported by a shared decision-making package and assessment of barriers and facilitators to implementation.
Introduction
People with severe mental illnesses (SMI) such as schizophrenia and bipolar affective disorder have higher rates of premature mortality than the rest of the population and much of this increased risk is attributed to cardiometabolic risk factors.1–4 Overweight and obesity contribute significantly to cardiometabolic risk, with obesity doubling the risk of all-cause mortality, coronary heart disease, stroke and type 2 diabetes (T2DM).4,5 An estimated three quarters of people with psychosis have co-morbid overweight or obesity.6 The trajectory of weight gain in people with SMI is rapid and nonlinear. For example, within a year of treatment, up to 80% of people with first episode psychosis will experience clinically significant weight gain, defined as ≥7% increase in baseline body weight.7–9 Furthermore, a UK longitudinal study of changes in weight in the 5 years after the first prescription for an antipsychotic found that over 50% of individuals in the healthy weight category at baseline progress to overweight/obesity.10 Despite greater awareness and recommendations for the monitoring and management of modifiable risk factors, this health inequality continues to widen, and premature mortality rates have risen consistently in recent years in people with SMI.1 Nonpharmacological factors such as lifestyle, genetics, and reduced access and/or utilization of healthcare services contribute to this health inequality. The net positive effect of antipsychotic treatment is demonstrated by the fact that those people with a diagnosis of schizophrenia who do not take medication have a shorter life expectancy than those who take medication.11,12 Nevertheless, antipsychotic medicines cause cardiometabolic side effects to varying degrees and interventions that reduce these side effects could improve physical health outcomes.13–15 Targeting antipsychotic-induced weight gain (AIWG) also promises to have a wider psychosocial impact. AIWG is one of the most commonly reported and distressing side effects of treatment16–18 and weight gain is related to poorer quality of life, as well as reduced medication adherence in people with schizophrenia19,20
Pharmacological and nonpharmacological interventions for the prevention and/or treatment of AIWG have been evaluated. Antipsychotic dose reduction, discontinuation or switching strategies have demonstrated statistically significant reductions in weight with mean changes in weight of −1.5 kg (95% CI −2.03 kg to −0.98 kg) compared to maintenance treatment. The clinical significance of these interventions is uncertain.21–23 Furthermore, antipsychotic switching will not be possible in some patients, eg, those receiving clozapine for treatment resistant schizophrenia.
Behavior change methods, including diet and lifestyle interventions should always be offered and continued alongside pharmacological interventions for weight gain.24 These interventions have a long-established evidence base for improving general health, quality of life and well-being in the general population. However, for people living with SMI, barriers in access to and utilization of these interventions precludes equity of access. Widespread replication of these intensive interventions is difficult. Furthermore, interventions for the prevention of weight gain are relatively understudied. The PHAsTER study, one of the few studies evaluating lifestyle interventions for the prevention of AIWG, found that physical health intervention failed to prevent significant metabolic complications of treatment, indicating a need for more intensive interventions.25
AIWG can be rapid and severe in the first weeks of treatment.7 Those who are acutely unwell may be unable to engage fully in these intensive interventions and pharmacological interventions may be required to mitigate against rapid weight gain. People with SMI have expressed that clinicians can overestimate the ability of dietary and lifestyle interventions and difficulies in accessing more effective interventions.26 The now outdated mantra of “eat less, move more” is no longer adequate when managing weight gain in people with SMI.24
Metformin, a biguanide licensed for T2DM, has a long-established safety profile and is, largely, well tolerated. Common, yet transient, side effects include nausea and gastrointestinal disturbance. Other possible complications of treatment include vitamin B12 deficiency and rarely, lactic acidosis or liver function test abnormalities. The unlicensed use of metformin for treating AIWG is associated with a mean reduction in weight of approximately 3 kg. The effect may be higher in people at the onset of SMI.27,28
A 2022 Cochrane review of pharmacological interventions for the prevention of AIWG found that metformin was the only pharmacological agent that may be effective for preventing weight gain when started with an antipsychotic.5 This was replicated in a large meta-analysis of metformin versus placebo commenced at the time of antipsychotic initiation, where metformin was associated with attenuation of weight gain and additional significant attenuation of derangement of fasting glucose levels, total cholesterol, and total triglyceride levels.29 Despite this, metformin for the prevention of AIWG is not routinely offered in psychiatric practice. Barriers to translation are likely multifactorial. Lack of knowledge, experience, and guidance are likely major factors. Therefore, we aimed to develop a clinical guideline for the use of metformin for the prevention of AIWG with specific and implementable recommendations on when and how to start metformin based on specific criteria.
Methods
An international steering group, part of the PROGRESS (Psychosis, Recovery, Optimization through Guidelines and Research-based Evidence in Service Strategies) project, identified metformin for the prevention of AIWG as an area of priority for translation of evidence into clinical practice guidelines. The steering group included international researchers and clinicians with expertise in psychiatry, physical and metabolic health and patient & public involvement (PPI) partners. A guideline development group (BOD, DK, AC, CH) with expertise in evidence synthesis, including systematic review, meta-analysis, the GRADE approach, implementation science, and translating research into practice, was chaired by the principal investigator (BOD). This research did not involve the collection of any patient identifiable data and research ethics committee approval was not required.
The appraisal of guidelines for research and evaluation II (AGREE II) checklist was used as a framework for the development of the guideline.30 Table 1 summarizes the guideline development process with each stage of the process mapped to AGREE II domains. A more detailed description of the process can be found in the supplementary file (Appendix 1). Key Health Questions were devised and listed in Table 2.
| Guideline development process . | |
|---|---|
| Stage . | AGREE II . |
| 1. Scope, purpose, objectives, and key health questions To address uncertainty around the use of metformin for preventing AIWG in adults and children, specific, implementable recommendations would be developed. Key health questions were agreed (Table 2). | Domain 1: Scope & Purpose |
| 2. Involve Stakeholders International researchers and clinicians with expertise in psychiatry, physical and metabolic health, general practice and clinical leads were invited to be involved. Patient and public partners were involved in the guideline development from concept. | Domain 2: Stakeholder Involvement Domain 6: Editorial Independence |
| 3. Evidence synthesis Literature was reviewed to address the key health questions. An information specialist was consulted in the development of the search strategies and search terms. Evidence profiles were produced. AC and DK independently rated the certainty of evidence using GRADE methodology. | Domain 3: Rigour of Development |
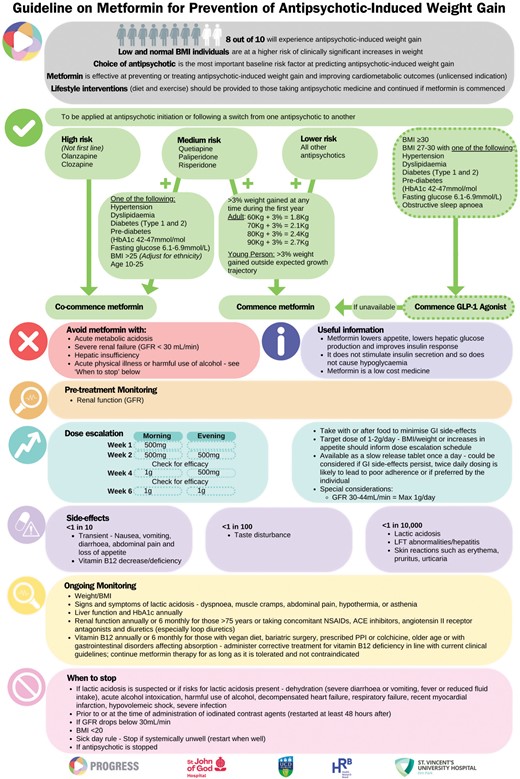
| 4. Develop recommendations and algorithm Core recommendations (Table 4) and good practice recommendations were agreed. An evidence-to-decision framework was populated (Supplementary file). An algorithm (Figure 1) was developed detailing the preliminary guidance. | Domain 4: Clarity of Presentation |
| 5. Preliminary review Evidence synthesis, evidence-to-decision framework and guideline recommendations were presented to endocrinology and international experts (DOS, DSiskind, AT, DShiers). All feedback was recorded and incorporated into the algorithm and evidence-to-decision framework. | Domain 3: Rigour of Development |
| 6. Consensus validation A consensus development meeting was held at the Schizophrenia International Research Society Conference 2024. Members of the consensus panel were asked to disclose any conflicts of interest. No members of the consensus panel had any direct conflicts relating to this guideline. Researchers had conducted research in the area of AIWG but none of that research was funded by pharmaceutical companies. In a manner of full disclosure, a list of all consultant and speaker fees can be found in the supplementary file. Members of the guideline development group presented the evidence summaries, evidence-to decision framework and the guideline algorithm. All feedback was recorded. Suggestions for change were discussed and agreed at the meeting. Attendees were invited to rate the strength of the recommendations according to the GRADE framework using an informal process. | Domain 2: Stakeholder Involvement Domain 3: Rigour of Development |
| 7. External reviews The guideline algorithm was presented for final external review to two external experts (AM and FG) and two PPI representatives (RB, MN). External reviewers were asked to comment on the rigour and transparency of the methods and the evidence-to decision framework. All feedback was recorded and, where applicable, incorporated into the algorithm and evidence-to-decision framework. | Domain 2: Stakeholder Involvement Domain 3: Rigour of Development |
| 8. Implementation An evaluation project is planned and the results will inform an updated version of the guideline in 2026. New or emerging evidence will be reviewed when updating the guideline. | Domain 5: Applicability |
| Guideline development process . | |
|---|---|
| Stage . | AGREE II . |
| 1. Scope, purpose, objectives, and key health questions To address uncertainty around the use of metformin for preventing AIWG in adults and children, specific, implementable recommendations would be developed. Key health questions were agreed (Table 2). | Domain 1: Scope & Purpose |
| 2. Involve Stakeholders International researchers and clinicians with expertise in psychiatry, physical and metabolic health, general practice and clinical leads were invited to be involved. Patient and public partners were involved in the guideline development from concept. | Domain 2: Stakeholder Involvement Domain 6: Editorial Independence |
| 3. Evidence synthesis Literature was reviewed to address the key health questions. An information specialist was consulted in the development of the search strategies and search terms. Evidence profiles were produced. AC and DK independently rated the certainty of evidence using GRADE methodology. | Domain 3: Rigour of Development |
| 4. Develop recommendations and algorithm Core recommendations (Table 4) and good practice recommendations were agreed. An evidence-to-decision framework was populated (Supplementary file). An algorithm (Figure 1) was developed detailing the preliminary guidance. | Domain 4: Clarity of Presentation |
| 5. Preliminary review Evidence synthesis, evidence-to-decision framework and guideline recommendations were presented to endocrinology and international experts (DOS, DSiskind, AT, DShiers). All feedback was recorded and incorporated into the algorithm and evidence-to-decision framework. | Domain 3: Rigour of Development |
| 6. Consensus validation A consensus development meeting was held at the Schizophrenia International Research Society Conference 2024. Members of the consensus panel were asked to disclose any conflicts of interest. No members of the consensus panel had any direct conflicts relating to this guideline. Researchers had conducted research in the area of AIWG but none of that research was funded by pharmaceutical companies. In a manner of full disclosure, a list of all consultant and speaker fees can be found in the supplementary file. Members of the guideline development group presented the evidence summaries, evidence-to decision framework and the guideline algorithm. All feedback was recorded. Suggestions for change were discussed and agreed at the meeting. Attendees were invited to rate the strength of the recommendations according to the GRADE framework using an informal process. | Domain 2: Stakeholder Involvement Domain 3: Rigour of Development |
| 7. External reviews The guideline algorithm was presented for final external review to two external experts (AM and FG) and two PPI representatives (RB, MN). External reviewers were asked to comment on the rigour and transparency of the methods and the evidence-to decision framework. All feedback was recorded and, where applicable, incorporated into the algorithm and evidence-to-decision framework. | Domain 2: Stakeholder Involvement Domain 3: Rigour of Development |
| 8. Implementation An evaluation project is planned and the results will inform an updated version of the guideline in 2026. New or emerging evidence will be reviewed when updating the guideline. | Domain 5: Applicability |
| Guideline development process . | |
|---|---|
| Stage . | AGREE II . |
| 1. Scope, purpose, objectives, and key health questions To address uncertainty around the use of metformin for preventing AIWG in adults and children, specific, implementable recommendations would be developed. Key health questions were agreed (Table 2). | Domain 1: Scope & Purpose |
| 2. Involve Stakeholders International researchers and clinicians with expertise in psychiatry, physical and metabolic health, general practice and clinical leads were invited to be involved. Patient and public partners were involved in the guideline development from concept. | Domain 2: Stakeholder Involvement Domain 6: Editorial Independence |
| 3. Evidence synthesis Literature was reviewed to address the key health questions. An information specialist was consulted in the development of the search strategies and search terms. Evidence profiles were produced. AC and DK independently rated the certainty of evidence using GRADE methodology. | Domain 3: Rigour of Development |
| 4. Develop recommendations and algorithm Core recommendations (Table 4) and good practice recommendations were agreed. An evidence-to-decision framework was populated (Supplementary file). An algorithm (Figure 1) was developed detailing the preliminary guidance. | Domain 4: Clarity of Presentation |
| 5. Preliminary review Evidence synthesis, evidence-to-decision framework and guideline recommendations were presented to endocrinology and international experts (DOS, DSiskind, AT, DShiers). All feedback was recorded and incorporated into the algorithm and evidence-to-decision framework. | Domain 3: Rigour of Development |
| 6. Consensus validation A consensus development meeting was held at the Schizophrenia International Research Society Conference 2024. Members of the consensus panel were asked to disclose any conflicts of interest. No members of the consensus panel had any direct conflicts relating to this guideline. Researchers had conducted research in the area of AIWG but none of that research was funded by pharmaceutical companies. In a manner of full disclosure, a list of all consultant and speaker fees can be found in the supplementary file. Members of the guideline development group presented the evidence summaries, evidence-to decision framework and the guideline algorithm. All feedback was recorded. Suggestions for change were discussed and agreed at the meeting. Attendees were invited to rate the strength of the recommendations according to the GRADE framework using an informal process. | Domain 2: Stakeholder Involvement Domain 3: Rigour of Development |
| 7. External reviews The guideline algorithm was presented for final external review to two external experts (AM and FG) and two PPI representatives (RB, MN). External reviewers were asked to comment on the rigour and transparency of the methods and the evidence-to decision framework. All feedback was recorded and, where applicable, incorporated into the algorithm and evidence-to-decision framework. | Domain 2: Stakeholder Involvement Domain 3: Rigour of Development |
| 8. Implementation An evaluation project is planned and the results will inform an updated version of the guideline in 2026. New or emerging evidence will be reviewed when updating the guideline. | Domain 5: Applicability |
| Guideline development process . | |
|---|---|
| Stage . | AGREE II . |
| 1. Scope, purpose, objectives, and key health questions To address uncertainty around the use of metformin for preventing AIWG in adults and children, specific, implementable recommendations would be developed. Key health questions were agreed (Table 2). | Domain 1: Scope & Purpose |
| 2. Involve Stakeholders International researchers and clinicians with expertise in psychiatry, physical and metabolic health, general practice and clinical leads were invited to be involved. Patient and public partners were involved in the guideline development from concept. | Domain 2: Stakeholder Involvement Domain 6: Editorial Independence |
| 3. Evidence synthesis Literature was reviewed to address the key health questions. An information specialist was consulted in the development of the search strategies and search terms. Evidence profiles were produced. AC and DK independently rated the certainty of evidence using GRADE methodology. | Domain 3: Rigour of Development |
| 4. Develop recommendations and algorithm Core recommendations (Table 4) and good practice recommendations were agreed. An evidence-to-decision framework was populated (Supplementary file). An algorithm (Figure 1) was developed detailing the preliminary guidance. | Domain 4: Clarity of Presentation |
| 5. Preliminary review Evidence synthesis, evidence-to-decision framework and guideline recommendations were presented to endocrinology and international experts (DOS, DSiskind, AT, DShiers). All feedback was recorded and incorporated into the algorithm and evidence-to-decision framework. | Domain 3: Rigour of Development |
| 6. Consensus validation A consensus development meeting was held at the Schizophrenia International Research Society Conference 2024. Members of the consensus panel were asked to disclose any conflicts of interest. No members of the consensus panel had any direct conflicts relating to this guideline. Researchers had conducted research in the area of AIWG but none of that research was funded by pharmaceutical companies. In a manner of full disclosure, a list of all consultant and speaker fees can be found in the supplementary file. Members of the guideline development group presented the evidence summaries, evidence-to decision framework and the guideline algorithm. All feedback was recorded. Suggestions for change were discussed and agreed at the meeting. Attendees were invited to rate the strength of the recommendations according to the GRADE framework using an informal process. | Domain 2: Stakeholder Involvement Domain 3: Rigour of Development |
| 7. External reviews The guideline algorithm was presented for final external review to two external experts (AM and FG) and two PPI representatives (RB, MN). External reviewers were asked to comment on the rigour and transparency of the methods and the evidence-to decision framework. All feedback was recorded and, where applicable, incorporated into the algorithm and evidence-to-decision framework. | Domain 2: Stakeholder Involvement Domain 3: Rigour of Development |
| 8. Implementation An evaluation project is planned and the results will inform an updated version of the guideline in 2026. New or emerging evidence will be reviewed when updating the guideline. | Domain 5: Applicability |
| Key health question 1 | Should metformin be used for the prevention of AIWG? |
| Key health question 2 | What predictors, thresholds and parameters should inform the decision of when to start metformin to prevent AIWG? |
| Key health question 3 | What dose of metformin should be used for the prevention of AIWG? |
| Key health question 4 | When should metformin be stopped when it is prescribed for the prevention of AIWG? |
| Key health question 1 | Should metformin be used for the prevention of AIWG? |
| Key health question 2 | What predictors, thresholds and parameters should inform the decision of when to start metformin to prevent AIWG? |
| Key health question 3 | What dose of metformin should be used for the prevention of AIWG? |
| Key health question 4 | When should metformin be stopped when it is prescribed for the prevention of AIWG? |
Abbreviation: AIWG, antipsychotic-induced weight gain.
| Key health question 1 | Should metformin be used for the prevention of AIWG? |
| Key health question 2 | What predictors, thresholds and parameters should inform the decision of when to start metformin to prevent AIWG? |
| Key health question 3 | What dose of metformin should be used for the prevention of AIWG? |
| Key health question 4 | When should metformin be stopped when it is prescribed for the prevention of AIWG? |
| Key health question 1 | Should metformin be used for the prevention of AIWG? |
| Key health question 2 | What predictors, thresholds and parameters should inform the decision of when to start metformin to prevent AIWG? |
| Key health question 3 | What dose of metformin should be used for the prevention of AIWG? |
| Key health question 4 | When should metformin be stopped when it is prescribed for the prevention of AIWG? |
Abbreviation: AIWG, antipsychotic-induced weight gain.
Results
Development of the Initial Guideline Contents
Key Health Question 1: Should Metformin be Used for the Prevention of AIWG?
Existing published guidelines were reviewed (Supplementary file).2,4,24,31–39 Canadian and Irish guidelines for obesity in adults refer to the early use of metformin for AIWG but don’t provide guidance on how and when to initiate metformin.24,31,32 Significant developments have been made in the area of weight management in recent years and some of the published guidelines pre-date these advances. Table 3 summarizes the evidence profile, including effect size and certainty of evidence for metformin in preventing AIWG.
Evidence Profile. Metformin compared to placebo or no treatment for the prevention of weight gain
| No. of participants taking metformin (studies) . | Mean difference change in weight (kg) 95% CI (kg) . | Certainty of evidence (grade) . |
|---|---|---|
| 65 (4 RCTs)17 | −4.03 (−5.78, −2.28) | ⊕⊕⊝⊝ Low |
| 563 (14 RCTs) | −3.12 (−4.22, −2.01) | ⊕⊝⊝⊝ Very low |
| 69 (1 cohort study)19 | −3.14 (−0.78, −5.52) | ⊕⊕⊝⊝ Low |
| No. of participants taking metformin (studies) . | Mean difference change in weight (kg) 95% CI (kg) . | Certainty of evidence (grade) . |
|---|---|---|
| 65 (4 RCTs)17 | −4.03 (−5.78, −2.28) | ⊕⊕⊝⊝ Low |
| 563 (14 RCTs) | −3.12 (−4.22, −2.01) | ⊕⊝⊝⊝ Very low |
| 69 (1 cohort study)19 | −3.14 (−0.78, −5.52) | ⊕⊕⊝⊝ Low |
Metformin compared to placebo or no treatment for the prevention of weight gain.
Outcome: Average endpoint change in body weight.
Evidence Profile. Metformin compared to placebo or no treatment for the prevention of weight gain
| No. of participants taking metformin (studies) . | Mean difference change in weight (kg) 95% CI (kg) . | Certainty of evidence (grade) . |
|---|---|---|
| 65 (4 RCTs)17 | −4.03 (−5.78, −2.28) | ⊕⊕⊝⊝ Low |
| 563 (14 RCTs) | −3.12 (−4.22, −2.01) | ⊕⊝⊝⊝ Very low |
| 69 (1 cohort study)19 | −3.14 (−0.78, −5.52) | ⊕⊕⊝⊝ Low |
| No. of participants taking metformin (studies) . | Mean difference change in weight (kg) 95% CI (kg) . | Certainty of evidence (grade) . |
|---|---|---|
| 65 (4 RCTs)17 | −4.03 (−5.78, −2.28) | ⊕⊕⊝⊝ Low |
| 563 (14 RCTs) | −3.12 (−4.22, −2.01) | ⊕⊝⊝⊝ Very low |
| 69 (1 cohort study)19 | −3.14 (−0.78, −5.52) | ⊕⊕⊝⊝ Low |
Metformin compared to placebo or no treatment for the prevention of weight gain.
Outcome: Average endpoint change in body weight.
A 2022 Cochrane review of pharmacological interventions for the prevention of AIWG found that metformin was the only pharmacological agent that may be effective for preventing weight gain.5 Four randomized controlled trials (RCTs) with a total of 131 participants assessed mean change in body weight in those prescribed risperidone (n = 2) or olanzapine (n = 2). The metformin group gained on average 4.03 kg (95% CI 5.78 kg to 2.28 kg) less than the control group.5 Co-commencement in this systematic review was defined as starting metformin “around the same time as an antipsychotic.”
This finding was replicated in a systematic review and meta-analysis of co-commencement of metformin at the time of initiation of an antipsychotic. In 14 RCTs, 11 of which were obtained from Chinese databases, involving 1126 participants, the metformin group gained on average 3.12 kg, (95% CI 4.22 kg to 2.01 kg) less than control. Clozapine (n = 3), olanzapine (n = 9), risperidone (n = 4) and sulpiride (n = 2) were among the antipsychotics studied.29
A large naturalistic cohort study involving 396 participants prescribed clozapine found that the metformin group gained on average 3.14 kg (95% CI .78 kg to 5.52 kg) less weight than the control group at 6 months and 5.38 kg (95% CI 2.65 kg to 8.13 kg) less weight at 12 months.40
In assessing the evidence, RCTs were initially rated as moderate certainty of evidence according to GRADE but downgraded to low certainty due to short study duration, small sample size, and variability in dosage used, follow-up, randomization, and blinding. Naturalistic studies were included in the evidence synthesis due to large sample size and follow-up periods and graded as low certainty. Overall, the certainty of evidence for metformin for prevention of AIWG was graded as low; however, the direction of evidence is in favor of the intervention, and the magnitude of effect across studies is high. Differences exist in the relative value of metformin for the prevention of weight gain and the treatment of weight gain. Effect size for the prevention of weight gain is not well described in the literature. The effect size for treatment of weight gain does not necessarily correspond with the effect size for the prevention of weight gain. Population-based cohort modeling studies have found that shifting mean population weight downwards by 5 (±2.0)% or 3–6 kg can prevent 42.4% (95% CI 24.3 to 56.1%) of T2DM cases. Achieving weight maintenance (±3.0%) can prevent 22.0% (95% CI 15.5 to 28.0%) of T2DM cases.41 Behavior change and lifestyle interventions for preventing weight gain in the general population describe effect sizes of 1.15 kg (95% CI 1.50 kg to 0.80 kg).42 NICE guidance on preventing excessive weight gain describes effect sizes of 1 kg as cost-effective, provided they do not cost in excess of £100 per individual.35 When presented to endocrinology experts, there was consensus that effect sizes seen for metformin for the prevention of weight gain (3–5 kg) are considered large effect sizes.
Key Health Question 2: What Predictors, Parameters, and Thresholds Should Inform the Decision of When to Intervene to Prevent AIWG?
Predictors for susceptibility to AIWG were extracted from the literature to address key health question 2.
Choice of Antipsychotic.
The choice of antipsychotic has been found to be the most important nongenetic factor at baseline for predicting AIWG.14,23,43–46 There is low certainty evidence for this association.46 The side effect profile of the antipsychotic agent is an important factor in the decision regarding which antipsychotic medication is commenced first.34,47–49 While not everyone prescribed antipsychotics with higher cardiometabolic adverse effect profiles will experience clinically significant weight gain, at present there is no accurate clinic-ready means to predict worse cardiometabolic outcomes in psychosis at the individual level. Prognostic tools for the psychosis population are advanced in the development pipeline and may be suitable for inclusion in future updates to this guidance.50 Nevertheless, modeling of over 1100 FEP cases in the UK found that even antipsychotics with higher cardiometabolic risk can be prescribed, and cardiometabolic risk lowered over time, so long as other factors, including weight gain are carefully addressed.51 Therefore, in lieu of a current clinically-available tool to predict cardiometabolic outcomes in this population, as part of shared decision-making, all people with first episode psychosis should be offered of an antipsychotic with low-risk of cardiometabolic side effects where possible at the lowest effective dose.52 Yet, if high- or medium-risk medicines are necessary, prevention strategies, for example, the early use of metformin, should be targeted at these groups.
Multiple network meta-analyses have reviewed the risk of cardiometabolic side effects with antipsychotics. All antipsychotic medicines can cause weight gain. Clozapine and olanzapine are consistently associated with the highest risk of weight gain. Quetiapine, risperidone, and paliperidone generally rank lower than clozapine and olanzapine for cardiometabolic side effects but still have significant potential to cause clinically significant weight gain.14,43–45,53
Young Age and Antipsychotic Naivety.
Younger age, first episode psychosis, and antipsychotic naivety are all predictors for susceptibility to AIWG, with age being a proxy for both first episode and antipsychotic naivety and vice versa. There is low-moderate certainty evidence for this association.46 Younger people are particularly susceptible to metabolic side effects of antipsychotic medicines. Children and adolescents have AIWG rates of up to twice that seen in adults.54 Young and antipsychotic naïve patients can gain 3–4-fold more weight irrespective of the specific antipsychotic.53 Rogdaki et al. 2024 report that children and adolescents exposed to antipsychotics such as clozapine experience 4.69 kg of weight gain in the first weeks of treatment. Whilst this figure matches that seen in adults, it is proportionally more significant given the lower body mass of children and adolescents.14,55 Predictive factors that were found to be relevant for the adult population, including baseline BMI, gender and ethnicity, were less associated with AIWG in children and adolescents, which reinforces the need for specific and separate considerations for the prevention of AIWG in younger populations. Antipsychotic prescribing in younger people has doubled since 2000, highlighting the need to act swiftly to monitor and manage metabolic side effects.56
Metabolic risk is accrued early in treatment with antipsychotics.57 For younger people, if not managed, this can lead to exposure to elevated cardiometabolic risk for extended periods. Health systems struggle to implement cardiometabolic monitoring and primary prevention recommendations, not least among those with SMI and younger populations.58–60 Younger people are underserved by guidelines for prevention of cardiometabolic disease, which, in many cases, target those over 40 years.3 Weight gain is a significant risk factor for cardiometabolic risk, with even 1 kg of weight gain translating to increases in CVD of 3.1%.61 Guidelines for prevention of AIWG in people of all ages, including young people, are lacking. Given that the age of onset of SMI is in early adulthood, the need to intervene early to prevent harm from weight gain is essential.
Cardiometabolic Risk Factors.
In addition to evaluating the evidence for metformin in preventing AIWG, the potential impact of metformin on cardiometabolic outcomes was extracted from the literature and an evidence profile was populated (Supplementary file). Metformin has a small to medium effect on cardiometabolic outcomes such as total cholesterol, triglycerides, fasting glucose, HOMA-IR, and triglycerides. The certainty of evidence was graded as very low.
Early Trends in Weight Gain.
Weight gain can be substantial and rapid early in the course of psychosis, the first year of treatment being critical.7 A naturalistic cohort study found that individuals with first episode psychosis gained on average 3.46 ± 7.81 kg of weight after a mean of 44.6 days of treatment.62 Trend in weight gain and change in BMI in the first weeks of treatment has been identified as the most clinically significant factor influencing longer-term risk of adverse cardiometabolic outcomes.46,63 In the case of BMI, there is moderate certainty evidence for this association.46
Eating Behavior and Early Appetite Increases.
Early increases in appetite or eating behavior may predict risk of weight gain, but the certainty of evidence is low and inconsistent. One study found that increased appetite at 4 weeks was associated with significantly greater weight gain at 12 weeks. Mean difference 2.67 kg (95% CI 1.20 to 4.15), P < .0001.64 Eating behavior and raised blood glucose were found to be potential predictors of weight gain.65
Parameters and Thresholds for Intervention
To further address key health question 2, parameters and thresholds for intervention were extracted from the literature. Various definitions exist for clinically significant weight gain ranging from 5% increase in baseline body weight to 10%.5,46,63,66–68 In trying to prevent clinically significant weight gain, there is no standardized threshold or timeframe for intervention, to our knowledge. One review defines long-term weight maintenance in adults as <3% change in body weight.66 Elsewhere, weight stability has been defined as +/−2 kg change in body weight over 6 months. Average 10-year weight gain among 13 802 US adults in the general population was reported as 4.2 ± 0.2 kg or 6.6% ± 0.2% of initial body weight.69 Younger adults experience more significant increases in body weight under the age of 25 years where the average 6-year weight gain is reported as 7.3 kg over 6 years.
Presentation of the evidence for preliminary review to endocrinology experts including the clinical lead for obesity in Ireland revealed that local metabolic clinics categorize weight stability as ±2.5% changes in body weight and a threshold of 3% for intervention for preventing AIWG was deemed acceptable. For the prevention of AIWG, the guideline development group proposed the threshold for intervention as an increase in baseline body weight of >3%. For children and adolescents, this was defined as >3% weight gain outside of the expected trajectory of weight increases. WHO growth charts for girls and boys aged 9–18 years should be used to assess expected trajectory weight increases. Adapted versions exist for some countries eg, UK and USA.70
When the guideline was presented at the consensus meeting, the timeframe for this threshold was left open. Experts at the consensus meeting suggested that a timeframe should be specified and it was agreed that this would be specific to the first year of treatment with an antipsychotic and would apply to the initiation or switching of antipsychotic medicines.
Three core recommendations were developed from key health questions 1 and 2 (Table 4).
| Recommendation . | Co-commence metformin with high-risk antipsychotics (olanzapine or clozapine) . |
|---|---|
| Strength: Strong recommendation for the intervention Certainty of evidence: ⊕⊕⊝⊝ Low | |
| Recommendation | Co-commence metformin with medium-risk antipsychotics (quetiapine, paliperidone or risperidone) in the presence of one or more cardiometabolic risk factorsa or in people aged 10–25 years |
| Strength: Strong recommendation for the intervention Certainty of evidence: ⊕⊕⊝⊝ Low | |
| Recommendation | Commence metformin with any antipsychotic if >3% increase in baseline body weight is observed during the first year of treatment with an antipsychotic |
| Strength: Strong recommendation for the intervention Certainty of evidence: ⊕⊕⊝⊝ Low | |
| Recommendation . | Co-commence metformin with high-risk antipsychotics (olanzapine or clozapine) . |
|---|---|
| Strength: Strong recommendation for the intervention Certainty of evidence: ⊕⊕⊝⊝ Low | |
| Recommendation | Co-commence metformin with medium-risk antipsychotics (quetiapine, paliperidone or risperidone) in the presence of one or more cardiometabolic risk factorsa or in people aged 10–25 years |
| Strength: Strong recommendation for the intervention Certainty of evidence: ⊕⊕⊝⊝ Low | |
| Recommendation | Commence metformin with any antipsychotic if >3% increase in baseline body weight is observed during the first year of treatment with an antipsychotic |
| Strength: Strong recommendation for the intervention Certainty of evidence: ⊕⊕⊝⊝ Low | |
aCardiometabolic risk factors to include one of: Hypertension, dyslipidaemia, diabetes (type 1 or 2), pre-diabetes (HbA1c 42–47 mmol/L or fasting glucose 6.1–6.9 mmol/L), BMI >25.
| Recommendation . | Co-commence metformin with high-risk antipsychotics (olanzapine or clozapine) . |
|---|---|
| Strength: Strong recommendation for the intervention Certainty of evidence: ⊕⊕⊝⊝ Low | |
| Recommendation | Co-commence metformin with medium-risk antipsychotics (quetiapine, paliperidone or risperidone) in the presence of one or more cardiometabolic risk factorsa or in people aged 10–25 years |
| Strength: Strong recommendation for the intervention Certainty of evidence: ⊕⊕⊝⊝ Low | |
| Recommendation | Commence metformin with any antipsychotic if >3% increase in baseline body weight is observed during the first year of treatment with an antipsychotic |
| Strength: Strong recommendation for the intervention Certainty of evidence: ⊕⊕⊝⊝ Low | |
| Recommendation . | Co-commence metformin with high-risk antipsychotics (olanzapine or clozapine) . |
|---|---|
| Strength: Strong recommendation for the intervention Certainty of evidence: ⊕⊕⊝⊝ Low | |
| Recommendation | Co-commence metformin with medium-risk antipsychotics (quetiapine, paliperidone or risperidone) in the presence of one or more cardiometabolic risk factorsa or in people aged 10–25 years |
| Strength: Strong recommendation for the intervention Certainty of evidence: ⊕⊕⊝⊝ Low | |
| Recommendation | Commence metformin with any antipsychotic if >3% increase in baseline body weight is observed during the first year of treatment with an antipsychotic |
| Strength: Strong recommendation for the intervention Certainty of evidence: ⊕⊕⊝⊝ Low | |
aCardiometabolic risk factors to include one of: Hypertension, dyslipidaemia, diabetes (type 1 or 2), pre-diabetes (HbA1c 42–47 mmol/L or fasting glucose 6.1–6.9 mmol/L), BMI >25.
Key Health Question 3: What Dose of Metformin Should be Used for the Prevention of AIWG?
The doses used in studies evaluating metformin for the prevention of AIWG ranged from 500 mg/day to 2 g/day. Titration protocols were not always reported, but where available, doses were increased at weekly increments as tolerated. To our knowledge, there are no dose finding studies for AIWG and time-to-effect is uncertain. Without intervention, the time taken for AIWG to plateau is also uncertain and may differ between antipsychotics.71 The manufacturer recommends initiating metformin at a dose of 500 mg bd and increasing at weekly increments. Discussion at the consensus and endocrinology meetings among clinicians who routinely prescribe metformin in clinics for prevention and/or treatment of weight gain revealed variation in practice, with some sites opting for fortnightly dose titration and others increasing over 6–12 weeks. Doses above 2 g/day are not commonly used in practice for this indication. The final algorithm recommends weekly titration until a dose of 1 g/day and fortnightly titration thereafter as tolerated. Dose reductions are required in renal impairment and this criterion is included in the algorithm. This good practice recommendation incorporates data from the literature, licensing recommendations, and feedback from clinical practice. (Good Practice Recommendation).
Key Health Question 4: When Should Metformin be Stopped When it is Prescribed for the Prevention of AIWG?
According to the license, metformin should be discontinued if lactic acidosis is suspected or risks for lactic acidosis are present, temporarily prior to or at the time of the administration of iodinated contrast agents or if estimated glomerular filtration rate drops below 30 mL/min.72 We also recommend that metformin is discontinued if BMI is below 20 or if the antipsychotic medicine is discontinued. (Good Practice Recommendation)
Metformin should be avoided where there is harmful use of alcohol72 eg, Alcohol User Disorders Identification Test (AUDIT) score of 8 or more.73 (Good Practice Recommendation)
Additional Guideline Considerations Outside of the Key Health Questions.
In addition to answering the key health questions, important prescribing information, such as common side effects and monitoring recommendations were included in the algorithm.
The scope of this guideline was limited to the use of metformin for the prevention of AIWG. However, the guideline development group was cognizant of the availability of more effective treatments for people living with overweight or obesity. Glucagon-like peptide-1 (GLP-1) agonists have demonstrated a larger effect size than metformin when treating overweight or obesity, including that caused by antipsychotics MD −6 kg (95% CI −10.8 kg to −1.36 kg).74–77 Failure to include this information in our algorithm could create hesitancy to intervene to effectively manage existing overweight or obesity in people living with SMI. These treatments should be equally available to people with SMI as the general population. We therefore included the recommendation to initiate GLP-1 agonists according to the licensed indication.78 The recommendation to initiate GLP-1 agonists is supported by high certainty evidence and these recommendations have been included in national obesity guidelines in Canada and Ireland.24,31 Supply problems, cost and prescribing restrictions in certain countries mean accessing these treatments can be challenging and we have therefore included the recommendation to start metformin if access is a problem.79
Incorporating the Values of People Living with SMI
Patient and public partners were involved in the guideline development from concept and throughout development to ensure the values of service users were incorporated. Clinical experience within the guideline development group and steering group meant there was an understanding of the importance and challenges in managing AIWG for both service users and clinicians. Literature was reviewed for evidence of the psychological impact of AIWG. People living with SMI place significant value on the avoidance of AIWG. Among 202 callers to a UK National Mental Health helpline, weight gain was the most distressing side effect of antipsychotic treatment, reported by 45% of callers, 84% of whom rated this as quite or extremely distressing.80 Weight and subjective distress from AIWG are predictors of poor adherence to antipsychotic medicines. People living with obesity are 2.5 times more likely to discontinue medicine than nonobese people.81 When adjuvant medicines such as metformin are initiated to improve weight outcomes in people with SMI, they have also been found to improve antipsychotic adherence.40 Qualitative studies exploring the service user experience of weight gain describe people with psychosis being “catapulted into obesity” and “rapid and uncontrollable weight gain” at an early stage of treatment. Psychosis and treatment of SMI dominate the early stages of acute illness, but service users value early provision of information and prevention strategies to manage weight gain.82
Despite knowledge of the importance of the avoidance of weight gain in people with SMI, there is a paucity of evidence for the acceptability of metformin among people with SMI and, in particular, among younger people for whom factors such as pill burden may be significant. Similar dropout rates were observed in RCTs for the prevention of weight gain in the metformin versus control groups (5.8% versus 5.9%), but qualitative studies assessing the acceptability of metformin are lacking.5
Consensus Validation
During the consensus meeting, suggestions for change were agreed including recommendations to slow the rate of metformin dose titration to improve tolerance, expanding the inclusion criteria from age >16 years to >10 years in line with the license for Diabetes and more specific recommendations on the time frame for interpreting >3% body weight gain in the first year of treatment. The need to adjust BMI thresholds for ethnicity was also raised. The use of a lower BMI thresholds of >23 is recommended by NICE when intervening to reduce the risk of T2DM for black African, African-Caribbean and South Asian and Chinese groups. Similar adjustments for the identification of overweight/obesity would be advocated for when initiating metformin for the prevention of weight gain.35
Agreement was achieved among the consensus panel on the strength of the recommendations using an informal process where all in attendance graded the recommendations as strong according to GRADE methodology. In grading the three core recommendations as strong, the consensus panel considered the whole body of evidence for AIWG. The risk of harm from AIWG, the high prevalence of obesity in SMI, and significant psychological impact were considered in the evidence-to-decision framework (Supplementary file). The risk of harm from metformin was also considered. Metformin has a long-established safety profile with a very rare likelihood of serious harm. Metformin is also inexpensive with no equity, acceptability or feasibility concerns. Each of these factors indicated a clear net health benefit from metformin and in such circumstances, GRADE allows the formulation of strong recommendations.83,84
External Reviews
External reviewers assessed the guideline development methods as robust and transparent. Feedback was collated and incorporated into the guideline algorithm as appropriate. External review with endocrinology colleagues confirmed that whilst co-morbid diabetes was not a contra-indication to starting metformin, it would be reasonable to liaise with endocrinology colleagues before making adjustments to diabetes treatment.
Evidence-to-Decision
The evidence-to-decision table (Supplementary file) details the body of evidence considered in making the recommendations.
Final Guideline Algorithm
The final guideline algorithm (Figure 1) makes specific recommendations for co-commencement of metformin at initiation with specific antipsychotics or commencement if certain criteria are present specific. Given the well-documented benefits of behavior change interventions, including lifestyle and diet interventions, we recommend that these should always be offered and continued wherever metformin is offered. Essential prescribing information to support implementation is included in the form of good practice recommendations.
Discussion
Statement of Principle Findings
Metformin is the only pharmacological agent with demonstrated efficacy for preventing AIWG. Co-commencement with antipsychotic medicines can reduce the extent of weight gain compared to controls and there is some evidence that longer durations can achieve larger effect sizes for high-risk antipsychotic medicines. We describe robust methods for the development of a guideline for the use of metformin for the prevention of AIWG incorporating recommendations for co-commencement of metformin at initiation with an antipsychotic or commencement if certain criteria are present.
Strengths and Weaknesses of the Study
Whist the guideline development benefited from the contributions of experts in the areas of antipsychotic prescribing and management of metabolic side effects, this in itself creates potential for bias. People who do research in this area are likely to be biased in favor of the intervention and those who oppose using metformin, including people living with SMI, are more likely to do so in their practice instead of vocally in academic settings. An implementation strategy will include a cluster RCT, behavior change methods and evaluation of barriers and facilitators to implementation.
Guidelines, by their nature, have limitations inherent in their development and implementation. Even with high certainty evidence, the recommendation for or against an intervention will involve subjective judgments among a panel weighing the potential benefits against the potential harms.85 Values will influence the recommendations, and the values of the panel may not match that of the service user or the clinicians applying the guideline in practice.85 The development of this guideline followed an evidence-based framework (AGREE II) including consensus validation, independent external reviews, and incorporated the values of service users by involving PPI partners from concept development.
There is low certainty evidence for the use of metformin for preventing AIWG. Adjunctive metformin has not been studied with all antipsychotics and there is heterogeneity among the populations studied. For example, the large cohort study by Stogios et al. had relatively high BMI at baseline, whereas the meta-analysis by Yu et al. included studies from China where the participants had normal BMI at baseline and low mean body weights.29,40 However, the overall body of evidence for metformin and clinical expertise was considered in an attempt to provide a pragmatic approach to addressing a problem that has significant long-term impact on health and well-being with a relatively low-risk intervention. The effect size for the prevention of weight gain is not described in the literature. Effect size as well as the certainty of evidence informs the strength of recommendations.83 The authors propose that 3–5 kg is a large effect size for the prevention of weight gain and the evidence-to-decision framework is based on this judgement.
Awaiting the funding and delivery of research needed to generate high certainty GRADE evidence will ultimately not serve people with SMI who are exposed to the risk of weight gain from antipsychotic medicine. GRADE encourages panels to “deal with discomfort” and make recommendations so that clinicians can offer treatment, patients can make choices, and policy makers can establish policies.86
Barriers and facilitators to implementation have been considered but will be further evaluated. Metformin is cost-effective and easily accessible, which should serve as a facilitator. The algorithm itself was noted to be a potential facilitator for implementation due to the clear, concise, and unambiguous illustration of the recommendations. Potential resource implications have been considered.
The perception that metformin may contribute to polypharmacy was raised at the consensus meeting as a potential barrier to implementation. Polypharmacy and potentially inappropriate prescribing have been areas of concern for decades, not least among people with SMI. More recently, the concept of appropriate polypharmacy has been recognized as the legitimate goal of managing multiple conditions in the same patient. It should no longer be assumed that polypharmacy is always harmful and clinical context should be considered where many medicines may need to be prescribed to manage co-morbid illness.87,88 The fact that people with SMI place significant value on the avoidance of weight gain and that metformin has been associated with improved antipsychotic adherence when prescribed alongside clozapine indicates strongly that this is appropriate polypharmacy but this will be evaluated as part of the implementation strategy.40
Unanswered Questions and Future Research
Shared decision-making, the joint process in which a healthcare professional works together with a person to make a decision about their care, is fundamental to guideline implementation.89 This supports a human-rights based approach where people living with SMI are empowered to make informed decisions about their treatment. Following feedback from PPI partners, a concise, plain English version of the algorithm has been developed into a video resource to support implementation. Shared decision-making support packages for clinicians and service users will also be developed. Future iterations of this guidance may include prognostic tools to help delineate a primary prevention approach encompassing features of universal and high-risk strategies, similar to guidelines for cardiometabolic primary prevention in the general population. eg, NICE Guideline for Cardiovascular Disease (NG238)58 Evaluation and review of the guideline will include a revision of the guideline in 3 years’ time with a review of emerging evidence for the use of metformin for the prevention of AIWG.
It has been suggested that people with SMI may benefit from the use of GLP-1 agonists at an earlier stage due to barriers in accessing specialist weight management services.79 As the evidence for the use of GLP-1 agonists for AIWG expands, future iterations of this guideline may include lower thresholds for GLP-1 agonists.
Conclusion
Metformin is the only pharmacological agent that has demonstrated efficacy for preventing AIWG and this research describes the development of a clinical practice guideline for this indication using robust and transparent methods following an evidence-based framework. The algorithm contains core recommendations for co-commencement of metformin at initiation with an antipsychotic or commencement if certain criteria are present. Essential prescribing information in the form of good practice recommendations has been included to support implementation. Implementation and evaluation of the guideline will be supported by a shared decision-making package and assessment of barriers and facilitators to implementation.
Author Contributions
B.O.D., D.K. and members of the Psychosis, Recovery, Optimisation through Guidelines and Research-based Evidence in Service Strategies (PROGRESS) steering group developed the concept for this research. A.C., C.H., D.K. and B.O.D. developed the guideline, conducted data extraction and evidence assessments. C.H., B.O.D. and D.K. participated in a substantive review of the manuscript. The authors read and approved the final manuscript.
Funding
This work is supported by the Health Research Board (grant no. APRO-2023-005). The views of the funding body have not influenced the research or the content of the guideline.
Data Availability
All research data is included in the supplementary file.



