-
PDF
- Split View
-
Views
-
Cite
Cite
Martin Schulte-Rüther, Ellen Greimel, Martina Piefke, Inge Kamp-Becker, Helmut Remschmidt, Gereon R. Fink, Beate Herpertz-Dahlmann, Kerstin Konrad, Age-dependent changes in the neural substrates of empathy in autism spectrum disorder, Social Cognitive and Affective Neuroscience, Volume 9, Issue 8, August 2014, Pages 1118–1126, https://doi.org/10.1093/scan/nst088
Close - Share Icon Share
Abstract
In typical development, empathic abilities continue to refine during adolescence and early adulthood. Children and adolescents with autism spectrum disorders (ASD) show deficits in empathy, whereas adults with ASD may have developed compensatory strategies. We aimed at comparing developmental trajectories in the neural mechanisms underlying empathy in individuals with ASD and typically developing control (TDC) subjects. Using an explicit empathizing paradigm and functional magnetic resonance imaging, 27 participants with ASD and 27 TDC aged 12–31 years were investigated. Participants were asked to empathize with emotional faces and to either infer the face’s emotional state (other-task) or to judge their own emotional response (self-task). Differential age-dependent changes were evident during the self-task in the right dorsolateral prefrontal cortex, right medial prefrontal cortex, right inferior parietal cortex, right anterior insula and occipital cortex. Age-dependent decreases in neural activation in TDC were paralleled by either increasing or unchanged age-dependent activation in ASD. These data suggest ASD-associated deviations in the developmental trajectories of self-related processing during empathizing. In TDC, age-dependent modulations of brain areas may reflect the ‘fine-tuning’ of cortical networks by reduction of task-unspecific brain activity. Increased age-related activation in individuals with ASD may indicate the development of compensatory mechanisms.
INTRODUCTION
Empathy can be defined as the result of psychological inferences about other persons’ mental and emotional states, allowing for socially appropriate emotional responses. Empathy is a multidimensional construct entailing emotional aspects (such as shared affect and emotional responses) as well as cognitive aspects (such as perspective-taking, self-other distinction, reflection about other people’s mental states and explicit self assessment of own evoked emotions) ( Davis, 1980 ; Decety and Jackson, 2004 ). Theory of mind (ToM) is closely related to cognitive aspects of empathy and is defined as the ability to represent other persons’ intentions, beliefs and desires as different from one’s own ( Premack and Woodruff, 1978 ). Although a lot of research has been conducted on the typical and atypical development of empathic abilities and ToM, less is known about the neurodevelopmental trajectories and their disturbance in atypical development such as autism spectrum disorder (ASD), in particular during late childhood and young adulthood.
Aspects of empathy are evident very early in development, e.g. contagious distress can be observed in newborns in response to other infants’ cries ( Dondi et al. , 1999 ). Basic ToM abilities typically have developed by 3–4 years of age ( Baron-Cohen et al. , 1985 ), paralleled by the emergence of empathic responses such as other-oriented behavior and instrumental acts of helping ( Thompson, 1987 ; Zahn-Waxler and Radke-Yarrow, 1990 ). Understanding of increasingly complex ToM tasks ( Wellman and Liu, 2004 ), e.g. social ‘faux-pas’ ( Baron-Cohen et al. , 1999 ) continues to develop into late adolescence and is closely linked to empathy( Ciaramelli et al. , 2013 ). Results from behavioral and questionnaire studies suggest improvement in empathic abilities after childhood ( Strayer, 1993 ; Dadds et al. , 2008 ). Furthermore, mature empathic understanding requires both the representation of other’s and one’s own emotional states without confusion of both ( Decety and Jackson, 2004 ). Thus, self-regulatory aspects of emotional processing are important for the acquisition of fully developed empathic abilities and these typically mature in late adolescence and young adulthood ( Diamond, 2002 ). Although several studies have elucidated the neural substrates of empathic processing in children, adolescents ( Decety et al. , 2008 ; Pfeifer et al. , 2008 ; Light et al. , 2009 ) and adults ( Carr et al. , 2003 ; Schulte-Rüther et al. , 2007 ), most do not take a developmental perspective (but see Decety and Michalska, 2010 ; Greimel et al. , 2010a ). Akin to the multidimensional concept of empathy, distinct brain regions have been implicated in distinct subcomponents of empathy ( Schulte-Rüther and Greimel, 2011 ): Affective components (in particular shared affect) have been linked to the human mirror neuron system, in concert with limbic structures and the insula ( Carr et al. , 2003 ; Schulte-Rüther et al. , 2007 ; Bastiaansen et al. , 2009 ). In contrast, cognitive components seem to draw upon brain regions also known to mediate ToM processing, i.e. medial prefrontal cortex (MPFC), superior temporal sulcus (STS), temporal poles (TP) and the temporoparietal junction (TPJ) ( Vogeley et al. , 2001 ; Frith and Frith, 2003 ). The MPFC, precuneus and inferior parietal cortex (IPC) have also been shown to play an important role for self-referential processing and self-other distinction ( Decety and Sommerville, 2003 ; Vogeley and Fink, 2003 ). Developmental change from childhood to adulthood suggests continuous refinements within this network ( Blakemore, 2008 ), e.g. due to accumulated expertise or a shift of cognitive strategies during empathizing ( Greimel et al. , 2010a ).
ASD are characterized by disturbances in the development of appropriate skills for social interaction and social communication. It has been suggested that many aspects of these problems can be explained by developmental delays in ToM abilities ( Baron-Cohen et al. , 1985 , 2000 ), which are evident even in subjects with otherwise high cognitive profiles ( Happé, 1994 ). Furthermore, atypical self-processing has been reported for ASD both on the neural ( Lombardo et al. , 2010 ) and behavioral level, and might be intrinsically linked to impaired empathic abilities ( Lombardo et al. , 2007 ). Atypical empathic behavior in early childhood is a key symptom of ASD in children ( Scambler et al. , 2007 ) and predicts later diagnosis ( Hutman et al. , 2010 ). However, empathic abilities ( Schwenck et al. , 2011 ), emotional responsiveness and social behavior ( Shattuck et al. , 2007 ; Farley et al. , 2009 ) improve during adolescence and early adulthood in patients with ASD. Furthermore, intact emotional empathic responses have been reported in adult individuals with ASD ( Dziobek et al. , 2008 ; Bird et al. , 2010 ) despite persistent deficits in ToM ( White et al. , 2011 ) or cognitive aspects of empathy ( Dziobek et al. , 2008 ). To date, little data are available regarding the development of empathic processing in ASD relative to typically developing individuals. In particular, it remains unclear whether in subjects with ASD improvements in ToM and empathy reflect a maturation of neural circuitries as can be observed in typical development ( Greimel et al. , 2010a ) or rather reflect compensatory processes due to, e.g. therapeutic interventions.
In ASD, most of the brain structures involved in empathic processing have been reported to show aberrant brain activation during empathy-related tasks. These include frontal components of the human mirror neuron system ( Iacoboni and Dapretto, 2006 ; Greimel, 2010b ), MPFC, STS, TP ( Happé et al. , 1996 ; Castelli et al. , 2002 ; Schulte-Rüther et al. , 2011 ), TPJ/IPC ( Schulte-Rüther et al. , 2011 ) and anterior insula ( Silani et al. , 2008 ; Bird et al. , 2010 ). Virtually nothing is known about differences in developmental trajectories of these networks in patients with ASD. A better understanding of disturbances or potential compensatory neural mechanisms is mandatory to (i) understand individual differences in the development of empathic abilities in ASD patients and (ii) to develop age-specific targeted interventions. The present study provides a first step toward the investigation of developmental trajectories in ASD by using a cross-sectional sample of children, adolescents and adults (aged 12–31 years), reflecting a particular interesting period of fundamental changes in networks related to social processing ( Blakemore, 2008 ) and potential improvements in patients with ASD ( Shattuck et al. , 2007 ). We employed a well-established empathizing task ( Schulte-Rüther and Greimel, 2011 ). This task has been shown to engage the diverse components of the brain network associated with empathizing (as reviewed earlier), as well as correlations of brain activation with individual empathic abilities and emotional reactivity ( Schulte-Rüther et al. , 2007 , 2008 , 2011 ; Greimel et al. , 2010b ). The task requires interactive assessment of the self- and other-perspective to allow for the construction of an interpersonal context. It taps on the understanding and perception of an emotional state (‘other-task’), as well as explicit emotional self-reference [such as the assessment of one’s own emotional reaction (‘self-task’)], two closely related aspects of empathic processing. We contrasted developmental trajectories of neural activation related to self- and other-tasks in participants with ASD and typically developing controls (TDC) to identify brain regions where covariation of neural activity with age was significantly different in both groups. We expected such differences mainly in brain regions associated with ToM, self-related processing, the mirror neuron system and the limbic system.
METHODS
Participants
Fifty-four male participants (aged 12–31 years) were included in the final functional magnetic resonance imaging (fMRI) data analysis. Twenty-seven participants were diagnosed with ASD (mean age ± SD = 18.52 ± 5.10 years; n = 15, age < 12–17 years; n = 12, age ≥ 18 years) and 27 were TDC participants (mean age ± SD = 18.22 ± 4.41 years; n = 15, age = 12–17 years; n = 12, age ≥ 18 years) without a history of neurological or psychiatric disease. Data presented here are a combined subset from the participants of two previous studies on empathy in children and adolescents ( Greimel et al. , 2010b ) and adults with ASD ( Schulte-Rüther et al. , 2011 ). Inclusion into this study depended upon a close match with respect to age for the comparison of patients and control subjects. To exclude psychiatric disorders in control subjects, a standardized semi-structured interview (K-SADS-PL) was conducted with children and adolescents, and the Brief Symptom Inventory ( Franke, 2000 ) was completed by adults. For all children and adolescent subjects, parents’ evaluations of psychopathology were obtained by the Child Behaviour Checklist ( Döpfner et al. , 1994 ). Both groups were comparable with respect to mean age ( t test for independent samples, T52 = 0.233, P > 0.817), age distribution (Kolmogorov–Smirnov test, Z = 0.544, P > 0.930), mean G-IQ (ASD: 107.04 ± 14.93 SD; TDC: 111.04 ± 9.44 SD; T52 = −1.177, P > 0.245) and G-IQ distribution (K-S Z = 0.816, P > 0.441). Only participants with a general IQ of at least 80 were included (WAIS III and WISC III).
ASD subjects were diagnosed by experienced clinicians (according to the criteria of ICD-10 and DSM-IV). For all participants, diagnosis was confirmed with the Autism Diagnostic Observation Schedule, conducted by trained examiners (E.G. and I.K.-B). Furthermore, the Autism Diagnostic Interview-Revised (ADI-R) was performed in children and adolescents ( n = 15) and in a subset of adult patients ( n = 6), if a qualified informant was available. Age did not significantly correlate with total ADOS score (Pearson’s R = 0.497, P > 0.497). Demographic and clinical data are summarized in Table 1 . At the time of examination, some subjects of the ASD group were medicated [atypical neuroleptics ( nadol. = 1; nadults = 1), typical neuroleptics ( nadol. = 1; nadults = 1), atomoxetine: ( nadol. = 2), antidepressant (SSNRI, NaSSA) ( nadults = 1)]. Medication with stimulants ( n = 3) was discontinued 48 h before testing. The study was approved by the local ethics committee (according to the Declaration of Helsinki), and all subjects or their parents/caregivers gave written informed consent (adults/caregivers) and assent (children and adolescents) prior to participation.
| . | TDC group ( n = 27) . | ASD group ( n = 27) . |
|---|---|---|
| Age, mean (SD) (years) | 18.22 (4.41) | 18.52 (5.10) |
| Age range (Min–Max) | 12–26 | 13–31 |
| V-IQ, mean (SD) | 112 (11.5) | 113 (17.3) |
| V-IQ range (Min–Max) | 89–137 | 84–144 |
| P-IQ, mean (SD) | 108 (9.3) | 99 (15.7) |
| P-IQ range (Min–Max) | 91–129 | 73–128 |
| G-IQ, mean (SD) | 111 (9.44) | 107 (14.9) |
| G-IQ range (Min–Max) | 95–133 | 80–134 |
| . | TDC group ( n = 27) . | ASD group ( n = 27) . |
|---|---|---|
| Age, mean (SD) (years) | 18.22 (4.41) | 18.52 (5.10) |
| Age range (Min–Max) | 12–26 | 13–31 |
| V-IQ, mean (SD) | 112 (11.5) | 113 (17.3) |
| V-IQ range (Min–Max) | 89–137 | 84–144 |
| P-IQ, mean (SD) | 108 (9.3) | 99 (15.7) |
| P-IQ range (Min–Max) | 91–129 | 73–128 |
| G-IQ, mean (SD) | 111 (9.44) | 107 (14.9) |
| G-IQ range (Min–Max) | 95–133 | 80–134 |
G-IQ, General IQ; V-IQ, Verbal IQ; P-IQ, Performance IQ.
| . | TDC group ( n = 27) . | ASD group ( n = 27) . |
|---|---|---|
| Age, mean (SD) (years) | 18.22 (4.41) | 18.52 (5.10) |
| Age range (Min–Max) | 12–26 | 13–31 |
| V-IQ, mean (SD) | 112 (11.5) | 113 (17.3) |
| V-IQ range (Min–Max) | 89–137 | 84–144 |
| P-IQ, mean (SD) | 108 (9.3) | 99 (15.7) |
| P-IQ range (Min–Max) | 91–129 | 73–128 |
| G-IQ, mean (SD) | 111 (9.44) | 107 (14.9) |
| G-IQ range (Min–Max) | 95–133 | 80–134 |
| . | TDC group ( n = 27) . | ASD group ( n = 27) . |
|---|---|---|
| Age, mean (SD) (years) | 18.22 (4.41) | 18.52 (5.10) |
| Age range (Min–Max) | 12–26 | 13–31 |
| V-IQ, mean (SD) | 112 (11.5) | 113 (17.3) |
| V-IQ range (Min–Max) | 89–137 | 84–144 |
| P-IQ, mean (SD) | 108 (9.3) | 99 (15.7) |
| P-IQ range (Min–Max) | 91–129 | 73–128 |
| G-IQ, mean (SD) | 111 (9.44) | 107 (14.9) |
| G-IQ range (Min–Max) | 95–133 | 80–134 |
G-IQ, General IQ; V-IQ, Verbal IQ; P-IQ, Performance IQ.
Experimental paradigm and stimuli
We used the same experimental paradigm and stimuli described in previous studies ( Greimel et al. , 2010b ; Schulte-Rüther et al. , 2011 ). In short, subjects were asked to empathize with emotional facial expressions presented on a computer screen by ‘feeling into’ the depicted person and either to judge the emotional state of each face (other-task), or to report the emotions elicited in themselves by the emotional faces (self-task). As a control task, a perceptual decision on the width of stimulus faces was used (see Figure 1 and Supplementary Material for more details). The software Presentation 9 (Neurobehavioral Systems, Albany, CA, USA; http://www.neurobs.com ) was used for stimulus presentation and response collection. Additionally, eye movement data were collected during the fMRI scan (see Supplementary Material for more details) to control for equal attention to facial stimuli across age and diagnostic group. After the fMRI experiment, subjects were questioned about their strategies used to perform the tasks and other performance-related aspects. Five ASD subjects and five TD subjects were unable to describe the difference between the self and the other task and indicated that they had always responded ‘according to how the other person felt’ without any reference to their own feelings. These were excluded from further analysis. Thus, from an original sample of n = 64 participants, 54 remained for fMRI data analysis. All reported results pertain to the sample of n = 54 participants.
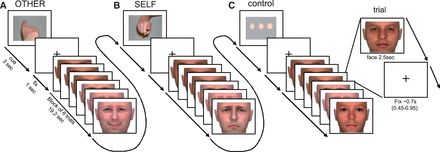
Time course of stimulus presentation during the scanning session. Subjects were instructed to empathize with the person presented on the screen and to (A) identify the emotional state (happy, neutral and sad) observed in the face (other-task) or (B) evaluate their own emotional response (happy, neutral and sad) to that face (self-task). As a control-task (C) a perceptual decision on the width of neutral faces (slim, normal wide) was used. Each block (19.2 s) was preceded by an instruction cue (3 s) and comprised six stimulus faces (each 2.5 s), separated by a fixation cross (jittered duration: 0.45–0.95 s). Instruction cues were pictures of a finger pointing towards the subject (self-task), pointing away from the subject (other-task) or three dots of increasing width (control-task). Each of n = 72 individual faces was presented once displaying a happy expression, once displaying a sad expression and once displaying a neutral expression. Faces had either a low or high intensity emotional expression. Tasks varied block-wise with 6 trials per block, resulting in 32 blocks and 192 trials overall. Prior to scanning, subjects were trained on the experimental tasks. Reprinted with permission from Schulte-Rüther, M., et al. (2011) . Dysfunction in brain networks supporting empathy: an fMRI study in adults with autism spectrum disorders. Social Neuroscience , 6 (1), 1-21, Copyright 2010 Taylor & Francis Ltd. ( http://www.tandfonline.com/doi/full/10.1080/17470911003708032 )
MR technical parameters
MR imaging was accomplished on a 1.5-Tesla Avanto MR scanner (Siemens, Erlangen, Germany) using a standard head coil. For functional imaging, gradient-echo, echoplanar T2*-weighted images were acquired (TE = 60 ms, TR = 3000 ms, α = 90°, FOV = 200 mm, voxel size = 3.1 × 3.1 × 4 mm 3 , matrix size = 64 × 64, 30 transversal slices, slice acquisition: ascending) in one session (∼14 min). Anatomical images were acquired using a T1-weighted 3D magnetization-prepared, rapid acquisition gradient echo (MP-RAGE) pulse sequence (TE = 3.93 ms, TR = 2200 ms, α = 15°, FOV = 256 mm, voxel size = 1 × 1 × 1 mm 3 , matrix size = 256 × 256, 160 sagittal slices, slice thickness = 1 mm).
Image processing and data analysis
Fifty-four subjects (27 TDC, 27 ASD) were included in the final sample for the analysis of the fMRI data. Functional volumes were analyzed with SPM5 (Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk/spm ) implemented in MATLAB 7 (The Mathworks Inc., Natick, MA, USA). Two hundred eighty-five images were realigned using rigid body transformation, normalized into the Montreal Neurological Institute (MNI) coordinate space and resampled at 2 × 2 × 2 mm 3 . Normalization parameters were determined. Prior to statistical analysis, functional volumes were smoothed with an 8 × 8 × 8 mm 3 Gaussian kernel (full width half maximum).
Boxcar functions of 19.2 s duration (corresponding to the onset of each experimental block, starting with the first presentation of a face) were convolved with a model of the hemodynamic response function (HRF) and its first-order temporal derivative. Movement parameters were included as additional regressors of no interest. A high-pass cut-off filter of 128 s was used. Parameter estimates of the resulting general linear model were calculated for each voxel and each regressor. Using the first regressor of the HRF model as an estimate of response height, the effect of both self- and other-task (relative to the control-task, respectively) was calculated and individual contrast images were created for each subject. Experimental conditions containing high and low intensity stimuli were modeled separately, however, high and low intensity conditions were thereafter combined because the initial assessment of the data indicated that statistical sensitivity was insufficient for separate analyses of low and high intensity conditions at a corrected threshold.
The following analysis focused on linear and non-linear developmental trajectories of neural activation patterns related to the empathizing tasks. A second-level analysis of covariance (ANCOVA) model was set up, i.e. individual images of the contrasts for the other task (other-task > control-task) and the self-task (self-task > control-task) were each entered into a ‘two-sample t test’-model with age as a covariate (separate for both groups, centered for overall mean). Furthermore, similar models were set up to test for the effect of age on the direct comparison of self- and other-task (i.e. self-task > other-task and other-task > self-task). Such models allow to test for a group difference in the linear effect of age on brain activation, irrespective of general group differences in activation. T-contrasts involving both covariate regressors were used to detect differences in the slope of the regression with age. For all models, both possible directions of slope differences were tested (ASD > TDC, TDC > ASD). Additionally, further models were set up that included the squared age regressor (separate for groups, centered for overall mean). In these models, F-contrasts were used to detect any group difference in quadratic age related effects (such as U-shape or inverted U-shape curves). Departures from sphericity assumptions were accommodated using the non-sphericity correction in SPM5. For these analyses, SPMs were thresholded at P < 0.005 (voxel level, uncorrected). We only report group differences that exceed a statistical threshold of P < 0.05, cluster level corrected for multiple comparisons (whole-brain). Additionally, we report significant peaks within predefined anatomical regions of interest that exceed a threshold of P < 0.05 (family-wise error (FWE)-corrected for ROI, voxel level). Anatomical ROIs were constructed using the software WFU pickatlas ( Tzourio-Mazoyer et al. , 2002 ) and included MPFC, precuneus, inferior frontal gyrus, IPC, fusiform gyrus (see Supplementary Material for more details).
At identified significant clusters, peak beta values were extracted for each individual to determine the direction and significance of developmental trajectories of the respective contrasts for each diagnostic group separately (linear regression models), and model comparisons were performed (see Supplementary Material ). To investigate the possibility of developmental delay in patients with ASD (e.g. similar patterns in ASD adults and TDC children/adolescents), further exploratory analyses were performed to compare developmental trajectories of TDC children/adolescents with ASD adults (see Supplementary Material ).
To assess the relationship of the identified clusters of differential developmental trajectories to individual empathic abilities and autistic symptoms, we correlated brain activation for the self task with individual self-rated empathic abilities (Empathy Quotient, EQ for adults [ Lawrence et al. , 2004 ]; Bryant Index of Empathy for children/adolescents [ Bryant, 1982 ] and ADOS score [in the ASD group]). Using SPM, regression analyses were performed separately for groups (ASD children/adolescents, ASD adults, TDC children/adolescents and TDC adults). We restricted the analyses to ROIs of the previously identified brain areas demonstrating differential developmental trajectories (5 mm spheres around peak coordinates).
Analysis of behavioral data
Behavioral data were analyzed with the software package SPSS 19 (SPSS Inc., Chicago, IL). Percentage of correct (i.e. correct attribution of the emotional state of a stimulus face in the other-task) or congruent responses (i.e. responses during the self-task mirroring the emotional state of a stimulus face) and mean reaction times (RTs) were calculated. As Kolmogorov–Smirnov tests indicated normal distribution of all variables of interest, parametric analyses were employed to test for statistically significant differences between groups and/or experimental conditions and demographical variables and to test for linear influences of age (mixed ANOVAs, t tests and ANCOVAs). For all behavioral analyses, significance was determined using two-tailed testing. To test for differential age effects on cognitive ability (IQ) and behavioral measures depending on group, data were entered into GLM analyses, modeling group as between subject factor, age as a covariate, and the interaction of group and age. If no interaction could be observed, data were entered into a standard ANCOVA analysis using group as the between subject factor and age as a covariate to test for group differences irrespective of age. To be consistent with the analysis of neuroimaging data, behavioral data were analyzed separately for self- and other-task but were collapsed across intensity levels.
RESULTS
Behavioral data
With respect to cognitive ability, the group × age interactions were not significant [G-IQ: F (1, 50) = 0.337, P = 0.564; P-IQ: F (1, 50) = 0.006, P = 0.937; V-IQ: F (1, 50) = 0.257, P = 0.615]. There were no group differences in V-IQ [ F (1, 51) = 0.115, P = 0.736] and G-IQ [ F (1, 51) = 1.322, P = 0.256]; however, a significant group difference emerged in P-IQ: F (1, 51) = 6.753, P < 0.05. A significant influence of the covariate age on IQ measures could not be observed [V-IQ: F (1, 51) = 1.082, P = 0.303; H-IQ: F (1, 51) = 0.051, P = 0.822; G-IQ: F (1, 51) = 0.335, P = 0.565)].
Percentage of congruent responses (i.e. same emotion indicated as evident in the stimulus face) for the self-task revealed no age × group interaction [ F (1, 50) = 1.062, P = 0.922], no influence of age [ F (1, 51) = 1.374, P = 0.247], but a significant effect of group [ F (1, 50) = 12.467, P < 0.001]. Control participants responded congruently in 68.08% (±16.73 SD), ASD participants in 45.68% (±28.93 SD) of the self-trials. Note, incongruent responses in both groups were almost exclusively neutral responses (on average less than 2.4% selection of the opposite emotional state). Percentage of correct responses for the other-task revealed no age × group interaction [ F (1, 50) = 0.000, P = 0.989], no influence of age [ F (1, 50) = 2.095, P = 0.154], and no group effect [ F (1, 50) = 0.502, P = 0.482]. Mean correct responses were 73.40% (± 10.69 SD) for controls and 75.37% (±8.51 SD) for participants with ASD in the other-trials. A scatterplot illustrating the relationship between performance and age is given in Figure 2 . The direct comparison between correct (other-task) and congruent responses (self-task) revealed significant differences in control participants [ T (26) = 2.452, P < 0.05], as well as in participants with ASD [ T (26) = 4.791, P < 0.001], suggesting that both groups were able to distinguish properly between both tasks.
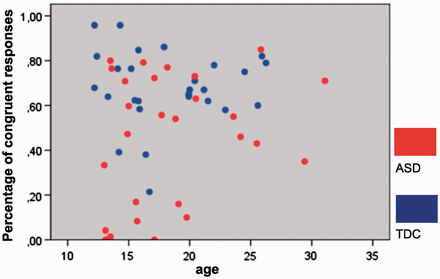
Covariation of behavioral performance and age. Scatterplot depicts behavioral performance during the self-task as a function of age, separately for each group (blue = TDC; red = individuals with ASD).
For RTs during the self-task, no group × age interaction could be observed [ F (1, 50) = 1.886, P = 0.176], a trend for a significant effect of age [ F (1, 51) = 3.434, P = 0.070], and no group effect [ F (1, 51) = 0.015, P = 902; mean controls = 1.193 s (±0.223 SD); mean ASD = 1.189 s (±0.297 SD)]. During the other-task, no group × age interaction effects [ F (1, 50) = 0.964, P = 0.331], no effect of age [ F (1, 51) = 1.678, P = 0.201], but a trend for a group difference [ F (1, 51) = 3.087, P = 0.085] was evident [mean controls : 1.123 s (±0.181 SD); mean ASD = 1.211 s (±0.181 SD]. The analysis of eyetracking data did not reveal any age effects or age × group interactions (see Supplementary Material for details).
Neuroimaging data
For the analyses aimed at detecting group differences for quadratic influences of age (such as U-shaped or inverted U-shaped functions) on brain activation, no significant results could be observed at the selected thresholds, neither for the self-task and other task (as compared with the control task), nor for the direct comparison of self-task versus other-task and vice versa. Concerning linear effects of age, significant group differences emerged for the comparison of the self-task vs. control-task. In the whole-brain analysis, significant differences in covariation of brain activation with age emerged in the occipital cortex (including area 17/18 and extending into the cerebellum), the anterior insula, right middle frontal gyrus and right inferior parietal lobule (extending into superior parietal lobe). Using a ROI approach, a significant cluster also emerged in the dorsal MPFC ( Table 2 ). No significant group differences were detected for the contrast other-control, other-self and self-other, neither in the whole brain analysis nor in the ROI analyses. At all coordinates of significant peak group difference, brain activation decreased significantly with age in TDC subjects (all R < −0.50; P < 0.01). In contrast, in ASD subjects, we observed a significant increase in brain activation for DMPFC ( R = 0.51; P < 0.01), rIPL ( R = 0.492; P < 0.01) and area 17 ( R = 0.51; P < 0.01), and a trend for increased activation in right anterior insula ( R = 0.345; P = 0.078). No significant effect of age was observed in ASD subjects for the middle frontal gyrus ( R = 0.24; P = 0.231). No group differences were detected that showed a reverse interaction pattern (i.e. decrease in ASD and/or increase in TDC) ( Figure 3 ). Model comparisons revealed that linear regression with age was an appropriate model fit for all brain regions and was not significantly improved by adding the age-squared regressor (see Supplementary Results ). Exploratory direct comparisons of differential trajectories between the subgroups ASD adults and TDC children/adolescents (with the hypothesis of the possibility of a developmental delay in ASD patients) revealed a similar pattern of differential age effects (see Supplementary Results ).
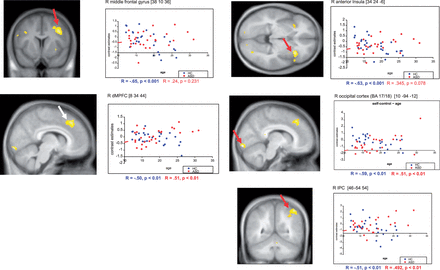
Covariation of brain activation and age. Statistical parametric maps of significant differences in covariation of brain activation with age (with respect to group) during the self task (see Methods section for details). SPMs are thresholded at P < 0.005 (voxel level, uncorrected) and overlayed on a mean anatomical T1 image of all participants. Depicted clusters (red arrows) were significant at the whole brain level ( P < 0.05, cluster-level corrected for multiple comparisons), except for the peak in the MPFC ( P < 0.05, voxel level corrected for multiple comparisons (ROI), white arrow). Correlation plots depict individual contrast estimates for the self task as a function of age in the activation peak. Correlation coefficients (R) and linear best fit estimates are given separately for each group (blue = TDC; red = individuals with ASD).
| Anatomical region . | H . | BA . | k . | x . | y . | z . | t . |
|---|---|---|---|---|---|---|---|
| Occipital cortex | R | 18/17 | 643 | 10 | −94 | −12 | 4.65 |
| Anterior insula | R | 48/47 | 357 | 34 | 24 | −6 | 4.26 |
| Middle frontal gyrus | R | 6/44 | 669 | 38 | 10 | 36 | 4.07 |
| Inferior parietal lobule | R | 39/40 | 590 | 46 | −54 | 54 | 4.04 |
| MPFC* | R | 8/32 | — | 8 | 34 | 44 | 4.13 |
| Anatomical region . | H . | BA . | k . | x . | y . | z . | t . |
|---|---|---|---|---|---|---|---|
| Occipital cortex | R | 18/17 | 643 | 10 | −94 | −12 | 4.65 |
| Anterior insula | R | 48/47 | 357 | 34 | 24 | −6 | 4.26 |
| Middle frontal gyrus | R | 6/44 | 669 | 38 | 10 | 36 | 4.07 |
| Inferior parietal lobule | R | 39/40 | 590 | 46 | −54 | 54 | 4.04 |
| MPFC* | R | 8/32 | — | 8 | 34 | 44 | 4.13 |
H, hemisphere; L, left; R, right; BA, Brodmann area; k , cluster size; peak activated voxels within significant clusters of brain activation ( P < 0.05 corrected for multiple comparisons (whole brain analysis) at the cluster level), and peak within the MPFC (*small volume correction for multiple comparisons (FWE, P < 0.05, voxel level), in an anatomical ROI of the MPFC) x , y , z refer to MNI coordinates of local peaks of activation for the interaction of age × group.
| Anatomical region . | H . | BA . | k . | x . | y . | z . | t . |
|---|---|---|---|---|---|---|---|
| Occipital cortex | R | 18/17 | 643 | 10 | −94 | −12 | 4.65 |
| Anterior insula | R | 48/47 | 357 | 34 | 24 | −6 | 4.26 |
| Middle frontal gyrus | R | 6/44 | 669 | 38 | 10 | 36 | 4.07 |
| Inferior parietal lobule | R | 39/40 | 590 | 46 | −54 | 54 | 4.04 |
| MPFC* | R | 8/32 | — | 8 | 34 | 44 | 4.13 |
| Anatomical region . | H . | BA . | k . | x . | y . | z . | t . |
|---|---|---|---|---|---|---|---|
| Occipital cortex | R | 18/17 | 643 | 10 | −94 | −12 | 4.65 |
| Anterior insula | R | 48/47 | 357 | 34 | 24 | −6 | 4.26 |
| Middle frontal gyrus | R | 6/44 | 669 | 38 | 10 | 36 | 4.07 |
| Inferior parietal lobule | R | 39/40 | 590 | 46 | −54 | 54 | 4.04 |
| MPFC* | R | 8/32 | — | 8 | 34 | 44 | 4.13 |
H, hemisphere; L, left; R, right; BA, Brodmann area; k , cluster size; peak activated voxels within significant clusters of brain activation ( P < 0.05 corrected for multiple comparisons (whole brain analysis) at the cluster level), and peak within the MPFC (*small volume correction for multiple comparisons (FWE, P < 0.05, voxel level), in an anatomical ROI of the MPFC) x , y , z refer to MNI coordinates of local peaks of activation for the interaction of age × group.
Brain behavior correlations
Significant positive correlations (self-rated empathic abilities ∼brain activation) emerged in the right inferior parietal lobe for ASD adults. Negative correlations could be observed in occipital cortex and right inferior parietal lobe for ASD children/adolescents and in right anterior insula for TDC children/adolescents. A significant negative correlation with autistic symptoms was found in the right inferior parietal lobe in adults with ASD.
DISCUSSION
To the best of our knowledge, this is the first study on neurofunctional developmental trajectories related to empathizing in individuals with ASD in comparison with TDC. At the behavioral level, we observed a group difference (ASD < TDC) for congruent responses (i.e. observed emotional state matches the emotional state indicated by the participant) during the self-task across all age groups. Note, differences were due to more ‘neutral’ responses of participants with ASD rather than inappropriate emotion choices, suggesting primarily a lack of emotional contagion. Accordingly, no group difference could be observed for correct answers (i.e., correct identification of observed emotional state during the other-task) see also ( Greimel, et al. , 2010b ; Schulte-Rüther et al. , 2011 ). These results suggest that participants with ASD were in principle able to identify the other person’s emotional states, but showed a deficit in the self-assessment of an appropriate emotional reaction. The finding is consistent with previous reports of reduced emotional contagion ( Scambler et al. , 2007 ) and reduced self-reports of empathy ( Baron-Cohen and Wheelwright, 2004 ) in individuals with ASD. There were no significant effects of age or group by age interactions. This pattern suggests that the empathizing task was suitable for all age levels and that age-related group differences at the neural level are due to differences in the neurofunctional development of these brain areas, rather than to performance differences in the empathizing task.
At the neural level, we found age by group interactions for the self-task, but no such effects for the other-task or the direct differential comparison of both tasks, suggesting that differences in developmental trajectories are most prominent for self-related components of empathizing (i.e. the explicit assessment of one’s own emotional reaction in an empathic face-to-face situation).
Cognitive control, mentalizing and empathy
Our results suggest atypical functional development of cognitive control and mentalizing processes in ASD during self-related empathizing, as reflected by differential trajectories of brain activation in lateral PFC and DMPFC.
Developmental changes in brain activation typically reflect the maturation of brain networks ( Casey et al. , 2005 ): Focal, specialized task-related areas show an increase in brain activation whereas activation within task-irrelevant brain areas declines with age ( Durston et al. , 2006 ). The lateral PFC has been implied in a wide range of tasks related to executive function and cognitive control ( Bunge et al. , 2002 ; Blasi et al. , 2006 ). The self-task poses considerable demands on executive control, i.e. the monitoring of internal evoked emotions needs to be coordinated with a continuous update of the observed stimuli and their respective emotional state. In TDC, this process may require fewer (neural) resources in adults, resulting in decreased activation. Accordingly, a decline of dorsolateral prefrontal brain activation with age has been shown to reflect decreased effort when solving a task ( Luna and Sweeney, 2001 ; Tamm et al. , 2002 ). Our results suggest that in subjects with ASD these refinements are impaired and that the executive demands for explicit empathizing persist into adulthood.
Similarly, our findings are in accordance with previous studies demonstrating a decrease of frontal brain activation for social-cognitive tasks from adolescence to early adulthood in the MPFC ( Wang et al. , 2006 ; Blakemore et al. , 2007 ; Pfeifer et al. , 2007 ; Kobayashi et al. , 2008 ; Gunther Moor et al. , 2011 ; see Blakemore 2008 for a review). These effects might sometimes be too subtle to detect ( Greimel et al. , 2010a ), but become evident in the direct comparison to a group with aberrant development such as ASD. Brain activation in the MPFC has consistently been reported for diverse ToM tasks ( Castelli et al. , 2000 ; Vogeley et al. , 2001 ; see Frith and Amodio, 2006 ; Frith and Frith, 2008 for reviews), and is particularly related to abstract mentalizing ( Gallagher and Frith, 2003 ). The peak of differential trajectories within DMPFC in the present study is located between anterior rostral MPFC and a bordering, more posterior region within DMPFC which has been implicated in goal directed behavior, including error monitoring and cognitive control ( Ridderinkhof et al. , 2004 ). The inverted pattern of developmental trajectories within this transition zone suggests that cognitive components of empathic self-reference (i.e. mentalizing and cognitive control) are increasingly engaged in ASD, whereas these components of empathizing may become more and more automated and less error-prone in TDC. In accordance with this interpretation, a shift from DMPFC to vMPFC activation for the self-task could be observed only in TDC adults, but not in adults with ASD ( Schulte-Rüther et al. , 2011 ).
Self- other distinction and explicit monitoring of emotional states
The IPC has been implicated in self referential processing ( Lou et al. , 2004 ; Uddin et al. , 2005 ) and in particular for the distinction between self- and other-perspective ( Decety and Sommerville, 2003 ; Schulte-Rüther et al. , 2007 ). During typical development, assessing the own emotional response may draw stronger on these networks in children and adolescents than in adults ( Greimel et al. , 2010a ), in line with behavioral studies suggesting that with increasing age empathic responses are more and more focused on other people’s inner states ( Strayer, 1993 ) rather than one’s own. The increase of activation in this brain region in participants with ASD may thus reflect that adults with ASD develop a different strategy for assessing their own emotion in response to a facial display. Enhanced distinction between self- and other perspective may contribute to a diminished capability of showing contagious emotional responses ( Schulte-Rüther et al. , 2011 ), in favor of a more cognitively biased understanding of the observed emotion. Interestingly, activation in the IPC was negatively correlated with ASD symptoms in adults, but positively with self-rated empathic abilities, suggesting that this strategy might be applied to a greater extent by higher functioning individuals and may result in a greater amount of self-ascribed emotional responsiveness. In adolescents with ASD, we observed a negative correlation with self-rated empathy, supporting the idea of age-dependent compensatory mechanisms in ASD that develop at the transition to adulthood.
The insular cortex is involved in viscero-sensory processing of internal body states, including states of emotional arousal. It has been suggested that the anterior insula is critically involved in interoception, emotional awareness, and self-recognition (see Craig, 2009 for a review), plays an important role in shared affect ( Carr et al. , 2003 ), especially concerning disgust ( Wicker et al. , 2003 ) and pain ( Singer et al. , 2004 ) and is related to dispositional differences in empathy ( Greimel et al. , 2010b ). Our results suggests that during typical development, the assessment of own emotional reactions during empathizing draws less on explicit processes (such as internal awareness of feelings and bodily sensations) with increasing age, as reflected by a decrease in brain activation. Typically developing children and adolescents encounter numerous socio-emotional situations involving face-to-face interactions during their lives, thus the monitoring of self-related emotional states may become increasingly automatic and less relevant for explicit empathizing. In support of that notion, we observed a negative correlation in anterior insula with self-rated empathy in TDC adolescents. In contrast, in patients with ASD, a more explicit processing of self-related emotional states may be maintained into adulthood.
Developmental differences in early visual processing
Unexpectedly, a significant differential effect of age on brain activation could also be observed in visual processing areas (including primary visual areas 17/18). Increased activation in primary visual areas in ASD during visual tasks might be associated with increased processing of specific local aspects of a visual stimulus ( Manjaly et al. , 2007 ; Brieber et al. , 2010 ). To a certain extent, our empathizing paradigm draws upon the visual processing of local facial features, because the facial emotion needs to be classified prior to eliciting an empathic response. Processing of local facial features during empathizing may decrease during typical development possibly reflecting a developmental shift from local to global processing of emotional faces. Enhanced visual processing in the adult participants with ASD may be explained by the use of compensatory strategies (e.g. attentional top-down control via DMPFC or lateral prefrontal cortex). However, whereas empathic abilities seem to be negatively associated with activation in early visual processing areas in ASD children and adolescents, this does not seem to be the case in adults with ASD. Tentatively, this pattern of results suggests a transition from disordered visual processing patterns in childhood to more controlled processing style in adults, potentially less interfering with empathic processing. However, these conclusions remain speculative and future research is needed to further explore developmental trajectories in primary visual areas in ASD and their relation to empathic processing.
Clinical implications
The finding of an overall pattern of age-related increases in brain activation in participants with ASD in several brain regions strongly suggest that these effects reflect compensatory processes. Increases in brain activation in ASD may result from a greater effort or enhanced supervisory strategies during situations that demand empathizing, in particular when associated with emotional self-reference. Most young adults with ASD have undergone behavioral therapeutic interventions (e.g. social skills training). Such interventions often target at paying explicit attention to social stimuli and situations, and teach explicit strategies to adapt one’s own thoughts and behavior (see e.g. Bock, 2001 ). Such strategies may contribute to differential developmental effects in neural networks of empathy, in particular for explicit assessment of one’s own emotional state. Our results indicate that in patients with ASD, the functional brain networks supporting ToM and empathy continue to develop into adulthood. Note, our findings are more in favor of qualitatively different neural compensatory mechanisms than a simple quantitative delay in development, also reflected by the finding that developmental trajectories also differ in the comparison of ASD adults and TDC children/adolescent. Consistent with the idea of additional compensatory processes, several studies showed that social behavior and emotional responsiveness improve during adolescence and adulthood ( Shattuck et al. , 2007 ; Farley et al. , 2009 ), but though individuals with ASD can develop higher-order ToM abilities during adolescence and adulthood they still lack intuitive ToM in dyadic social interactions ( Bowler, 1992 ; Happé, 1994 ), including access to self-referential emotions during empathizing. Furthermore, we found stable behavioral differences for explicit empathizing between TDC and ASD across the life-span (i.e. no behavioral age-by-group interactions). If the changes in neural activations from adolescence to early adulthood reflect compensatory mechanisms as hypothesized, these do not seem to be sufficient to normalize behavioral empathic performance. However, a measure of categorical affect match, as employed in our paradigm, may not be sensitive enough to detect subtle age-related improvements in individuals with ASD. Thus, socio-emotional processing might have improved during the course of development and as a result of behavioral interventions (e.g. recognition of emotional faces, perspective taking abilities and social skills), despite persistent reduced emotional contagion. Though speculative at present, this finding suggests that training of explicit mentalizing and ToM abilities throughout adolescence is important, but probably not sufficient to enhance emotional contagion in individuals with ASD in empathic situations. Ultimately, such questions can only be answered in a longitudinal design employing a variety of empathic performance measures. Furthermore, future studies need to include individuals with ASD at younger ages. The age range in our sample is particularly suited to detect compensatory changes during adolescence and young adulthood. However, ToM abilities ( Baron-Cohen et al. , 1985 ), as well as early empathic understanding ( Thompson, 1987 ) are rooted much earlier in development which is likely also reflected in the neurodevelopmental trajectories of empathic abilities ( Greimel et al. , 2010a ). The identification of differential neural trajectories during earlier stages of typical and atypical development might be particularly informative for early age-adapted interventional strategies.
CONCLUSIONS
Our results provide first evidence for developmental changes in the neural substrates of empathic processing in ASD. In a cross-sectional approach including children, adolescents and adults, we observed that the developmental trajectories of TDC subjects and ASD subjects differ from each other and that atypical brain activation patterns extend into adulthood. Furthermore, our data show that the refinement of functional brain networks related to social-cognitive abilities in ASD continues into adulthood. Interestingly, a decrease in brain activation with age in controls was paralleled by an increase in brain activation in individuals with ASD, mainly in brain regions relevant for cognitive control and explicit monitoring of emotional states. These data therefore strongly suggest that during the course of development, individuals with ASD may be able to acquire a cognitive-biased strategy to gain access to other people’s emotions.
The authors are grateful to all volunteers who took part in this study. They thank their colleagues in the Brain Imaging Physics (INM-4) and Cognitive Neuroscience groups (INM-3) at the Research Center Jülich (Institute of Neuroscience and Medicine) and the Child Neuropsychology section at the University Hospital RWTH Aachen for their support and helpful advice. K.K. and B.H.-D. were supported by the Bundeministerium für Bildung und Forschung grant 01GW0751. M.P. was supported by a grant from the Hans-Lungwitz-Foundation.


