-
PDF
- Split View
-
Views
-
Cite
Cite
Paige Ethridge, Aislinn Sandre, Melanie A Dirks, Anna Weinberg, Past-year relational victimization is associated with a blunted neural response to rewards in emerging adults, Social Cognitive and Affective Neuroscience, Volume 13, Issue 12, December 2018, Pages 1259–1267, https://doi.org/10.1093/scan/nsy091
Close - Share Icon Share
Abstract
Anhedonia is associated with multiple forms of psychopathology, yet relatively, little is known about how anhedonia develops. Emerging evidence suggests that anhedonia is the result of interactions between life stress and the brain’s reward systems, and that social stress, in particular, may drive these processes. One potent form of social stress is peer victimization, but very little research has focused on peer victimization beyond adolescence, and even less has examined the associations between peer victimization and neural response to rewards. The present study sought to identify associations between past-year history of peer victimization and neural response to rewards in emerging adults (N = 61). Relational and physical forms of victimization were assessed separately since these distinct types of social stress have different trajectories across development and different associations with psychopathology. Reward sensitivity was indexed with the event-related potential component known as the reward positivity, which was elicited using a forced-choice monetary reward guessing task. Results demonstrated that past-year relational, but not physical, victimization was associated with a blunted neural response to rewards. These findings provide insight into one potential mechanism in the etiology of anhedonia, which may, in turn, help us to better identify pathways to multiple psychopathologies.
Past-year relational victimization predicts a blunted neural response to rewards in emerging adults
Anhedonia, or the inability to experience pleasure (Ribot, 1896), is associated with multiple forms of psychopathology, including schizophrenia (Blanchard and Cohen, 2006), substance use disorders (Koob and Le Moal, 1997) and depression (Pechtel et al., 2013). Anhedonia is a fundamental symptom of some disorders (e.g. depression; American Psychiatric Association, 2013) and greater anhedonia has been associated with increased disorder severity (Gabbay et al., 2015) as well as poorer treatment response (McMakin et al., 2012; Vrieze et al., 2014). In efforts to understand how anhedonia develops, a promising line of research has focused on associations between anhedonia and abnormal responses in neural networks critical for reward processing. Specifically, rodent, non-human primate and human studies have implicated the striatum and the prefrontal cortex in reward processing (O’Doherty, 2004; Balleine et al., 2007; Haber and Knutson, 2010) and evidence suggests that abnormal activation of these brain regions in response to rewards is associated with anhedonia (e.g. Keedwell et al., 2005). Importantly, stress also plays a fundamental role in the emergence of anhedonia (Berenbaum and Connelly, 1993; Anisman and Matheson, 2005), and in fact , it has been suggested that anhedonia is a result of interactions between life stress and the brain’s reward systems (e.g. Pizzagalli, 2014). Experimental data support this view; for instance, several studies have shown that acute laboratory stressors reduced activation in the prefrontal cortex and the striatum to monetary rewards (Ossewaarde et al., 2011; Porcelli et al., 2012).
However, the body’s stress response systems, including the hypothalamic–pituitary–adrenal (HPA) axis, which plays a major role in hormonal responses to stress, are activated by multiple diverse triggers (McRae et al., 2006; Schwabe et al., 2008). Research investigating the association between stress and reward insensitivity has largely been conducted using laboratory stressors, such as the cold pressor test (Lighthall et al., 2012) or threat of shock (Bogdan and Pizzagalli, 2006). While these measures are typically methodologically rigorous and easy to administer, they may lack ecological validity and therefore provide an incomplete understanding of how real-world stress leads to reward insensitivity. This limitation is important given the evidence that not all stressors are equivalent in their impact on HPA axis functioning. In particular, the HPA axis is exquisitely sensitive to social stress (e.g. negative social evaluation; Dickerson et al., 2008; Schwabe et al., 2008) and social stress has been linked to maladaptive psychological outcomes (Crick and Grotpeter, 1995; Prinstein et al., 2001; Desjardins and Leadbeater, 2011; Wu et al., 2015). In fact, cortisol, the primary glucocorticoid in humans indicating activation of the HPA axis, may be a key mediator in the effects of stress on cognitive and emotional processing (Erickson et al., 2003), and laboratory data suggest that social evaluative stress tasks result in greater cortisol elevation than non-social stress tasks (McRae et al., 2006). Consistent with this, there is longstanding evidence that interpersonal stressors better predict the onset of depression and substance use disorders than other types of stressors (Wagner and Compas, 1990; Rudolph and Hammen, 1999; Low et al., 2012).
Peer victimization, a potent and commonly occurring interpersonal stressor, has been shown to prospectively predict negative psychological outcomes, including symptoms of psychopathology (Reijntjes et al., 2010; Reijntjes et al., 2011) and poor psychosocial adjustment (Hodges and Perry, 1999). However, much of this research has been limited to child and adolescent populations (e.g. Casper and Card, 2017) and little is known about the impact of this type of social stress across the lifespan, even though there is evidence that peer victimization persists into adulthood (e.g. Linder et al., 2002; Verona et al., 2008; Sandstrom and Cillessen, 2010; Ostrov et al., 2011; Leadbeater et al., 2014). Moreover, the links between peer victimization and symptoms of psychopathology may strengthen as youth develop from childhood to adolescence (Casper and Card, 2017), suggesting it is critical that we examine these associations during subsequent developmental phases such as emerging adulthood (i.e. the distinct developmental period between ages 18 and 25 years; Arnett, 2007).
Although the links between peer victimization and poor psychological adjustment, including disorders characterized by anhedonia, have been well documented (Hawker and Boulton, 2000; Card et al., 2008; Reijntjes et al., 2010; Reijntjes et al., 2011; Casper and Card, 2017), very few studies have investigated the association between peer victimization and neural correlates of anhedonia (e.g. reduced neural response to anticipation or receipt of rewards). One longitudinal study assessed associations between social stress, broadly defined, in early adolescence (ages 11 and 12 years) and neural response to rewards at age 16 years, and found that peer victimization was modestly associated with decreased response to rewards in the medial prefrontal cortex (Casement et al., 2014). To our knowledge, no studies have assessed the associations between these experiences and neural processes in adults. The present study, therefore, sought to extend the work by Casement et al. (2014) by evaluating associations between peer victimization and reward-related neural processes in emerging adults.
In the current study, we examined both physical (e.g. hitting) and relational (e.g. social exclusion) victimization. Evidence suggests that the trajectories of these two types of victimization are not equivalent across development (Côté et al., 2007; Cleverley et al., 2012) and that they may be differently associated with psychosocial outcomes (Card et al., 2008). Indeed, some research has shown that relational victimization is more strongly associated with internalizing symptoms than is physical victimization (Leadbeater et al., 2014) and this association becomes stronger with age (Casper and Card, 2017). Moreover, physical victimization decreases with age, and emerging adults report relatively little physical victimization (Leadbeater et al., 2014). Together, these findings suggest that understanding associations between peer victimization and neural response to rewards, especially in emerging adulthood, requires studying physical and relational victimization separately.
The neural response to rewards used in the present study was the event-related potential (ERP) component often referred to as the reward positivity (RewP). The RewP is a positive-going component that is maximal at frontocentral sites on the scalp ∼250–350 ms following the onset of reward-related feedback. The RewP is thought to be enhanced (i.e. more positive) to rewards compared to losses (Proudfit, 2015). Previous research has demonstrated associations between the RewP and self-reported and behavioral measures of reward sensitivity (Bress and Hajcak, 2013), and studies have demonstrated that a reduced magnitude of these responses is associated with acute stress (Bogdan et al., 2011; Banis and Lorist, 2012).
Given previous research suggesting that social stress may play an important role in the emergence of anhedonia via its influences on neural responses to rewards (Pizzagalli, 2014) and the paucity of research studying these processes as individuals transition to adulthood, the present study had two primary aims: (i) to identify whether self-reported past-year peer victimization was associated with a blunted RewP in an emerging adult sample and (ii) to identify whether sensitivity to rewards was differentially associated with physical and relational victimization. Although neural response to reward-related feedback is only one factor subsumed under the umbrella of anhedonia, the present study focused on the RewP in order to be better able to identify specific associations between life stress and correlates of anhedonia. Because peer victimization is present in emerging adulthood (Linder et al., 2002; Verona et al., 2008; Sandstrom and Cillessen, 2010; Ostrov et al., 2011; Leadbeater et al., 2014) and previous research has shown that, in younger samples, peer victimization is associated with disrupted reward processing (Casement et al., 2014), we hypothesized that greater past-year peer victimization would be associated with a more blunted RewP. Since relatively little research has investigated differential associations between physical and relational forms of peer victimization, we conducted exploratory analyses regarding these distinct types of social stress. However, in line with evidence suggesting that relational victimization is more strongly associated with depressive cognitions and symptoms than physical victimization (Sinclair et al., 2012; Leadbeater et al., 2014), we predicted that a similar pattern would be observed here, such that relational victimization would be more strongly associated with reward insensitivity (i.e. a blunted RewP).
Method
Participants
Seventy-one participants were recruited from the McGill University psychology human participant pool or from flyers posted around the McGill University campus. Participants either received course credit or monetary compensation for their participation in a 2 h study. All participants provided written informed consent after review of the protocol and all procedures were approved by the Research Ethics Board at McGill University.
Data from one participant were excluded because of excessive noise in one of the mastoid reference channels (TP9); one participant was excluded because they selected ‘prefer not to answer’ for the demographic question related to gender; one participant was excluded because his age was more than three s.d. above the mean (M) sample age; and seven participants were excluded because they did not follow instructions while completing the questionnaires, leaving 61 participants. The mean age of the sample was 20.20 years (s.d. = 1.51; 18–25 years of age); 54 participants (89%) were female; 54% were Caucasian, 13% were Chinese, 10% were Arab/West Asian, 10% were Korean, 2% were South Asian, 2% were South East Asian, 2% were Hispanic and 8% indicated that they were another ethnicity.
Measures
Past-year peer victimization was assessed using nine items modified and updated from the Peer Experiences Scale (Vernberg et al., 1999). Items assessed victimization via physical aggression from others [three items; e.g. ‘During the past 12 months, how many times has another person been physically rough with you (pushed you, hit you, etc.)?’] and relational aggression from others (six items; e.g. ‘During the past 12 months, how many times has another person prevented you from being part of his/her group or team when you wanted to?’). Items were assessed on a three-point scale (1 = ‘never’, 2 = ‘once or twice’, 3 = ‘more often’). Cronbach’s alphas were good for the full scale (0.88), the physical victimization subscale (0.86) and the relational victimization subscale (0.82; Nunnally, 1978).
Task and materials
To elicit the RewP, the present study employed the doors task. This forced-choice guessing task, during which participants win or lose money, is well validated and has been shown to elicit neural responses with good psychometric properties (Levinson et al., 2017; Luking et al., 2017; Ethridge and Weinberg, 2018). In the present sample, neural responses to gains and losses demonstrated good to excellent internal consistency reliability, with split-half estimates (computed as the correlation between odd- and even-numbered trials, corrected using the Spearman–Brown prophecy formula) of r = 0.95 and r = 0.88, respectively (Cicchetti, 1994).
The task consisted of 40 trials, presented in two blocks of 20. At the beginning of each trial, participants were presented with an image of two doors and were instructed to choose one door by clicking the left or right mouse button. The doors remained on the screen until the participant responded. Next, a fixation mark (+) appeared for 1000 ms, followed by feedback presented for 2000 ms. Participants were told that they could either win $0.50 or lose $0.25 on each trial. A win was indicated by a green ‘↑’ and a loss was indicated by a red ‘↓’. A fixation mark then appeared for 1500 ms and was followed by the message ‘Click for the next round’, which remained on the screen until the participant responded and the next trial began. Across the task, 20 gain and 20 loss trials were presented in a random order and all participants received $3.00 immediately after the task concluded.
Electroencephalographic recording and data processing
Continuous electroencephalogram (EEG) was recorded using a 32-electrode cap and a BrainVision actiCHamp system (Brain Products GmbH, Munich, Germany) based on the standard 10/20 layout and a ground electrode at Fpz. The electrooculogram (EOG) generated from eye movements and blinks was recorded using facial electrodes placed ∼1 cm above and below the left eye and referenced to an electrode on the back of the neck. All electrode impedances were <10 kΩ and data were recorded with a sampling rate of 1000 Hz using a 60 Hz low-pass filter.
Offline analysis was conducted using BrainVision Analyzer software (Brain Products GmbH). Data were referenced to an average of the left (TP9) and right (TP10) mastoids and band-pass filtered with low and high cut-offs of 0.01 and 30 Hz (24 dB/octave). Eye-blink and ocular corrections were conducted using EOG per a modification of the original algorithm published in Gratton et al. (1983). A semi-automatic procedure was employed to detect and reject artifacts. The criteria applied were a voltage step of >50.0 μV between sample points, a voltage difference of 300.0 μV within a trial and a maximum voltage difference of <0.50 μV within 100 ms intervals. These intervals were rejected from individual channels in each trial using the ‘Individual Channel Mode’ in BrainVision Analyzer. Visual inspection of the data was then conducted to detect and reject any remaining artifacts in individual channels (see Brain Products GmbH, 2017, pp. 250–262, for a detailed description of the artifact rejection procedure). Using this procedure, almost all data for most participants remained in the channels of interest (Cz, FC1 and FC2) after artifact rejection (MGain trials = 19.46, s.d. = 1.30, minimum = 12, maximum = 20; MLoss trials = 19.37, s.d. = 1.19, minimum = 14, maximum = 20).
The EEG was segmented into 1200 ms windows for each trial, beginning 200 ms before presentation of feedback and continuing for 1000 ms following feedback. The 200 ms window from −200 to 0 ms prior to feedback onset served as the baseline. The RewP appears maximal ∼300 ms at frontocentral sites; therefore, the RewP was scored as the average activity at a pooling of electrodes Cz, FC1 and FC2 between 250 and 350 ms following the receipt of gain and loss feedback.
Pearson’s correlations were used to assess bivariate associations between variables. In order to examine specific associations between victimization and the RewP, we conducted a simultaneous multiple regression with the neural response to gains as the dependent variable and the neural response to losses, relational victimization, physical victimization and gender as predictors. Gender was entered as a covariate because our sample was predominantly female and previous studies have demonstrated gender differences in the effect of stress on reward processing (Lighthall et al., 2012) as well as in responses to peer victimization (Paquette and Underwood, 1999; Dirks et al., 2014). Since previous research has shown that difference scores (e.g. the ∆ RewP) are poor measures of unique effects (Meyer et al., 2017), we opted instead to control for neural response to loss to examine specific associations with neural response to reward.
Results
Descriptive statistics of predictor variables and neural responses to gains and losses
| . | Mean . | s.d. . |
|---|---|---|
| Neural response to gains (μV) | 15.31 | 8.56 |
| Neural response to losses (μV) | 10.85 | 7.05 |
| Relational victimization | 9.70 | 2.42 |
| Physical victimization | 3.28 | 0.71 |
| . | Mean . | s.d. . |
|---|---|---|
| Neural response to gains (μV) | 15.31 | 8.56 |
| Neural response to losses (μV) | 10.85 | 7.05 |
| Relational victimization | 9.70 | 2.42 |
| Physical victimization | 3.28 | 0.71 |
Note and Sources. Neural responses are reported in μVs. The minimum and maximum possible values are 6 and 18 for the relational victimization scale and 3 and 9 for the physical victimization scale. The values in the present sample ranged from 6 to 17 for the relational victimization scale and from 3 to 6 for the physical victimization scale.
Descriptive statistics of predictor variables and neural responses to gains and losses
| . | Mean . | s.d. . |
|---|---|---|
| Neural response to gains (μV) | 15.31 | 8.56 |
| Neural response to losses (μV) | 10.85 | 7.05 |
| Relational victimization | 9.70 | 2.42 |
| Physical victimization | 3.28 | 0.71 |
| . | Mean . | s.d. . |
|---|---|---|
| Neural response to gains (μV) | 15.31 | 8.56 |
| Neural response to losses (μV) | 10.85 | 7.05 |
| Relational victimization | 9.70 | 2.42 |
| Physical victimization | 3.28 | 0.71 |
Note and Sources. Neural responses are reported in μVs. The minimum and maximum possible values are 6 and 18 for the relational victimization scale and 3 and 9 for the physical victimization scale. The values in the present sample ranged from 6 to 17 for the relational victimization scale and from 3 to 6 for the physical victimization scale.
Simultaneous regression predicting the neural response to gains from neural response to losses, gender and relational and physical victimization.
| Predictor . | b (standard error) . | β . |
|---|---|---|
| Neural response to losses | 1.08 (0.08) | 0.89*** |
| Gender | 2.41 (1.78) | 0.09 |
| Relational victimization | −0.56 (0.27) | −0.16* |
| Physical victimization | 1.28 (0.92) | 0.11 |
| Total R2 = 0.76; F(4, 60) = 43.61, P < 0.001 |
| Predictor . | b (standard error) . | β . |
|---|---|---|
| Neural response to losses | 1.08 (0.08) | 0.89*** |
| Gender | 2.41 (1.78) | 0.09 |
| Relational victimization | −0.56 (0.27) | −0.16* |
| Physical victimization | 1.28 (0.92) | 0.11 |
| Total R2 = 0.76; F(4, 60) = 43.61, P < 0.001 |
*indicates P < 0.05, ***indicates P < 0.001.
Simultaneous regression predicting the neural response to gains from neural response to losses, gender and relational and physical victimization.
| Predictor . | b (standard error) . | β . |
|---|---|---|
| Neural response to losses | 1.08 (0.08) | 0.89*** |
| Gender | 2.41 (1.78) | 0.09 |
| Relational victimization | −0.56 (0.27) | −0.16* |
| Physical victimization | 1.28 (0.92) | 0.11 |
| Total R2 = 0.76; F(4, 60) = 43.61, P < 0.001 |
| Predictor . | b (standard error) . | β . |
|---|---|---|
| Neural response to losses | 1.08 (0.08) | 0.89*** |
| Gender | 2.41 (1.78) | 0.09 |
| Relational victimization | −0.56 (0.27) | −0.16* |
| Physical victimization | 1.28 (0.92) | 0.11 |
| Total R2 = 0.76; F(4, 60) = 43.61, P < 0.001 |
*indicates P < 0.05, ***indicates P < 0.001.
Discussion
Anhedonia is of central importance to the study of psychopathology but pathways to anhedonia are not well understood. However, some evidence suggests that anhedonia may be a result of interactions between life stress and the brain’s reward circuitry (e.g. Pizzagalli, 2014). To advance this line of research, the present study sought to identify associations between past-year peer victimization and sensitivity to reward-related feedback, an important facet of anhedonia, and to determine whether these associations differed based on the type of victimization experienced. Results demonstrated that past-year experience of relational, but not physical, victimization was associated with a blunted RewP. The present findings underscore the fact that real-world social stress is associated with disrupted processing of rewards and may therefore be an important predictor of anhedonia during the transition to adulthood.
Anhedonia is the focus of significant research attention primarily due to its associations with mental illnesses such as depression. The data reported here further our understanding of how disrupted reward processing, which is often associated with anhedonia and depression, is related to peer victimization in emerging adulthood. Ample evidence has demonstrated that stressful life events precede depressive episodes (Paykel et al., 1969; Billings and Moos, 1984; Lewinsohn et al., 1988; Kendler et al., 1999; Monroe and Harkness, 2005) and that depression is associated with increased generation of, and sensitivity to, stressful life events (Hammen, 1991; Adrian and Hammen, 1993; Rudolph and Hammen, 1999; Rudolph et al., 2000). Despite the strong link between stress and depression, mechanisms by which stress leads to depression remain unclear. Stress-induced reward insensitivity, indexed by blunted neural responses to rewards, as reported here, is a plausible mechanism linking stress and depression. Indeed, pre-clinical studies have reported that stress induces reward insensitivity (Anisman and Matheson, 2005) and, in humans, stress-induced anhedonia has been shown to be associated with a family history of depression (Berenbaum and Connelly, 1993) and with symptoms of depression (Bogdan and Pizzagalli, 2006; Dillon et al., 2009) especially for women (Lighthall et al., 2012). Moreover, multiple studies have demonstrated an association between depression and a blunted RewP (Foti and Hajcak, 2009; Bress et al., 2012; Bress et al., 2013); thus, it is possible that this reduced neural response to rewards mediates the relationship between life stress and the development of depression (Hanson et al., 2015). In the present sample, however, we did not find that depressive symptoms were significantly correlated with neural responses nor did we find that depressive symptoms moderated the relationship between relational victimization and neural response to rewards (see Supplementary data for details). While this may seem contrary to the aforementioned literature, little evidence suggests that current symptoms of depression in a non-clinical sample are predictive of disrupted reward processing (Kujawa et al., 2014; Weinberg et al., 2015). This study, nevertheless, provides an important link between relational aggression, a potent social stressor, and reward insensitivity, as measured by the RewP, which may help us gain a nuanced understanding of the development of depression.
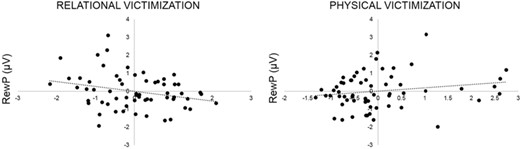
Partial regression plots depicting associations between the RewP (measured at a pooling of Cz, FC1 and FC2) and relational and physical forms of peer victimization, controlling for the other independent variables entered into the regression model (Table 2; neural response to losses, gender and the alternate type of victimization).
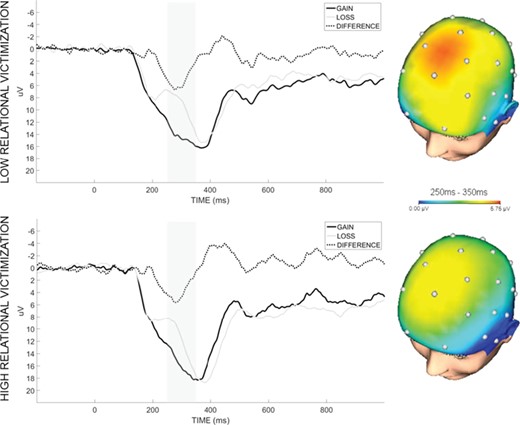
Waveforms and scalp topographies depicting neural response to gain and loss feedback at a pooling of electrodes Cz, FC1 and FC2, in participants high and low on past-year relational victimization based on a median split. Shaded bars demarcate the 250–350 ms time window used for analysis. Scalp topographies show Δ RewP (gain minus loss).
While anhedonia is commonly discussed with reference to depression, it is not unique to depression and has also been observed in substance use disorders (Pozzi et al., 2008; Garfield et al., 2014; Garfield et al., 2017) and schizophrenia (Martin et al., 1977; Wolf, 2006; Strauss and Gold, 2012). Likewise, interpersonal stress has been shown to precipitate both substance use disorders (Wills, 1986; Turner and Lloyd, 2003; Lehavot and Simoni, 2011) and schizophrenia (Nuechterlein and Dawson, 1984; Walker and Diforio, 1997; Rosenfarb et al., 2000; van Winkel et al., 2008). It is possible that the association observed in the present study between relational victimization and reward insensitivity represents a pathway common to all of these disorders. However, while substance use disorders and schizophrenia are frequently comorbid with depression (Regier et al., 1990; Swendsen and Merikangas, 2000; Hasin et al., 2007; Buckley et al., 2009) and with each other (Regier et al., 1990; Buckley et al., 2009; Zhornitsky et al., 2012), they also manifest in distinct symptoms and behaviors (American Psychiatric Association, 2013). Future research in large clinical samples should consider how these common factors can lead to clinically divergent phenotypes (Nolen-Hoeksema and Watkins, 2011; Corral-Frías et al., 2015).
Although the present study provided valuable evidence linking peer victimization and an important correlate of anhedonia (i.e. blunted neural response to rewards), some important considerations remain. For instance, experience of physical victimization in the past year was positively but not significantly associated with the magnitude of the RewP. We would note here that our sample reported low levels of physical victimization, limiting the variance in this measure. This is not surprising given the composition of our sample (89% female, emerging adults) and evidence that rates of physical victimization remain relatively low for females across development and decrease with age for males (Leadbeater et al., 2014). Nonetheless, the association with physical victimization was of a similar magnitude to that of relational victimization but in the opposite direction, which suggests some intriguing possibilities. For example, it is possible that the experience of increased physical aggression follows the enactment of greater physical aggression. Evidence suggests that those who experience greater physical aggression also tend to respond aggressively toward others (e.g. Dirks et al., 2017) and that the exhibition of physical aggression is positively correlated with measures of appetitive motivation and reward sensitivity (Harmon-Jones, 2003; Carlson et al., 2013). Taken together, this may explain the trend toward a positive association between physical victimization and neural response to rewards. Still, future studies using samples with greater ranges of peer victimization experiences, and with additional measures of aggressive behaviors, may be better able to delineate associations between distinct types of social stress and neural responses to rewards.
Another important consideration is that our sample was almost entirely female. Evidence suggests that there are gender differences in types of peer victimization experienced (Leadbeater et al., 2014) and in responses to victimization (Nansel et al., 2001). In fact, females tend to be more invested in their relationships and report greater distress from experiences of relational victimization than males (Paquette and Underwood, 1999; MacEvoy et al., 2012). There have also been gender differences reported in the effects of stress on neural responses to rewards (Lighthall et al., 2012). So, although we did not find that gender was a significant predictor of the RewP, future studies with a larger sample of males could test interactions between gender and relational or physical victimization.
A final consideration relates to the nature of the peer victimization data in this sample; namely, that they are cross-sectional retrospective self-reports and represent relatively low levels of peer victimization. Future work should include daily diary or other-reported (e.g. from a close friend) data, as well as longitudinal measures of peer victimization with detailed information about timing and duration of social stressors, in samples with greater ranges of peer victimization experiences. Importantly, research suggests that there are sensitive periods in development during which stress has a greater impact on psychological outcomes (e.g. Blakemore and Mills, 2012; Simpson et al., 2012) as well as reward processing (Novick et al., 2018). Given evidence that anhedonia is associated with poor social functioning and increased generation of stress (Hammen, 1991; Hankin et al., 2010), longitudinal examinations would be necessary to establish a causal association from stress (e.g. peer victimization) to anhedonia (e.g. blunted neural response to rewards). Indeed, an alternative explanation for the observed association is that anhedonic individuals, characterized by a blunted RewP, generate more interpersonal stress in their lives (Hammen, 1991). Nevertheless, this study advances our understanding of associations between interpersonal stress and neural response to rewards.
In sum, this study represents an initial step toward identifying how real-world interpersonal stress might influence neural reward systems to lead to the development of anhedonia. This may be helpful in future studies seeking to understand how life stress gets `under the skin’ of individuals, by demonstrating that real-world life stressors are associated with impaired reward processing; future longitudinal research might examine whether this mediates the association between stress and mental illness. This work, therefore, supports continued efforts to understand pathways to psychopathologies such as depression, which may, in turn, help us to more effectively prevent and intervene in these debilitating disorders.
References


