-
PDF
- Split View
-
Views
-
Cite
Cite
Steven J Russell, Robert Sala, Philip G Conaghan, George Habib, Quang Vo, Rickey Manning, Alan Kivitz, Yvonne Davis, Joelle Lufkin, James R Johnson, Scott Kelley, Neil Bodick, Triamcinolone acetonide extended-release in patients with osteoarthritis and type 2 diabetes: a randomized, phase 2 study, Rheumatology, Volume 57, Issue 12, December 2018, Pages 2235–2241, https://doi.org/10.1093/rheumatology/key265
Close - Share Icon Share
Abstract
Approximately 30% of patients with type 2 diabetes mellitus have knee osteoarthritis. IA corticosteroids used to manage osteoarthritis pain can elevate blood glucose in these patients. We compared blood glucose levels following intra-articular injection of triamcinolone acetonide extended-release (TA-ER), an extended-release, microsphere-based triamcinolone acetonide formulation, vs standard triamcinolone acetonide crystalline suspension (TAcs) in patients with knee osteoarthritis and comorbid type 2 diabetes.
In this double-blind, randomized, parallel-group, phase 2 study (NCT02762370), 33 patients with knee osteoarthritis (American College of Rheumatology criteria) and type 2 diabetes mellitus (HbA1c 6.5–9.0% [48–75 mmol/mol]; 1–2 oral hypoglycaemic agents) were treated with intra-articular TA-ER (32 mg n = 18) or TAcs 40 mg (n = 15). Continuous glucose monitoring-measured glucose (CGMG) was assessed from 1 week pre-injection through 2 weeks postinjection. Endpoints included change in average daily CGMG from baseline (days −3 to −1) to days 1–3 postinjection (CGMGdays1–3) (primary) and percent time average hourly CGMG levels remained in prespecified glycaemic ranges.
The change CGMGdays1–3 was significantly lower following TA-ER vs TAcs (14.7 vs 33.9 mg/dl, least-squares-mean-difference [95% CI]: −19.2 [−38.0, −0.4]; P = 0.0452). The percentage of time over days 1–3 that CGMG was in the target glycaemic range (70–180 mg/dl) was numerically greater for TA-ER (63.3%) vs TAcs (49.7%), and that CGMG was >180 mg/dl was lower for TA-ER (34.5%) vs TAcs (49.9%). Non-glycaemic adverse events were mild and comparable between groups.
TA-ER may enable intra-articular corticosteroid treatment with minimal blood glucose disruption in patients with knee osteoarthritis and type 2 diabetes mellitus.
ClinicalTrials.gov, https://clinicaltrials.gov, NCT02762370.
Intra-articular CS injection can elevate blood glucose in patients with OA and type 2 diabetes.
Glucose levels were more stable in patients with OA and type 2 diabetes treated with extended-release versus standard triamcinolone acetonide.
Patients with OA and type 2 diabetes had minimal glycaemic control disruption following triamcinolone acetonide extended-release injection.
Introduction
Approximately 30% of patients with type 2 diabetes mellitus have osteoarthritis of the knee, a painful condition and a leading cause of disability in the United States [1–3]. Limitation of mobility in diabetic patients with osteoarthritis is a risk factor for diabetes-related complications including hyperglycaemia [4].
IA corticosteroids are commonly used to treat osteoarthritic pain [5]. However, rapid and substantial efflux of corticosteroids from the joint begins within hours of IA injection of standard crystalline formulations [6], limiting the magnitude and persistence of pain reduction and potentially causing systemic effects [7]. Blood glucose (BG) elevation has been observed in diabetic patients following IA delivery of traditional corticosteroids [8–11]. In one study, ∼23% of patients receiving IA triamcinolone acetonide crystalline suspension (TAcs) to treat knee osteoarthritis had significant increases in BG levels, peaking at 24–32 h postinjection, that did not return to normal until 2.5–4 days postinjection [10]. As such, potential loss of BG control poses an important clinical challenge for patients with type 2 diabetes mellitus also receiving IA corticosteroids to treat knee osteoarthritis.
Triamcinolone acetonide extended-release (TA-ER, formerly FX006) is a novel, microsphere-based formulation of triamcinolone acetonide. In phase 2b and 3 clinical trials, IA administration of TA-ER 32 mg produced clinically meaningful pain relief and functional improvement relative to saline-placebo and standard TAcs 40 mg in patients with knee osteoarthritis [12, 13]. Pharmacokinetic evaluations demonstrated that a single IA injection of TA-ER 32 mg was associated with prolonged joint residency, diminished peak plasma levels and reduced systemic exposure of triamcinolone acetonide compared with TAcs 40 mg [14]. We hypothesized that BG levels in diabetic patients would be less impacted by TA-ER than TAcs. The current study was conducted to compare BG levels over time in patients with knee osteoarthritis and type 2 diabetes mellitus following a single IA injection of TA-ER 32 mg vs TAcs 40 mg.
Methods
Study design and participants
This phase 2, double-blind, randomized, parallel-group, single-dose, multicentre study (ClinicalTrials.govidentifier: NCT02762370) was conducted per Declaration of Helsinki and International Conference on Harmonisation Good Clinical Practice guidelines. A central institutional review board (Schulman Central IRB, Cincinnati, OH, USA) approved the protocol (IRB No. 201601317). All patients provided written informed consent.
Eligible participants were aged ⩾40 years, with symptomatic osteoarthritis of the knee ⩾6 months meeting American College of Rheumatology clinical and radiographic criteria [15] at screening and with type 2 diabetes mellitus ⩾1 year prior to screening not managed by injectable agents. Participants were receiving treatment with 1–2 oral hypoglycaemic agents (stable doses ⩾2 months) and had haemoglobin-A1c (HbA1c) levels between 6.5–9.0% (48–75 mmol/mol). Only patients with adequate screening continuous glucose monitoring-measured glucose (CGMG) data, i.e. ≥ 1 full day of data with no gaps >3 h and with confirmation of two calibrations performed within that 24-h period, were eligible for study participation. Additional eligibility criteria and CGMG collection methods are provided in the supplementary data, section Methods, available at Rheumatology online.
Study medication
Patients were randomized to receive IA administration of TA-ER 32 mg (5 ml) or TAcs 40 mg (Kenalog-40; Bristol-Myers Squibb Company, Princeton, NJ, USA; 1 ml). TA-ER and TAcs were not identical in appearance at the time of administration. For that reason, treatments were prepared and administered by designated unblinded site personnel who had no other contact with participants or study personnel, and the injection syringe was concealed from study participants. The individual responsible for clinical assessments and safety monitoring was blind to treatment. For additional details see supplementary data, section Study Medication, available at Rheumatology online.
Blood glucose monitoring
In this study, BG was assessed with the Dexcom G4 Platinum Professional CGM, which employs the same technology as the Dexcom G4 Platinum and has been shown to be highly accurate with an average error of ∼11% [16]. The CGM device, which has the advantage of capturing a comprehensive representation of glucose variability over the entire day and night, was calibrated with the Bayer Contour NEXT glucose-measuring metre, one of the most accurate glucose metres available, with an average error of ∼6% [17].
Procedures
Following screening (days −21 to −7), CGMG data were collected, as described above, from pre-treatment (days −7 to −1) through 2 weeks postinjection (days 1–15). Follow-up visits were scheduled for day 8, day 15 and week 6 post-IA injection. Safety evaluations, including a final safety visit, were scheduled for 6 weeks postinjection. For additional details see supplementary data, section Additional procedures, available at Rheumatology online.
Outcomes
The primary outcome was the change in average daily CGMG from baseline (days −3 to −1) to days 1–3 following IA injection of TA-ER vs TAcs. Secondary outcomes included percentage of time average hourly CGMG levels were in or above the target glycaemic range (70–180 mg/dl) [18] during days 1–3 and during days 1–15; area under the effect (AUE) curves for average CGMG and glycaemic variability (coefficient of variation over hours 1–24, days 1–2, days 1–3 and days 1–7 following administration of TA-ER vs TAcs.) For additional details see supplementary data, section Exploratory outcomes, available at Rheumatology online.
Safety outcomes were summarized for the Safety Population (i.e. participants who provided informed consent and received study medication). Analyses of AEs were performed for events considered treatment-emergent (i.e. with onset after the administration of study treatment) or which were present at baseline but worsened in intensity during the study.
Statistical analyses
Approximately 36 participants were to be randomized/treated to ensure ⩾30 evaluable people (15/treatment group). This sample size provided 72.8% power to reject the null hypothesis of equal means with a population mean difference of 28.0 mg/dl in CGMG change from baseline (days −3 to −1) to days 1–3, with a common SD of 30, at an alpha level of 0.05 for a two-sample t test. For additional details see supplementary data, sections Prespecified interim analysis and Statistical analyses, available at Rheumatology online.
Results
Patient disposition and baseline characteristics
Between 8 April and 30 September 2016, 98 people were screened and 33 were enroled/randomized (TA-ER, n = 17; TAcs, n = 16; Fig. 1). Three participants were randomized correctly but received the incorrect treatment, resulting in 18 TA-ER-treated and 15 TAcs-treated participants. Thirty-two (97.0%) of 33 patients completed the study through week 6 (final safety follow-up visit completed on 11 November 2016). One person in the TA-ER group did not return at week 6 but completed the study through day 15. All participants were analysed as treated for planned and post hoc analyses (Fig. 1).

Trial profile
a3 patients were randomized correctly, but received the incorrect treatment. b1 patient was not willing to return for the final week 6 visit, but did complete the study through day 15. TAcs: triamcinolone acetonide crystalline suspension; TA-ER: triamcinolone acetonide extended-release injectable suspension.
Participants had a mean (range) age of 61 (46–83) years; the mean times since osteoarthritis and type 2 diabetes mellitus diagnoses were 9.2 and 8.6 years, respectively; and 72.7% of participants were obese (body mass index 30.0–39.9 kg/m2). Baseline average BG categories were not balanced across treatment groups due to incorrect treatment assignment for three patients. More patients treated with TA-ER (n = 5) than TAcs (n = 1) had baseline BG in the 157–177 mg/dl category. Many participants had received prior osteoarthritis treatment; 30.3% had received IA corticosteroids in the index knee at any time prior to screening (Supplementary Table S1, available at Rheumatology online).
CGMG levels
Change in average daily CGMG from baseline to days 1–3 was significantly lower postinjection of TA-ER than TAcs (14.7 vs 33.9; LSM [95% CI] difference of −19.2 mg/dl [−38.0, −0.4]; P = 0.0452). Mean average daily CGMG increased from baseline (days −3 to −1) to days 1−3 by 8.2 mg/dl in TA-ER (155.2–163.4 mg/dl) and by 37.1 mg/dl in TAcs (161.7–198.8 mg/dl) patients (Figs 2 and 3A). For additional details see supplementary data, section Completeness of CGMG data collection, and Supplementary Tables S2 and S3, available at Rheumatology online.
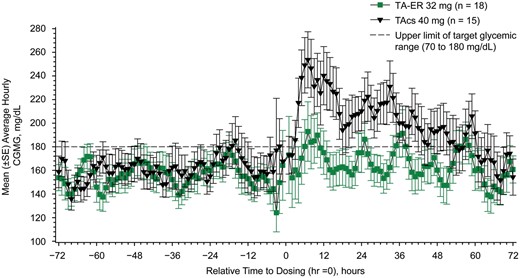
Mean average hourly CGMG levels (FAS; n = 33)
CGMG: continuous glucose monitoring-measured glucose; FAS: full analysis set; LSM: least squares mean; SE: standard error; TAcs: triamcinolone acetonide crystalline suspension; TA-ER: triamcinolone acetonide extended-release.
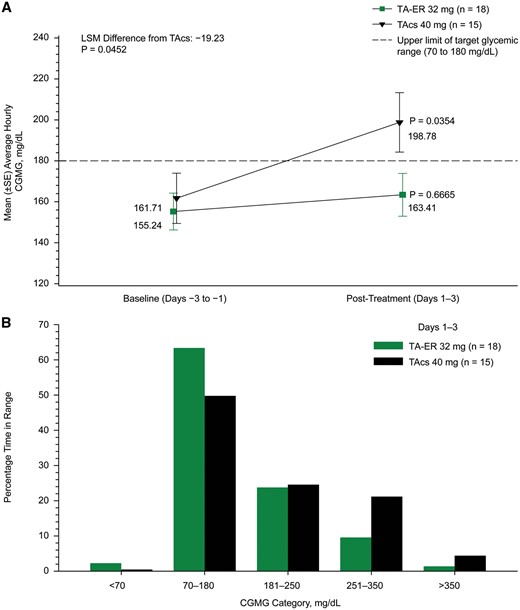
Changes in blood glucose profile for days 1–3 with TA-ER vs TAcs
(A) Mean change from baseline to days 1–3 for the average CGMG. (B) Percentage of time in target glycaemic range for hourly the average CGMG during days 1–3 (FAS; N = 33). CGMG: continuous glucose monitoring-measured glucose; FAS: full analysis set; LSM: least squares mean; SE: standard error; TAcs: triamcinolone acetonide crystalline suspension; TA-ER: triamcinolone acetonide extended-release.
The percentage of time that average hourly CGMG levels were in the target glycaemic range (70–180 mg/dl) during days 1–3 was numerically greater after TA-ER than TAcs (63.3 vs 49.7%), and the percentage of time that hourly CGMG levels were above the target range (>180 mg/dl) was numerically less following TA-ER than TAcs (34.5 vs 49.9%; Fig. 3B). Post hoc analyses indicated that the within-group CGMG increase was statistically significant in TAcs-treated, but not in TA-ER-treated, participants (P = 0.0354 and P = 0.6665, respectively). For additional details see supplementary data, section CGMG levels and Supplementary Figs S1 and S2, available at Rheumatology online.
Safety and tolerability
AEs were reported by 11.1% (2/18) and 13.3% (2/15) of TA-ER- and TAcs-treated participants, respectively (Table 1). All AEs were Grade 1 or 2 (Table 1). No TA-ER-treated person experienced an index-knee-related or injection-related AE, whereas one TAcs-treated person had Grade 1 ecchymosis at the index-knee injection site. One TA-ER-treated patient had an exacerbation of pre-existing hyperglycaemia reported as an AE; no other clinical laboratory AEs were reported.
| . | Treatment group . | |
|---|---|---|
| TA-ER 32 mg n = 18 . | TAcs 40 mg n = 15 . | |
| Patients with ≥1 AE | 2 (11.1) | 2 (13.3) |
| Grade 1 | 0 | 2 (13.3) |
| Grade 2 | 2 (11.1) | 0 |
| Not related | 1 (5.6) | 1 (6.7) |
| Probably related | 0 | 1 (6.7) |
| Definitely related | 1 (5.6)a | 0 |
| Patients with ≥1 SAE | 0 | 0 |
| Patients discontinuing from study due to an AE | 0 | 0 |
| Patients with ≥1 index-knee AE | 0 | 1 (6.7)b |
| Patients with ≥1 AE related to injection procedure | 0 | 1 (6.7)b |
| . | Treatment group . | |
|---|---|---|
| TA-ER 32 mg n = 18 . | TAcs 40 mg n = 15 . | |
| Patients with ≥1 AE | 2 (11.1) | 2 (13.3) |
| Grade 1 | 0 | 2 (13.3) |
| Grade 2 | 2 (11.1) | 0 |
| Not related | 1 (5.6) | 1 (6.7) |
| Probably related | 0 | 1 (6.7) |
| Definitely related | 1 (5.6)a | 0 |
| Patients with ≥1 SAE | 0 | 0 |
| Patients discontinuing from study due to an AE | 0 | 0 |
| Patients with ≥1 index-knee AE | 0 | 1 (6.7)b |
| Patients with ≥1 AE related to injection procedure | 0 | 1 (6.7)b |
Data presented are n (%).
Grade 2 hyperglycaemia assessed by the investigator as related to study drug.
Grade 1 ecchymosis at the injection site assessed by the investigator to be probably related to study agent injection.
AE: adverse event; SAE: serious adverse event; TAcs: triamcinolone acetonide crystalline suspension; TA-ER: triamcinolone acetonide extended-release.
| . | Treatment group . | |
|---|---|---|
| TA-ER 32 mg n = 18 . | TAcs 40 mg n = 15 . | |
| Patients with ≥1 AE | 2 (11.1) | 2 (13.3) |
| Grade 1 | 0 | 2 (13.3) |
| Grade 2 | 2 (11.1) | 0 |
| Not related | 1 (5.6) | 1 (6.7) |
| Probably related | 0 | 1 (6.7) |
| Definitely related | 1 (5.6)a | 0 |
| Patients with ≥1 SAE | 0 | 0 |
| Patients discontinuing from study due to an AE | 0 | 0 |
| Patients with ≥1 index-knee AE | 0 | 1 (6.7)b |
| Patients with ≥1 AE related to injection procedure | 0 | 1 (6.7)b |
| . | Treatment group . | |
|---|---|---|
| TA-ER 32 mg n = 18 . | TAcs 40 mg n = 15 . | |
| Patients with ≥1 AE | 2 (11.1) | 2 (13.3) |
| Grade 1 | 0 | 2 (13.3) |
| Grade 2 | 2 (11.1) | 0 |
| Not related | 1 (5.6) | 1 (6.7) |
| Probably related | 0 | 1 (6.7) |
| Definitely related | 1 (5.6)a | 0 |
| Patients with ≥1 SAE | 0 | 0 |
| Patients discontinuing from study due to an AE | 0 | 0 |
| Patients with ≥1 index-knee AE | 0 | 1 (6.7)b |
| Patients with ≥1 AE related to injection procedure | 0 | 1 (6.7)b |
Data presented are n (%).
Grade 2 hyperglycaemia assessed by the investigator as related to study drug.
Grade 1 ecchymosis at the injection site assessed by the investigator to be probably related to study agent injection.
AE: adverse event; SAE: serious adverse event; TAcs: triamcinolone acetonide crystalline suspension; TA-ER: triamcinolone acetonide extended-release.
Discussion
In this randomized, double-blind, parallel group study in people with osteoarthritis of the knee and type 2 diabetes mellitus, treatment with TA-ER 32 mg (a dose that demonstrated improved pain relief and improved function relative to TAcs 40 mg in previous clinical trials) [12, 13] resulted in a statistically significant reduction in CGMG relative to TAcs 40 mg over days 1–3 following IA injection. In addition, the within-group CGMG increase after IA injection was statistically significant in TAcs-treated, but not in TA-ER-treated, participants, suggesting that TA-ER did not significantly disrupt the glycaemic control of participants with type 2 diabetes mellitus. The perturbation of glucose control produced by TAcs appeared to be maximal during the first 2 days postinjection, an effect consistent with prior literature [9].
The average daily and average hourly CGMG levels during days 1–3 following TA-ER injection were well within the target glycaemic range of 70–180 mg/dl recommended by the American Diabetes Association [18]. Conversely, participants who received TAcs had average daily and average hourly CGMG levels above that range, often exceeding 200 mg/dl (Figs 2 and 3).
These observations are consistent with the pharmacokinetic profiles for TAcs and TA-ER generated in a recent phase 2 open-label study in 63 patients with knee osteoarthritis. In this study, peak plasma exposure in the days following a single IA injection was 966.7 pg/ml for TA-ER (32 mg) and 11, 064.7 pg/ml for TAcs (40 mg) [14]. In the earlier study, peak plasma TA concentrations were observed by 4 h postinjection among the 18 people who received IA TAcs. Conversely, IA TA-ER gradually increased plasma triamcinolone acetonide concentrations, which did not peak until 24 h postinjection; at this time point, plasma triamcinolone acetonide levels associated with TA-ER were ∼80% lower than those associated with TAcs [14]. These pharmacokinetic data are consistent with a slow release of triamcinolone acetonide from TA-ER, whereby only triamcinolone acetonide crystals near the surface of the poly (lactic-co-glycolic acid) (PLGA) microsphere dissolve initially, resulting in a low absorption into the systemic circulation following IA injection (Data on File, Flexion Therapeutics). Furthermore, the same study showed that IA triamcinolone acetonide concentrations at 6 weeks postinjection were notably higher following TA-ER (3, 590.0 pg/ml) than TAcs (7.7 pg/ml) [14]. The systemic and IA pharmacokinetic profiles of TA-ER are consistent with slow release of triamcinolone acetonide from the PLGA matrix in the joint, and the resulting slow efflux of triamcinolone acetonide from the joint, an effect that limits peak systemic levels.
In the current study, both TA-ER and TAcs were similarly well tolerated, with no SAEs reported. These findings are consistent with previous reports from phase 2 and 3 studies in people with knee osteoarthritis, which showed similar AE profiles for TA-ER 32 mg, TAcs 40 mg and saline-placebo [12, 13, 19].
This study has limitations in sample size, the diabetic population studied and the treatment comparisons. The sample size of 33 patients for this phase 2 pharmacodynamic study provided sufficient power to demonstrate statistically significant differences for the primary and some secondary outcomes. The study also assessed the responses to IA injection only in people with type 2 diabetes mellitus not managed by injectables, receiving 1–2 oral agents and with HbA1c levels between 6.5–9.0% (48–75 mmol/mol). The applicability of these findings to diabetic patients not meeting these criteria is unknown. An imbalance in treatment assignment is noted in patients with BG of >157.33 to <177.43, which is attributable to the incorrect treatment assignment at the time of randomization in three patients. All patients were analysed as treated. All other baseline characteristics were well-balanced. The two IA treatments used different injection volumes based upon product preparation: TA-ER is reconstituted and injected as 5 ml, while TAcs is injected as 1 ml. However, the syringe was concealed from the patient during the injection and a blinded-assessor technique was employed to minimize the potential for bias while allowing comparison of TA-ER and TAcs delivered in their respective recommended volumes.
In conclusion, TA-ER resulted in statistically significant and clinically relevant mitigation of CGMG elevation, with more time in target range, compared with TAcs over the first several days postinjection. These findings support previously identified disruption of glycaemic control with TAcs in diabetic patients; this is the first study to show negligible effects on CGMG after an IA corticosteroid (TA-ER) injection. The results are consistent with the relatively low systemic exposure to TA afforded by the extended-release formulation TA-ER, and suggest that TA-ER may be administered with minimal disruption of glycaemic control in people with knee osteoarthritis and type 2 diabetes mellitus.
Acknowledgements
The authors thank the patients who participated in this trial and their enroling investigators. Some of the information in this paper was presented in abstract form at the 77th Scientific Sessions of the American Diabetes Association, and this abstract was published in the journal Diabetes [20]. The authors also thank Karen Ozer, Teresa Curto, and Ashish Aggarwal of Cytel, Inc., Waltham, MA, USA for statistical analyses. Professional medical writing and editing assistance was provided by Michelle Perate, MS, and assistance with graphics was provided by ApotheCom (Yardley, PA, USA); this support was funded by Flexion Therapeutics, Inc. (Burlington, MA, USA). P.G.C. is supported in part by the National Institute for Health Research (NIHR) Leeds Biomedical Research Centre, Leeds, UK. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health. S.J.R., R.S., J.L., J.R.J. and N.B. contributed to trial conception and design; R.S., Q.V., R.M., A.K., Y.D., J.L. and N.B. contributed to data acquisition; and S.J.R., R.S., P.G.C., G.H., Q.V., R.M., A.K., Y.D., J.R.J., S.K. and N.B. interpreted the data. All authors were involved in drafting the article or revising it critically for important intellectual content; provided final approval of the version to be submitted/published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: This work was supported by Flexion Therapeutics, Inc.
Disclosure statement: S.J.R. has served on advisory panels for Companion Medical, Tandem Diabetes Care, Inc., and Unomedical A/S, as a consultant for Beta Bionics and Flexion Therapeutics, Inc., has received either financial or in-kind research support from Abbott Diabetes Care Inc., Dexcom, Inc., Eli Lilly and Company, Senseonics, SweetSpot Diabetes, Tandem Diabetes Care, Inc. and Zealand Pharma A/S, owns stock/shares in Companion Medical and has received honoraria for lectures from Ascensia Diabetes Care, Dexcom, Inc., Eli Lilly and Company, Novo Nordisk, Roche Diabetes Care, Sanofi-Aventis, and Tandem Diabetes Care, Inc. R.S. is employed by Dexcom, Inc. P.G.C. has received compensation for consultancies from AbbVie, Flexion Therapeutics, Inc., Infirst, Medivir, Merck Serono and ONO Pharmaceutical Co. Q.V. has received research support from BioDelivery Science International, GlaxoSmithKline, Oramed Pharmaceuticals, Inc. and Sanofi. R.M. has received research support from Novo Nordisk, Inc. and Sanofi. J.L., S.K. and N.B. are employed by, and/or own stock in, Flexion Therapeutics Inc., Burlington, MA, USA. J.R.J. was employed by Flexion Therapeutics, Inc. at the time of data analysis, is currently employed by Summit Analytical, LLC and has received personal fees for statistical and pharmacokinetic support from Acura Pharmaceuticals, Flexion Therapeutics, Inc., Iroko Pharmaceuticals, IX Biopharma, GNC and Tolmar, Inc. All other authors have declared no conflicts of interest.
References
- adrenal corticosteroids
- diabetes mellitus, type 2
- glucose
- glucocorticoids
- comorbidity
- hemoglobin a, glycosylated
- intra-articular injections
- microspheres
- osteoarthritis
- knee osteoarthritis
- pain
- suspensions
- triamcinolone acetonide
- blood glucose
- mineralocorticoids
- oral hypoglycemic agents
- trigeminal autonomic cephalalgias
- focal electrical alternating current therapy
- surrogate endpoints
- adverse event
- crystal structure
- american college of rheumatology
- teller acuity cards




Comments