-
PDF
- Split View
-
Views
-
Cite
Cite
A L Peralta-Amaro, S Triana-González, M F Manzo-Carballo, O L Vera Lastra, J García-Chávez, A Lucas-Hernández, Antiphospholipid syndrome and lupus anticoagulant-hypoprothrombinemia, QJM: An International Journal of Medicine, Volume 116, Issue 4, April 2023, Pages 308–309, https://doi.org/10.1093/qjmed/hcac243
Close - Share Icon Share
Learning points for clinicians
Although lupus anticoagulant-hypoprothrombinemia syndrome is a rare cause of bleeding in patients with antiphospholipid syndrome, clinicians should always have it in mind to initiate treatment as soon as possible. Treatment should be individualized, and Factor Eight Inhibitor Bypassing Activity could be an option if surgery is required to save the patient’s life.
Introduction
Antiphospholipid syndrome (APS) is an autoimmune disease characterized by thrombosis; hemorrhagic events can occur in about 10%.1,2 One of the causes of bleeding in APS, is the lupus anticoagulant-hypoprothrombinemia syndrome (LAHPS), an extremely rare entity that depends on the presence of non-neutralizing antibodies against coagulation factor II (FII).3 We present the case of a patient with APS and LAHPS that presented intracranial hemorrhage, treated with Factor Eight Inhibitor Bypassing Activity (FEIBA).
Case presentation
A 48-year-old man with primary APS began with a throbbing headache that progressed to right hemiparesis in 24 h. Laboratory tests showed prolongation of prothrombin time (PT) and activated partial thromboplastin time (aPTT). Brain computed tomography showed a hemorrhagic lesion in the left cerebellar hemisphere with left occipitotemporal parenchymal hematoma and subarachnoid extension. A mixing plasma study was performed and revealed PT correction (dilution 1:4, PT 16.5 s) and no correction of aPTT. The activity of the coagulation factors was measured; finding a decline in the FII, thus the diagnosis of LAHPS was concluded.
Treatment was initiated with methylprednisolone and immunoglobulin due to the necessity for neurological improvement. On the sixth day of hospitalization, he began with motor aphasia, decreased strength in the left leg and extension of the intraparenchymal hemorrhage (Figure 1) with the indication of urgent neurosurgery. Due to the high risk for intraoperative bleeding, preoperative management with FEIBA, an activated prothrombin complex concentrate was initiated. He presented a favorable evolution in the following 48 h and continued immunosuppressive treatment with prednisone and azathioprine. On the 13th day of hospitalization, the patient died from sudden massive gastrointestinal bleeding.
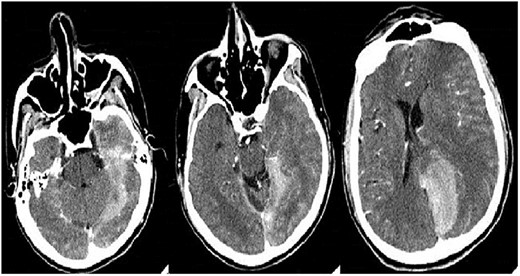
Control brain computed tomography shows the extension of the hyperdense lesion with left occipital-temporal parenchymal hemorrhage, with ventricular breakthrough and midline deviation.
Discussion
LAHPS is an extremely rare entity first described in 1960.4 In adults, LAHPS is associated with autoimmune diseases, mainly Lupus, and less frequently with primary APS.5 FII is synthesized in the liver, it is cleaved by factor Xa to form thrombin, thus polymerization of fibrinogen into fibrin, enabling platelet aggregation. In LAHPS, antiphospholipid antibodies (APAs) bind to the carboxy-terminal portion of FII forming antigen–antibody complexes that are cleared by the reticuloendothelial system, conditioning hypoprothrombinemia and thus a bleeding tendency. In a scenario of APAs and PT prolongation, LAHPS should be suspected. The diagnostic consists of performing dilution tests with plasma, then measuring coagulation factors activity, when FII is decreased, LAHPS is confirmed. The clinical spectrum of LAHPS is broad, from non-bleeding to life-threatening hemorrhages.2,5,6
Cases associated with viral infections are usually benign; however, in patients with moderate to severe bleeding, and preoperative prophylaxis, immunosuppressive treatment is indicated.1–5 Steroids represent the first line to reach FII level activity of 20–40% and reduce bleeding risk. In case of biochemical or clinical relapse (hemorrhage) associated with steroid reduction, adjuvant immunosuppressive therapy is recommended. Intravenous immunoglobulin has been used as first-line therapy before elective surgeries or after the failure of the initial therapeutic scheme.5
The prognosis of LAHPS is generally favorable, perhaps when associated with autoimmune or haemato-oncological causes, relapses and complications associated with the treatment have been identified. Although our patient died due to a sudden and fatal relapse of LAHPS, FEIBA resulted in a treatment that diminished the risk of severe bleeding during surgery.
Conclusions
We describe the case of a patient with life-threatening bleeding that underwent urgent surgery, and it was decided to use FEIBA with a favorable transurgical outcome. According to the review we made, FEIBA has not been used previously. It is of great importance to let know the therapeutic complexity, including neurosurgical, due to the fragile balance between thrombotic and hemorrhagic factors.
Conflict of interest: None declared.



