-
PDF
- Split View
-
Views
-
Cite
Cite
S Ito, K Ichimura, Y Takaku, K Abe, M Harada, M Ikeda, H Ito, Y Kishimoto, Y Nakajima, T Okada, H Sekiya, Improved method for measuring low-concentration radium and its application to the Super-Kamiokande Gadolinium project, Progress of Theoretical and Experimental Physics, Volume 2020, Issue 9, September 2020, 093H02, https://doi.org/10.1093/ptep/ptaa105
Close - Share Icon Share
Abstract
Chemical extraction using a molecular recognition resin named “Empore Radium Rad Disk” was developed to improve sensitivity for the low concentration of radium (Ra). Compared with the previous method, the extraction process speed was improved by a factor of three and the recovery rate for |$^{226}$|Ra was also improved from |$81\pm4\%$| to |$>99.9\%$|. The sensitivity on the |$10^{-1}$| mBq level was achieved using a high-purity germanium detector. This improved method was applied to determine |$^{226}$|Ra in Gd|$_2$|(SO|$_4$|)|$_3{\cdot}$|8H|$_2$|O which will be used in the Super-Kamiokande Gadolinium project. The improvement and measurement results are reported in this paper.
1. Introduction
Detection of radium (Ra) is important not only for environmental studies, medical science and biology, but also for non-accelerator particle physics experiments (e.g. neutrino measurements and dark matter searches), which require a low background experimental environment. In general, short-lifetime radioactive materials (e.g. |$^{226}$|Ra) are measured using particle counters such as |$\alpha$|-spectrometers, liquid scintillators, and germanium detectors. However, the sensitivity is generally limited by the detection efficiency related to the sample size, i.e. the self-shielding effect and geometrical acceptance. To minimize these problems and improve sensitivity, chemical extraction is often used. For example, Dulansk|$\acute{\rm a}$| et al. [1] have used a molecular recognition resin named “AnaLig-Sr01”, which is a product of IBC Advanced Technologies [2]. They determined the concentration of |$^{226}$|Ra included in rocks or building materials in the range of |$5.2$|–|$165.0\>$|Bq|$\>$|kg|$^{-1}$|. Other chemical extraction techniques have been developed using the “Empore Radium Rad Disk” produced by 3M Corporation [3] (see the following sections for more details) to determine |$^{226}$|Ra in water at the |$10^{2}-10^{4}\>$|mBq|$\>$|L|$^{-1}$| level [4–6]. However, the chemical extraction of |$^{226}$|Ra from a very high matrix sample with a much lower concentration has not been established.
The Super-Kamiokande Gadolinium (SK-Gd) project is an upgrade of the Super-Kamiokande (SK) detector [7], with the final goal of dissolving gadolinium sulfate octahydrate (Gd|$_2$|(SO|$_4$|)|$_3 \cdot$|8H|$_2$|O) into the SK detector up to the |$0.2\%$| concentration [8,9]. One of the main physics targets of SK-Gd is to discover supernova relic neutrinos and study star formation of the universe [10]. The measurements of solar neutrinos with a low energy threshold of |$\sim3.5\>$|MeV [11] will also be continued in SK-Gd: therefore several radio impurities (e.g. |$^{226}$|Ra, |$^{238}$|U, and |$^{232}$|Th) in Gd|$_2$|(SO|$_4$|)|$_3{\cdot}$|8H|$_2$|O should be minimized before loading into SK. The maximum allowed level of these radio impurities in Gd|$_2$|(SO|$_4$|)|$_3{\cdot}$|8H|$_2$|O [12,13] and the typical example of a commercially available product are summarized in Table 1. For the measurement of |$^{238}$|U and |$^{232}$|Th, the procedure was developed using inductively coupled plasma-mass spectrometry (ICP-MS) with chemical extraction (see Ref. [14] for more details).
| . | |$^{226}$|Ra . | |$^{238}$|U . | |$^{232}$|Th . |
|---|---|---|---|
| Requirement for SK-Gd | 0.5 | 5 | 0.05 |
| The typical concentration of | 5 | 50 | 100 |
| commercially available products |
| . | |$^{226}$|Ra . | |$^{238}$|U . | |$^{232}$|Th . |
|---|---|---|---|
| Requirement for SK-Gd | 0.5 | 5 | 0.05 |
| The typical concentration of | 5 | 50 | 100 |
| commercially available products |
| . | |$^{226}$|Ra . | |$^{238}$|U . | |$^{232}$|Th . |
|---|---|---|---|
| Requirement for SK-Gd | 0.5 | 5 | 0.05 |
| The typical concentration of | 5 | 50 | 100 |
| commercially available products |
| . | |$^{226}$|Ra . | |$^{238}$|U . | |$^{232}$|Th . |
|---|---|---|---|
| Requirement for SK-Gd | 0.5 | 5 | 0.05 |
| The typical concentration of | 5 | 50 | 100 |
| commercially available products |
As shown in Table 1, SK-Gd requires a method to determine low-concentration |$^{226}$|Ra. However, the sensitivity for |$^{226}$|Ra of the previous method with the molecular recognition resin “AnaLig-Ra01” was on the |$1$|-mBq level [15]. In addition, the recovery rate of |$^{226}$|Ra with the previous method was |$81\,\pm\,4\%$| and the process time of the sample was |$1\>$|L per hour. To achieve the required sensitivity of SK-Gd, it was necessary to increase the concentration rate with a higher recovery rate and a shorter processing time. In this study, the procedure of chemical extraction was improved to achieve the sensitivity on the |$10^{-1}\>$|mBq|$\>$|kg|$^{-1}$| level by solving these problems: the improved method was applied to SK-Gd to determine the concentration of |$^{226}$|Ra in Gd|$_2$|(SO|$_4$|)|$_3{\cdot}$|8H|$_2$|O.
2. Experimental equipment
2.1. Chemical equipment
An “Empore Radium Rad Disk” [3] was used for chemical extraction. Resin with the same chemical features as AnaLig-Ra01 was positioned on a filter (|$47\>$|mm diameter and |$0.5\,\mu$|m thickness) made of polytetrafluoroethylene fibrils.1Figure 1 shows the experimental setup of the chemical extraction using a vacuum filtration system with the disk. The disk was placed on a holder with volume of |$800\>$|mL (Advantech Toyo Ltd. [16], KP-47), and the holder was connected to a vacuum container (Advantech Toyo Ltd. [16], VT-500). The solution passes through the disk, and |$^{226}$|Ra in the solution is adsorbed by the resin bedded to the disk. The concentration of |$^{226}$|Ra can be determined by measuring the disk directly using an HPGe detector.
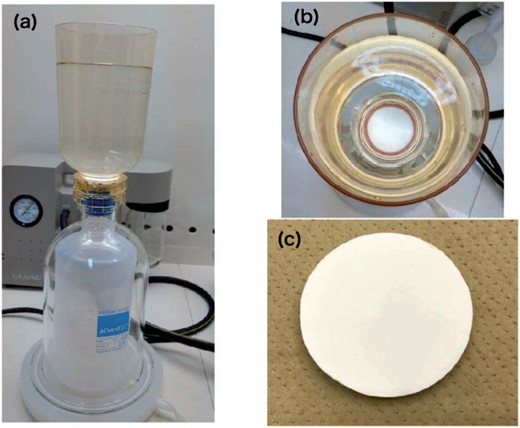
(a) Photograph of the entire experimental setup. (b) Top view of the setup. (c) Top view of the disk (|$47\>$|mm diameter).
To produce solutions with low contamination, ultra-pure SK water [7] was used. Electronic grade |$70\%$| nitric acid (HNO|$_3$|) (Wako Pure Chemical Industries Ltd. [17]) was used to wash the disk and efficiently dissolve Gd|$_2$|(SO|$_4$|)|$_3{\cdot}$|8H|$_2$|O in the SK water.
To check the concentration of |$^{226}$|Ra in Gd|$_2$|(SO|$_4$|)|$_3{\cdot}$|8H|$_2$|O easily, |$^{226}$|Ra-rich hot spring water from the Kawakita hot spring in Ishikawa, Japan [18] was used as the calibration solution. The concentration of |$^{226}$|Ra in the hot spring water was 112|$^{+34}_{-12}\>$|mBq|$\>$|L|$^{-1}$|, which was determined by the HPGe detector measurement. The uncertainty was mainly due to the systematic uncertainty of the HPGe detector (|$+30\%$| or |$-10\%$|) and statistics [15]. The sampled hot spring water was filtrated by membrane filters with pore size of |$0.45\,\mu$|m and acidified to pH |$\simeq1$| by HNO|$_3$| for preservation.
Because barium (Ba) and Ra have similar chemical features and ionic radii, Ba is frequently used as a tracer for Ra analysis to estimate the recovery rate [19]. Thus, |$1000\>$|mg|$\>$|L|$^{-1}$| Ba of standard solution (Merck Ltd. [20]) was used to estimate the recovery rate. The details of the recovery rate studies are described in Sect. 3. An ICP-MS “Agilent 7900” [21] was used to measure the concentration of Ba to estimate the recovery rate of |$^{226}$|Ra. The performance of this ICP-MS is described in Ref. [14].
2.2. High-purity germanium detector and its detection efficiency
The HPGe detector used for this measurement was a coaxial p-type HPGe crystal manufactured by CANBERRA France [22]. The dimension of sample chamber was |$23\times 23 \times 48\>$|cm|$^3$|. The details of the performance of the HPGe detector are described in Ref. [15]. The samples measured by the HPGe detector were covered by an ethylene vinyl alcohol bag to keep radon from samples inside the bag (Fig. 2).
The concentration of |$^{226}$|Ra was evaluated using the characteristic |$\gamma$|-lines of |$^{214}$|Pb (|$609\>$|keV) and |$^{214}$|Bi (|$352\>$|keV and |$1764\>$|keV), which are daughter nuclei of |$^{226}$|Ra, by considering of their branching ratios and detection efficiencies. Figure 3 shows the typical observed energy spectra for |$^{214}$|Bi |$352\>$|keV measurements. The detection efficiency was evaluated by a Monte Carlo simulation [23]. For example, the detection efficiency of |$352$|-keV gamma-rays originating from |$^{214}$|Bi with the chemical extraction procedure using the disk was found to be |$15.9\%$|. On the other hand, the detection efficiency of |$352$|-keV gamma-rays for |$^{226}$|Ra was determined to be |$0.8\%$| for the direct measurement of |$5\>$|kg of Gd|$_2$|(SO|$_4$|)|$_3{\cdot}$|8H|$_2$|O without a chemical extraction procedure. The detection efficiency was improved by a factor of 20 owing to the smaller volume of the sample using the disk.
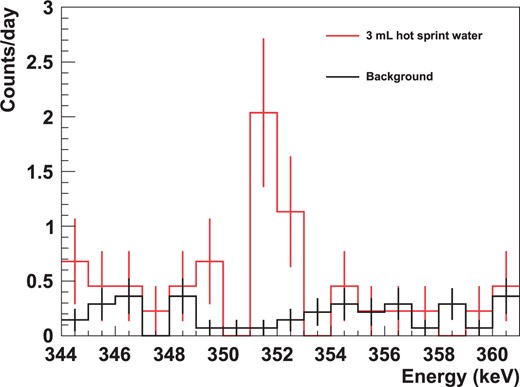
Energy spectra obtained by the HPGe detector around |$^{214}$|Bi |$352\>$|keV. The black line shows the background and the red line indicates the extracted |$^{226}$|Ra from |$3\>$|mL of hot spring water (corresponding to |$0.33\>$|mBq). The error bar at each bin represents only statistical uncertainty.
3. Chemical extraction of |$^{226}$|Ra from Gd|$_2$|(SO|$_4$|)|$_3{\cdot}$|8H|$_2$|O and its performance
The setup shown in Fig. 1 was connected to a vacuum pump: the solution loaded into the holder could pass through the disk. The disk was initially washed by loading |$50\>$|mL of |$3\>$|mol|$\>$|L|$^{-1}$| HNO|$_3$| and |$50\>$|mL of the ultra-pure SK water into the holder. A total of |$500\>$|g Gd|$_2$|(SO|$_4$|)|$_3{\cdot}$|8H|$_2$|O was dissolved in |$10\>$|L of a |$0.2\>$|mol|$\>$|L|$^{-1}$| HNO|$_3$| solution. Then, the sample solution was loaded into the holder, and the vacuum pressure was adjusted to produce a flow rate of |$50\>$|mL|$\>$|min|$^{-1}$| (|$3\>$|L|$\>$|hr|$^-1$|). The processing time for the developed method is three times faster than that used in the previous method [15] because the batch method was used in the previous study and it needed more than one hour to collect |$^{226}$|Ra with recovery rate |$>80\%$|. Compared with the batch method, the vacuum filtration method is more efficient since the sample solution directly passes through the resin bedded to the disk. Then, the disk was directly placed on the HPGe detector and measured.
In the previous method [15], the amount of |$^{226}$|Ra for the procedure blank was estimated to be |$0.3\,\pm\,0.2\>$|mBq by measuring |$2.0\>$|g of the resin “AnaLig-Ra01”. For a more accurate estimate of the blank amount of |$^{226}$|Ra in the disk, a lot of disks (17 disks) were measured using the HPGe detector and the value of |$1.9^{+0.6}_{-0.4}\>$|mBq was obtained for the 17 disks (corresponding to |$0.11^{+0.04}_{-0.02}\>$|mBq for each disk). Therefore, the value of the blank was more precisely estimated and the detection limit was also improved.
The recovery rate of this procedure was evaluated using the |$^{226}$|Ra-rich hot spring water. A volume of |$3\>$|mL and |$100\>$|mL of the hot spring water was added to |$10\>$|L of a Gd|$_2$|(SO|$_4$|)|$_3{\cdot}$|8H|$_2$|O dissolved sample solution. Then, the concentration of |$^{226}$|Ra adsorbed by the disk was measured using the HPGe detector for |$4.5\>$|d. As shown in Table 2, the results of the measurements were consistent with the expected amount of |$^{226}$|Ra, and the achieved sensitivity was on the |$10^{-1}$| mBq level owing to the development of this chemical extraction procedure.
Summary of the study for the recovery rate. The blank of the disk was already subtracted.
| Hot spring water . | Expected amount . | Results . |
|---|---|---|
| (mL) . | of |$^{226}$|Ra (mBq) . | (mBq) . |
| 3 | 0.33|$^{+0.10}_{-0.04}$| | 0.4|${\pm}0.2$| |
| 100 | 11.2|$^{+3.4}_{-1.2}$| | 11.3|$^{+3.4}_{-1.1}$| |
| Hot spring water . | Expected amount . | Results . |
|---|---|---|
| (mL) . | of |$^{226}$|Ra (mBq) . | (mBq) . |
| 3 | 0.33|$^{+0.10}_{-0.04}$| | 0.4|${\pm}0.2$| |
| 100 | 11.2|$^{+3.4}_{-1.2}$| | 11.3|$^{+3.4}_{-1.1}$| |
Summary of the study for the recovery rate. The blank of the disk was already subtracted.
| Hot spring water . | Expected amount . | Results . |
|---|---|---|
| (mL) . | of |$^{226}$|Ra (mBq) . | (mBq) . |
| 3 | 0.33|$^{+0.10}_{-0.04}$| | 0.4|${\pm}0.2$| |
| 100 | 11.2|$^{+3.4}_{-1.2}$| | 11.3|$^{+3.4}_{-1.1}$| |
| Hot spring water . | Expected amount . | Results . |
|---|---|---|
| (mL) . | of |$^{226}$|Ra (mBq) . | (mBq) . |
| 3 | 0.33|$^{+0.10}_{-0.04}$| | 0.4|${\pm}0.2$| |
| 100 | 11.2|$^{+3.4}_{-1.2}$| | 11.3|$^{+3.4}_{-1.1}$| |
To estimate the recovery rate more accurately, a Ba standard solution was added to the sample solution. The sample solution with the concentration of |$4.0{\times}10^{-8}\>$|g (Ba) mL|$^{-1}$| was loaded into the holder, and the solution (which passed through the disk) was collected and measured using the ICP-MS. The concentration of remaining Ba in the solution was |$<0.1\%$|. Those studies indicated that high matrix elements (Gd|$_2$|(SO|$_4$|)|$_3{\cdot}$|8H|$_2$|O) did not interfere with the extraction of |$^{226}$|Ra and the recovery rate obtained using the developed method was estimated to be |$>99.9\%$|. This is a significant improvement from the recovery rate obtained using the previous method (|$81\,\pm\,4\%$|) [15].
Table 3 shows the comparison of performance between the previous method in Ref. [15] and the developed method in this study. The sensitivity of the improved method is on the |$10^{-1}\>$|mBq level owing to the improvements shown in Table 3 and sufficient for measuring the experimentally allowed level of |$^{226}$|Ra for SK-Gd.
| . | Previous method . | Developed method . |
|---|---|---|
| Recovery rate (%) | 81|$\,\pm\,$|4 | |$>$|99.9 |
| Process time for sample (L per hour) | 1 | 3 |
| Procedure blank (mBq) | 0.3|$\,\pm\,$|0.2 | 0.11|$^{+0.04}_{-0.02}$| |
| Sensitivity of the test sample (mBq) | 0.9|$\,\pm\,$|0.5 | 0.4|$\,\pm\,$|0.2 |
| . | Previous method . | Developed method . |
|---|---|---|
| Recovery rate (%) | 81|$\,\pm\,$|4 | |$>$|99.9 |
| Process time for sample (L per hour) | 1 | 3 |
| Procedure blank (mBq) | 0.3|$\,\pm\,$|0.2 | 0.11|$^{+0.04}_{-0.02}$| |
| Sensitivity of the test sample (mBq) | 0.9|$\,\pm\,$|0.5 | 0.4|$\,\pm\,$|0.2 |
| . | Previous method . | Developed method . |
|---|---|---|
| Recovery rate (%) | 81|$\,\pm\,$|4 | |$>$|99.9 |
| Process time for sample (L per hour) | 1 | 3 |
| Procedure blank (mBq) | 0.3|$\,\pm\,$|0.2 | 0.11|$^{+0.04}_{-0.02}$| |
| Sensitivity of the test sample (mBq) | 0.9|$\,\pm\,$|0.5 | 0.4|$\,\pm\,$|0.2 |
| . | Previous method . | Developed method . |
|---|---|---|
| Recovery rate (%) | 81|$\,\pm\,$|4 | |$>$|99.9 |
| Process time for sample (L per hour) | 1 | 3 |
| Procedure blank (mBq) | 0.3|$\,\pm\,$|0.2 | 0.11|$^{+0.04}_{-0.02}$| |
| Sensitivity of the test sample (mBq) | 0.9|$\,\pm\,$|0.5 | 0.4|$\,\pm\,$|0.2 |
4. Application to SK-Gd and results of measuring Gd|$_2$|(SO|$_4$|)|$_3{\cdot}$|8H|$_2$|O
SK-Gd will be conducted in several experimental phases. For the first experimental phase, 13 tons of Gd|$_2$|(SO|$_4$|)|$_3{\cdot}$|8H|$_2$|O will be dissolved in the SK tank corresponding to a |$50\%$| neutron tagging efficiency [9]. Gd|$_2$|(SO|$_4$|)|$_3{\cdot}$|8H|$_2$|O was produced with many lots: thus, all the lots should be measured before loading. Currently, 14 tons of Gd|$_2$|(SO|$_4$|)|$_3{\cdot}$|8H|$_2$|O are being measured using the developed method to confirm that their radio impurities are below the experimentally allowed levels (see Table 1). The concentration of |$^{226}$|Ra in Gd|$_2$|(SO|$_4$|)|$_3{\cdot}$|8H|$_2$|O (unit: mBq|$\>$|kg|$^{-1}$|) can be obtained from the amount of |$^{226}$|Ra measured in the disk divided by the weight of Gd|$_2$|(SO|$_4$|)|$_3{\cdot}$|8H|$_2$|O. Table 4 shows the results of the measurement of |$^{226}$|Ra in Gd|$_2$|(SO|$_4$|)|$_3{\cdot}$|8H|$_2$|O for several production lots determined using the improved chemical extraction method. The signals of |$^{214}$|Pb and |$^{214}$|Bi were not observed above the statistical uncertainty. The concentrations of |$^{226}$|Ra in the measured products were confirmed to be below the experimentally allowed level.
Summary of the measurements of Gd|$_2$|(SO|$_4$|)|$_3{\cdot}$|8H|$_2$|O. The upper limits represent |$90\%$| confidence level.
| Lot No. . | Concentration of |$^{226}$|Ra . | Measurement time . |
|---|---|---|
| . | (mBq kg|$^{-1}$|) . | (d) . |
| 1 | |$<0.4$| | 6.0 |
| 2 | |$<0.3$| | 11.0 |
| 3 | |$<0.3$| | 8.8 |
| 4 | |$<0.2$| | 9.6 |
| 5 | |$<0.5$| | 8.7 |
| 6 | |$<0.2$| | 13.0 |
| Lot No. . | Concentration of |$^{226}$|Ra . | Measurement time . |
|---|---|---|
| . | (mBq kg|$^{-1}$|) . | (d) . |
| 1 | |$<0.4$| | 6.0 |
| 2 | |$<0.3$| | 11.0 |
| 3 | |$<0.3$| | 8.8 |
| 4 | |$<0.2$| | 9.6 |
| 5 | |$<0.5$| | 8.7 |
| 6 | |$<0.2$| | 13.0 |
Summary of the measurements of Gd|$_2$|(SO|$_4$|)|$_3{\cdot}$|8H|$_2$|O. The upper limits represent |$90\%$| confidence level.
| Lot No. . | Concentration of |$^{226}$|Ra . | Measurement time . |
|---|---|---|
| . | (mBq kg|$^{-1}$|) . | (d) . |
| 1 | |$<0.4$| | 6.0 |
| 2 | |$<0.3$| | 11.0 |
| 3 | |$<0.3$| | 8.8 |
| 4 | |$<0.2$| | 9.6 |
| 5 | |$<0.5$| | 8.7 |
| 6 | |$<0.2$| | 13.0 |
| Lot No. . | Concentration of |$^{226}$|Ra . | Measurement time . |
|---|---|---|
| . | (mBq kg|$^{-1}$|) . | (d) . |
| 1 | |$<0.4$| | 6.0 |
| 2 | |$<0.3$| | 11.0 |
| 3 | |$<0.3$| | 8.8 |
| 4 | |$<0.2$| | 9.6 |
| 5 | |$<0.5$| | 8.7 |
| 6 | |$<0.2$| | 13.0 |
On the basis of these studies and measurements, a highly sensitive method for measuring low-concentration |$^{226}$|Ra was established. This method can be applied to other non-accelerator particle physics experiments as well. For example, the XENON-1T detector will be upgraded to the XENON-nT detector with a neutron veto system which is based on a high-purity Gd-loaded water Cherenkov detector [24].
5. Conclusion
The method for measuring |$^{226}$|Ra using an HPGe detector with chemical extraction was improved to determine low-concentration |$^{226}$|Ra in a high matrix sample. More than |$99.9\%$| of |$^{226}$|Ra was recovered from the high matrix sample with a shorter processing time of chemical extraction, which resulted in the sensitivity on the |$10^{-1}\>$|mBq level. The improved method is being applied to SK-Gd to determine |$^{226}$|Ra in Gd|$_2$|(SO|$_4$|)|$_3{\cdot}$|8H|$_2$|O. Currently, all the measured Gd|$_2$|(SO|$_4$|)|$_3{\cdot}$|8H|$_2$|O products, which will actually be loaded into the SK tank, were confirmed to be below the maximum allowed |$^{226}$|Ra concentration level.
Acknowledgements
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grants Grant-in-Aid for Scientific Research on Innovative Areas No. 26104004, 26104006, 19H05807, and 19H05808, Grant-in-Aid for Specially Promoted Research No. 26000003, Grant-in-Aid for Young Scientists No. 17K14290, and Grant-in-Aid for JSPS Research Fellow No. 18J00049.
Footnotes
1 This is generally called “disk” and simply called disk in this paper from now on.
References
Advantec Toyo Ltd. (available at http://www2.advantec.co.jp/en/, date last accessed
Wako Pure Chemical Ltd. (available at http://www.wako-chem.co.jp/, date last accessed
International Atomic Energy Authority,
Merck Ltd. (available at http://www.merck.co.jp/en/company/merck_ltd/merck_ltd.html, date last accessed
Agilent Technologies,



