-
PDF
- Split View
-
Views
-
Cite
Cite
Maria-Angelica Sanclemente, ERF-VIIs and ORA59: Dual regulators of plant hypoxia and defense pathways during Botrytis cinerea infection, Plant Physiology, Volume 197, Issue 3, March 2025, kiaf086, https://doi.org/10.1093/plphys/kiaf086
Close - Share Icon Share
Oxygen deficiency (hypoxia) can be defined as an oxygen concentration below the ambient oxygen levels of 20.9% (Rolletschek et al. 2025). Diverse effects of hypoxia in plants can occur whenever levels drop below the physiological threshold needed to sustain aerobic metabolism (between 1% and 5%) and signaling (Loreti and Perata 2020). Even modest disruption of energy homeostasis can severely impact most plant cells. Despite the negative implications of hypoxia, low oxygen concentrations are common and needed for the development of plant heterotrophic tissues with high metabolic demands and cell density such as seeds, fruits, and meristems (van Dongen and Licausi 2015). In these tissues, oxygen gradients form due to high respiratory rates and restriction of oxygen diffusion and gas exchange (Rolletschek et al. 2025).
Tissue hypoxia can also develop due to exogenous factors such as soil flooding or pathogen infections. These environmental pressures can create conditions that lower oxygen availability similar to those encountered in endogenous hypoxic sites. However, exogenous hypoxia is sensed by most plants as stress (Chung and Lee 2020; Loreti and Perata 2020).
Regardless of the source, hypoxia leads to genome and metabolic reprogramming that induces the expression of core hypoxic genes associated with sugar metabolism, energy production, and overall cellular function (Mustroph et al. 2009). These responses are mediated by transcription factors belonging to the group VII of ETHYLENE RESPONSE FACTORS (ERF-VIIs) that are stabilized under low oxygen and relocated to the nucleus where they induce hypoxia responsive genes (HRGs).
ERF-VIIs are also important components of plant responses to pathogens and disease resistance (Kim et al. 2018). Their contributions to biotic stress tolerance are partly through their interaction with OCTADECANOID-RESPONSIVE ARABIDOPSIS 59 (ORA59) (Kim et al. 2018). In Arabidopsis, both ORA59 and the ERF-VII RELATED TO AP2.3 (RAP2.3) are required for pathogen resistance (Kim et al. 2018). Moreover, overexpression of RAP2.2 and RAP2.3 increases infection tolerance to the necrotrophic fungus Botrytis (Botrytis cinerea) (Zhao et al. 2012; Li et al. 2022).
In this issue of Plant Physiology, Brunello et al. (2024) investigated the effect of the ERF-ORA59 interactions on defense mechanisms and the extent of overlap between ERF-VIIs and ORA59 contributions to pathogen and low oxygen responses in Botrytis-induced hypoxic sites.
The authors showed that ORA59 is induced during Botrytis-induced hypoxia and interacts with 3 of the 5 members of the ERF-VIIs (Fig.). However, these interactions partially repress ERF-VIIs activity during Botrytis infection. As a result, only 15 (group A) of the 49 core HRGs were induced. The rest of the HRGs (group B = 34) were repressed, including classical markers for low oxygen such as alcohol dehydrogenase (ADH) and sucrose synthase. These observations are puzzling since ERF-VIIs activity is required for Botrytis tolerance, yet ORA59 induction dampens their effect (Brunello et al. 2024).
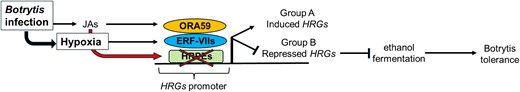
Convergence of hypoxic and defense responses in Arabidopsis. Botrytis infection induces JA accumulation and limits oxygen availability (hypoxia) at the site of infection. Low oxygen activates the expression of HRGs via ERF-VIIs transcription factors. Concurrently, increased JA levels activate defense mechanisms and ORA59 activity. The interaction between ORA59 and ERF-VIIs inhibits expression of most HRGs (group B) including those associated with ethanolic fermentation. Only a few (15 out of 49) HRGs are expressed (Group A). The repression of group B HRGs is partly associated with high levels of JA that reduce the binding of ERF-VIIs to HRPEs in the HRGs. The inhibition of ethanol production increases plant tolerance to Botrytis.
Despite the fact that Botrytis-induced hypoxia stabilized ERF-VIIs and repressed the HYPOXIA RESPONSE ATTENUATOR 1 (HRA1), a regulator that normally diminishes ERF-VIIs activities, many HRGs are not induced during Botrytis infection, suggesting that additional factors impact their expression. It further suggests that although hypoxia is induced by Botrytis infection, the hypoxia response is detrimental for Botrytis tolerance. The authors tested this hypothesis using mutants for enzymes that regulate ethanol production, including ADH and PYRUVATE DECARBOXYLASE (PDC1 and PDC2). Both adh and pdc1pdc2 mutants showed increased tolerance to botrytis. Moreover, ethanol treatment of Botrytis-infected leaves reduced tolerance to infection, indicating that fermentation pathways mediated by PDC and ADH during hypoxia impaired tolerance.
A key finding of Brunello et al. (2024) is that jasmonic acid (JA) acts as a nexus for botrytis and hypoxia responses in Arabidopsis (Fig. 1). JA signals are central for activation of Botrytis defense mechanisms and are an important component of hypoxia (Shukla et al. 2020). Arabidopsis treatment with methyl jasmonate (MeJA) induced ORA59 and reduced HRGs expression. Consistently, in ora59 lines, expression of the group B HRGs is enhanced, indicating that ORA59 acts as a repressor of those genes. Moreover, luciferase assays showed that hypoxia treatment of plants exposed to MeJA had reduced luminesce signal in reporter lines for HRGs and hypoxia-responsive promoter elements (HRPEs), which are important ERF-VII interacting sites for HRGs induction. It is unclear whether MeJA competes with ERF-VIIs for binding HRPEs. However, MeJA reduced binding of ERF-VIIs with HRPEs and consequently expression of most HRGs.
In separate experiments, authors showed that reoxygenation after hypoxia also leads to JA accumulation and induction of ORA59. Transition to normoxia typically destabilizes ERF-VIIs and downregulates the expression of HRGs. In ORA59-deficient plants recovery of HRGs levels was slow and their expression remained elevated, indicating a role for ORA59 in the modulation of HRGs expression during recovery.
Although many aspects of the interplay between Botrytis and hypoxia responses require further investigation, the work of Brunello et al. (2024) provides valuable insights into how individual components converge within the ERF-VII/ORA59 module and the role of JA connecting these pathways. The work is also significant to develop future strategies to enhance plant recovery from events that create localized oxygen gradients such as flooding and pathogen infections. For instance, excessively wet conditions following floods favor the growth of pathogens and disease incidence, which exacerbates post-flooding damage hindering plant recovery. Understanding how pathogen and hypoxia responses overlap is thus crucial for improving plant resilience to biotic and abiotic stresses.
Data availability
Data presented here is available in Brunello et al., (2024) https://doi.org/10.1093/plphys/kiae677.
References
Author notes
Conflict of interest statement. None declared.



