-
PDF
- Split View
-
Views
-
Cite
Cite
Xubo Ke, Junjun Shen, Yuqian Niu, Hongjiao Zhao, Yalu Guo, Piaoyun Sun, Tongwen Yang, Yanxin Jiang, Bosi Zhao, Zheng Wang, Tao Wu, Huasen Wang, Zheng Li, Cucumber NUCLEAR FACTOR-YC2/-YC9 target translocon component CsTIC21 in chloroplast photomorphogenesis, Plant Physiology, Volume 192, Issue 4, August 2023, Pages 2822–2837, https://doi.org/10.1093/plphys/kiad296
Close - Share Icon Share
Abstract
Light signals promote photomorphogenesis and photosynthesis, allowing plants to establish photoautotrophic growth. Chloroplasts are organelles responsible for photosynthesis in which light energy is converted into chemical energy and stored as organic matter. However, how light regulates chloroplast photomorphogenesis remains unclear. Here, we isolated a cucumber (Cucumis sativus L.) mutant albino seedling (as) from an ethyl methane sulfonate mutagenesis library with an albino phenotype. Map-based cloning revealed that the mutation occurred in a component of cucumber translocon at the inner membrane of chloroplasts (CsTIC21). Subsequently, virus-induced gene silencing and CRISPR/Cas9 analyses confirmed the association between the mutant gene and the as phenotype. Loss-of-function of CsTIC21 induces malformation of chloroplast formation, leading to albinism and death in cucumber. Notably, CsTIC21 transcription was very low in etiolated seedlings grown in the dark and was upregulated by light, with expression patterns similar to those of Nuclear factor-YC (NF-YC) genes. Here, 7 cucumber NF-YC family genes (CsNF-YC) were identified, among which the expression of 4 genes (CsNF-YC1, -YC2, -YC9, and -YC13) responded to light. Gene silencing of all CsNF-YC genes in cucumber indicated that CsNF-YC2, -YC9, -YC11-1, and -YC11-2 induced distinct etiolated growth and decreased chlorophyll content. Interaction studies verified that CsNF-YC2 and CsNF-YC9 target the CsTIC21 promoter directly and promote gene transcription. These findings provide mechanistic insights on the role of the NF-YCs–TIC21 module in chloroplast photomorphogenesis promoted by light in cucumber.
Introduction
The long-term evolution of land plants has resulted in numerous growth and development-related adaptations to different environments. Plants sense and adapt to external light signals during growth, and light is one of the most important environmental factors driving plant growth and development (Han et al. 2019). Light provides energy for plant growth and is a signal factor regulating various plant development stages (de Wit et al. 2016). In plants, the photosynthetic system plays an important role in the morphogenesis of chloroplast structure, which regulates normal growth. Specifically, light regulates gene transcription, chlorophyll synthesis, and protein degradation in the chloroplasts (Cackett et al. 2021). However, the detailed mechanisms via which light signals regulate chloroplast photomorphogenesis remain unclear.
Chloroplasts are organelles found in the green tissues of plants. Nuclear-encoded chloroplast proteins with transit peptides as targeting signals are imported from the cytosol to the chloroplast in a process mediated by the translocon at the outer and inner envelope membranes of chloroplasts (TOC–TIC) complexes (Bédard and Jarvis 2005; Kessler and Schnell 2006; Lee et al. 2017; Lee and Hwang 2018). Therefore, TOC and TIC play indispensable roles in chloroplast function and homeostasis. Recently, cryoelectron microscopic structures of 14 TOC–TIC super-complexes in Chlamydomonas (Chlamydomonas reinhardtii) have been reported (Jin et al. 2022). The structures indicate that the TOC–TIC complex functions through a hybrid β-barrel co-assembly of TOC120 and TOC75 and transmembrane helices of TIC20 and YlmG; however, the mechanism of regulating the expression of the genes has not been studied comprehensively. To date, 12 TIC components (TIC110, TIC20, TIC22, TIC40, TIC55, TIC32, TIC21, TIC62, TIC214, TIC56, TIC236, and TIC12) have been identified in Arabidopsis (Jackson et al. 1998; Kouranov et al. 1998; Stahl et al. 1999; Jackson-Constan and Keegstra 2001; Hörmann et al. 2004; Teng et al. 2006; Stengel et al. 2008; Kikuchi et al. 2013; Köhler et al. 2015; Chen et al. 2018; Zhao et al. 2022). In Arabidopsis (Arabidopsis thaliana), TIC21 is located on the inner membrane of the chloroplast participating in precursor protein transport, while a tic21 mutation (also named chloroplast import apparatus 5, cia5) results in mutants defective in chloroplast protein import (Sun et al. 2001; Teng et al. 2006; Kikuchi et al. 2009). The tic21 mutant exhibits a pronounced albino phenotype, defective in plastid protein transport. The growth of the mutant is limited considerably at the seedling stage, leading to massive seedling deaths. However, on a medium supplemented with sucrose, the mutant developed albino leaves and even inflorescences, implying that the tic21 loss-of-function mutant has normal growth potential and only lacks the photoautotrophic ability (Teng et al. 2006). Besides, TIC21 is a ferroportin that maintains metal homeostasis in cells, which regulates protein transport into chloroplasts (Duy et al. 2007). However, the response of the TIC21 gene to the light signal pathway in plants has not been reported.
Nuclear factor-Y (NF-Y) is a type of transcription factor found in all plants. It binds the target genes promoter CCAAT box to facilitate a permissive chromatin modification (Siefers et al. 2009; Petroni et al. 2012; Nardini et al. 2013). The NF-Y transcription factor complex comprises 3 subunits: NF-YA, NF-YB, and NF-YC. In Arabidopsis, 36 NF-Y transcription factor subunits, including 10 NF-YAs, 13 NF-YBs, and 13 NF-YCs, have been identified (Siefers et al. 2009). Among them, NF-YC1, NF-YC3, NF-YC4, and NF-YC9 are expressed in light- and dark-grown seedlings, which actively regulate photomorphogenesis through histone deacetylation and the HY5-independent light signaling pathway. In addition, they redundantly regulate the light-mediated inhibition of the brassinolide (BR) biosynthesis and signaling pathways (Myers et al. 2016; Tang et al. 2016; Zhang et al. 2021). Generally, the Arabidopsis NF-Y family is involved in light-mediated gene regulation, chlorophyll synthesis, and chloroplast development (Hackenberg et al. 2012; Hou et al. 2014; Liu et al. 2016). Moreover, rice (Oryza sativa L.) plants with antisense or RNA interference constructs of OsNF-YB2 have reduced chlorophyll content and degenerated chloroplasts. In addition, OsNF-YB2, OsNF-YB3, and OsNF-YB4 regulate several photosynthesis genes, including chlorophyll a/b binding protein (Miyoshi et al. 2003). In wheat (Triticum aestivum L.), TaNF-YB2 overexpression increases chlorophyll content, photosynthesis rate, and early growth substantially (Stephenson et al. 2011). Furthermore, 5 NF-YC genes (TaNF-YC5, -YC8, -YC9, -YC11, and -YC12) are upregulated under light, while TaNF-YC11 is co-regulated with photosynthesis-related genes in wheat (Stephenson et al. 2010).
Considering the high lethality of albino mutants at the seedling stage, it is difficult to preserve such chloroplast formation mutants. Therefore, every albino mutant is precious material in chloroplast developmental research. In the present study, we report the identification, fine-mapping, and functional characterization of a cucumber (Cucumis sativus L.) albino mutant gene. Gene silencing and CRISPR/Cas9-mediated knock-out assays verified that the cucumber TIC21 homologous gene (CsTIC21) was associated with the albino phenotype. In addition, the NF-YC2 and NF-YC9 homologs of cucumber function independently in the light-mediated TIC pathway by directly binding the CsTIC21 promoter and regulating its transcription. Our findings revealed that the NF-YC–TIC21 module mediates light-induced chloroplast photomorphogenesis in cucumber, which may provide mechanistic insights into light-regulated chloroplast formation in plants.
Results
Phenotypic analysis of albino seedling mutation in cucumber
An albino mutant plant was identified in an M1 family of an ethyl methane sulfonate (EMS) library generated using cucumber inbred line 649. Compared with that of the wild-type plant (inbred line 649, CK), the mutant cotyledon was pale yellow (etiolated) at germination and transitioned gradually into albino (Fig. 1, A to C). Subsequently, the mutant seedling withered slowly and died at the first true-leaf stage (Fig. 1, D to F). We named the mutant as (albino seedling) in the present study. At the cotyledon stage, the mutant hypocotyl length was slightly longer, the length of the roots was significantly shorter, the cotyledons were smaller, and the number of lateral roots was less than that in the CK (Supplemental Fig. S1). In addition, the chlorophyll a and b contents were significantly lower than those in CK (Fig. 1, G and H). Transmission electron microscopy (TEM) revealed that the CK chloroplasts were ellipsoid, with a normally stacked grana lamellar structure. In the mutant, the chloroplasts were abnormal, with malformed structure and less thylakoid accumulation (Fig. 1, I and J).

Phenotypic characterization of the as mutant. CK A) and as mutant seedling B) phenotypes at 5 d after sowing (DAS). C) Magnifications of the as mutant apexes at 5 DAS. CK D) and as mutant seedling E) phenotypes at 10 DAS. F) Magnifications of the as mutant apexes at 10 DAS. G and H) Chlorophyll contents in the CK and as mutant cotyledons. Error bars represent mean ± Sd (n = 9). Student's t-test was performed, and statistically significant differences were indicated by **P < 0.01. Chloroplast ultrastructure of CK I) and as mutant J) at 10 DAS. Chl, chlorophyll; DAS, days after sowing; FW, fresh weight; CK, wild-type 649 cultivar; as, albino seedling mutant. Scale bar = 1 cm in A to F) and 1 μm in I and J).
Subsequently, we tested the growth potential of the as mutant in an energy-supplied condition. On the sugar-supplemented medium, the as mutant cotyledons were light yellow-green and grew for a slight long period, even producing 4 to 5 true leaves. However, the leaf color was lighter than that of the CK plants (Fig. 2, A and B). Observations of the CK true-leaf chloroplast ultrastructure under TEM revealed that the chloroplasts were fully developed, regular in shape, and neatly arranged. The normal stacking of basal lamellae and starch granules was also observed (Fig. 2C). In the as mutant, fewer chloroplasts were observed in the mesophyll cells, the arrangement of grana lamella was loose, and the starch grains were fewer, when compared with those in the CK (Fig. 2D). Besides, the net photosynthetic rate in the as mutant was significantly lower than that in the CK (Fig. 2E). Overall, the results implied that as mutation affected the photoautotrophic process, leading to abnormal chloroplast development and insufficient photosynthesis.
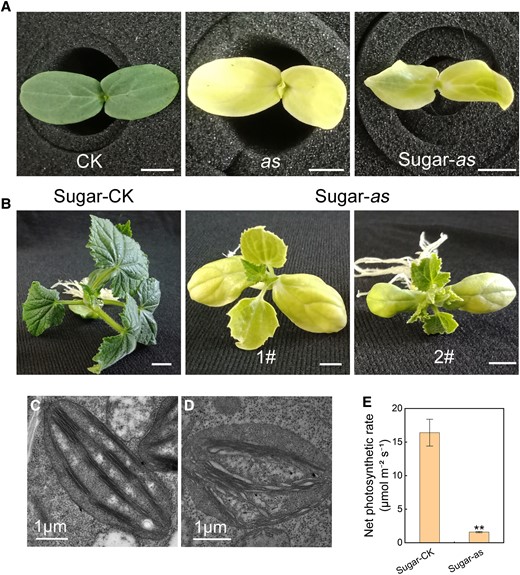
Growth characteristics of the as mutant in sugar-supplied culture. A) Cotyledon phenotypes of the CK grown on MS basic medium, the as mutant grown on MS basic medium, and the as mutant grown in MS medium with sugar (from left to right). B) Seedling phenotypes of the CK and as mutant grown on MS medium supplemented with sugar. Chloroplast ultrastructure of CK C) and as mutant D) grown on sugar-supplied medium. E) Comparison of the net photosynthetic rate between CK and as mutant cultured on sugar-supplied medium. Error bars represent mean ± Sd (n = 3). Student's t-test was performed and statistically significant differences were indicated by **P < 0.01. Scale bar = 1 cm in A and B) and 1 μm in C and D).
Mutation in CsTIC21 was associated with the as mutant
Two segregating populations were developed by crossing the heterozygous plants (named 649 M) in the M1 pedigree (with green/albino segregation) with cucumber model lines 9930 and Gy14. Genetic analyses of the F1 and F2 populations of Gy14 × 649 M and 9930 × 649 M revealed that the as mutant phenotype was controlled by a Mendel's recessive nuclear gene (Supplemental Table S1). Further, bulked segregant analysis (BSA) of the 2 F2 populations mapped the as locus, and the single nucleotide polymorphism (SNP) index revealed an interval on cucumber chromosome 7, with highly contrasting patterns between the WT and as bulks (Supplemental Fig. S2). Preliminary marker analysis using 96 mutants from the 9930 × 649 M F2 population revealed that the as locus was located at an 8.6-cM interval, flanked by SSR0649 and SSR9705 markers (Fig. 3A). Additional analysis using 518 mutant plants in the 2 F2 populations and 7 additional markers narrowed the interval into a 2.0-cM region between UW4918 and InDel0418 markers. The physical distance between the 2 markers was 165.5 kb (Fig. 3B). All putative SNPs in the region were predicted and screened using the cucumber reference genome sequences, and the polymorphic markers between Gy14 and 649 M were identified. Two SNP markers, SNP7349 and SNP0787, were developed to map the as locus into a final genomic interval of 63.44 kb (Fig. 3C). Genome annotation revealed that there were 10 genes in the final interval, including Csa7G071680, which was predicted to encode a chloroplast inner membrane protein (Fig. 3C; Supplemental Table S2). The protein sequence had the highest similarity to Arabidopsis TIC21, which was subsequently named CsTIC21 in cucumber. The full-length genomic sequence of CsTIC21 was 5,165 bp, with 4 predicted exons. The genome sequence analysis between CK and the as mutant identified only one nucleotide mutation occurring in the exon 4 (G to T), which altered the protein C-terminal sequence (glycine to tryptophan) (Fig. 3, D and E; Supplemental Fig. S3).
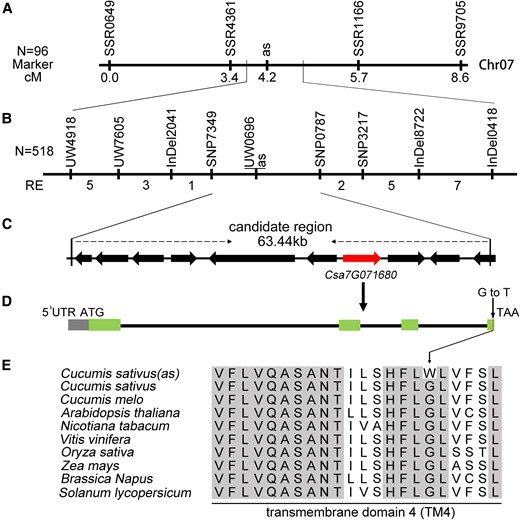
Map-based cloning of genes in the as locus. A) Primary mapping using 96 mutant plants from the 9930 × 649 M F2 population. B) Fine-mapping using 518 mutant plants from the Gy14 × 649 M F2 and 9930 × 649 M F2 populations. The numbers indicate the recombinant events. C) Annotated genes in the final interval. Csa7G071680 is cucumber TIC21 putative ortholog. D) Exon–intron prediction of CsTIC21 and the SNP (G in CK to T in as mutant) between CK and as. E) Alignment of TIC21 homologs from diverse species. Amino acid residues with 100% identity between the homologs are dark background. cM, centimorgan.
Amino acid alignment of the homologous proteins revealed that the C-terminal region in TIC21, including the transmembrane domain 4 (TM4), is highly conserved in all species tested, including cucumber, melo (Cucumis melo L.), Arabidopsis, tobacco (Nicotiana tabacum L.), grape (Vitis vinifera L.), maize (Zea mays L.), rice, rape (Brassica napus L.), and tomato (Solanum lycopersicum L.) (Supplemental Fig. S4). The results implied that the mutation in the TM4 domain (as mutant) might produce a defective protein (Supplemental Fig. S5 and Table S3). In addition, the genome-wide analysis revealed that there was only one TIC21 homologous sequence in the cucumber genome. Therefore, Cstic21 mutation should exhibit a defective phenotype similar to that of the as mutant.
Functional verification of CsTIC21 by virus-induced gene silencing and CRISPR/Cas9 assays
Most recently, the tobacco ringspot virus (TRSV)-based virus-induced gene silencing (VIGS) system has been developed for cucurbits and used to verify the functions of CsHOX3 and CsbHLH1 in nonglandular hair elongation and glandular hair formation in cucumbers, respectively (Fang et al. 2021; Dong et al. 2022). We performed a preliminary experiment with CsPDS (Csa4G011080) as a positive control (Fang et al. 2021). Among the 50 TRSV-CsPDS-treated plants, the first true leaves of 30 plants were photobleached, implying the VIGS system was suitable for functional verification of the leaf color mutant gene, CsTIC21 (Fig. 4A; Supplemental Fig. S6). A total of 52 TRSV-CsTIC21-treated seedlings were harvested, 16 of which had visible yellowing phenotypes (Fig. 4, B to D). All the 16 plants had significantly down-regulated CsTIC21 transcription and significantly lower chlorophyll contents than the TRSV-CK (Fig. 4, E and F). The results indicated that the CsTIC21-silencing in cucumber produced similar etiolation as in the as mutant seedlings.
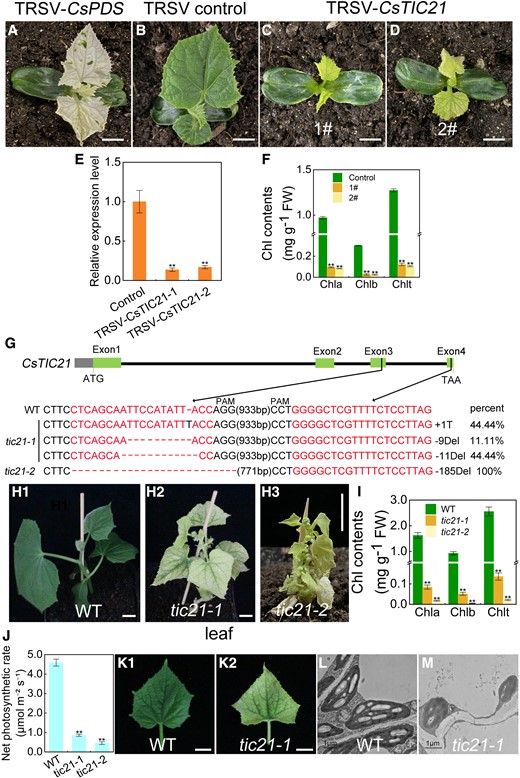
Functional verification of CsTIC21 in cucumber. A) The phenotype of TRSV-CsPDS-infected cucumber plants. Phenotypes of young cucumber seedlings treated with TRSV-CK B) and TRSV-CsTIC21C and D) at 20 d post-infection. E)CsTIC21 expression changes in the TRSV-CsTIC21 cucumber plants showed in C and D). Error bars represent mean ± Sd (n = 3). F) Chlorophyll contents in the TRSV-CK and TRSV-CsTIC21 cucumber plants. Error bars represent mean ± Sd (n = 9). G) Illustration of the sequences insertion/deletion patterns of CsTIC21-edited cucumber mutant. H1 to H3) Phenotypes of the WT (PS76 line) and 2 CsTIC21 knock-out mutants (tic21-1 and tic21-2) obtained by CRISPR/Cas9. I) Chlorophyll contents in the WT cucumber and knock-out mutants. Error bars represent mean ± Sd (n = 9). J) Comparison of the net photosynthetic rate between the WT and knock-out plants. Error bars represent mean ± Sd (n = 3). K1 and K2) Leaves in the WT and tic21-1 mutant line. Images were digitally extracted for comparison. Chloroplast ultrastructure in WT L) and tic21-1 knock-out mutant leaves M) observed under transmission electron microscopy. Student's t-test was performed and statistically significant differences were indicated by **P < 0.01 E, F, I and J). Scale bar = 1 cm in A to D), H1 to H3), K1 to K2), and 1 μm in L and M).
This association was further verified using the CRISPR/Cas9 gene editing system. Two guide RNA targets (Targets 1 and 2) located in the third and fourth exons of CsTIC21 were assembled into the CRISPR/Cas9 vector pBSE402 (Supplemental Fig. S7). Two CsTIC21 knock-out lines, tic21-1 and tic21-2, were obtained following the transformation of the cucumber germplasm PS76 (Fig. 4, G to I). The tic21-1 was a CsTIC21 heterozygous knock-out line. However, after subcloning for DNA sequencing (18 random clones), only 3 types of mutant sequences (8 clones with 1 bp insertion, 2 clones with 9 bp deletion with a frameshift open reading frame, and 8 clones with 11 bp deletion) were obtained, implying that the tic21-1 line possessed mutant Cstic21 alleles. The tic21-2 mutant phenotype was produced by a large segment (185 bp) homologous deletion in CsTIC21 (Fig. 4G).
We examined 6 homologous sequences of the CsTIC21 gene targets and did not find any off-target results (Supplemental Table S4). The tic21-1 seedlings had an albino phenotype and developed true leaves in a culture medium supplemented with sugar (Fig. 4, H1 and H2). Comparatively, a severe mutant appearance, including extremely dwarf and fully albino blades, was observed in tic21-2 (Fig. 4H3). The chlorophyll contents of tic21-1 and tic21-2 were significantly lower than those of the PS76 inbred line, and the tic21-1 and tic21-2 almost lost their photosynthetic capacity (Fig. 4, I and J). Regrettably, the tic21-2 seedlings could not survive in a medium supplemented with sugar and died soon after transplanting into the soil. Therefore, only the leaf morphology and chloroplast structure in tic21-1 were observed. Comparatively, the tic21-1 blade had green veins, indicating minimal autotrophic photosynthetic potential; hence, it was a milder mutant phenotype compared to the as and tic21-2 mutants (Fig. 4, K1 and K2). The chloroplast ultrastructure of tic21-1 was characterized by round or nearly round plastids, and the thylakoid membranes were barely detectable (Fig. 4, L and M). The results are almost consistent with the observations in the as mutant seedlings, implying that Cstic21 is the gene associated with the albino phenotype in the cucumber line 649 M.
CsTIC21 light-responsive assay
The cotyledons of Cstic21 mutant seedlings had a mimic etiolation phenotype with severe chloroplast developmental damage. To establish whether CsTIC21 mediated the light-responsive chloroplast photomorphogenesis, CsTIC21 expression in cucumber seedlings grown in the dark was analyzed (Fig. 5, A and B). Reverse transcription quantitative PCR (RT-qPCR) analysis revealed that the CsTIC21 expression in dark-grown seedlings was significantly lower than in light-grown seedlings (Fig. 5C). However, CsTIC21 expression was recovered after the plants were re-exposed to light (Fig. 5D). The results imply that CsTIC21 responds to light signals. Further analysis of the 2-kb sequence of the CsTIC21 promoter revealed several putative light-responsive cis-elements. Among them, 2 CCAAT-box elements gave rise to a potential interactive regulation from the NF-Y gene family (Supplemental Fig. S8).
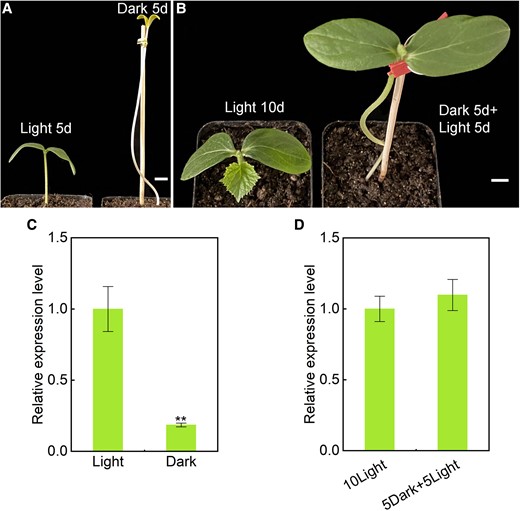
Dark treatment of cucumber seedlings and CsTIC21 gene expression analysis. A and B) Comparison of the cucumber seedlings grown in different conditions. Images were digitally extracted for comparison. A) Wild-type (WT) cucumber seeds were planted in the light (left) and dark (right) for 5 d, and then grown in light for the next 5 d B). C) RT-qPCR analysis of CsTIC21 gene expression in the seedlings grown in light and dark conditions for 5 d. D) RT-qPCR analysis of CsTIC21 gene expression in the seedlings grown in light for 10 d and 5 d light + 5 d dark. Data represent mean ± Sd of 3 biological replicates C and D). Student's t-test was performed and statistically significant differences were indicated by **P < 0.01 C and D). Scale bar = 1 cm in A and B).
Cucumber NF-YC gene family identification and functional analysis
Genome-wide gene identification identified 7 NF-YC genes in cucumber (Supplemental Table S5). Based on the phylogenetic tree constructed using the Arabidopsis and cucumber NF-YC gene family and the gene annotation from the cucumber database, we renamed the cucumber NF-YC genes in the present study (Supplemental Fig. S9 and Table S6). RT-qPCR analysis revealed that the levels of expression of CsNF-YC1, CsNF-YC2, CsNF-YC9, and CsNF-YC13 in dark-grown seedlings were significantly lower than in light-grown seedlings, which were consistent with that of CsTIC21 (Fig. 6A). In addition, CsNF-YC11-2 was upregulated in the dark, and there were no significant changes in the levels of expression of CsNF-YC10 and CsNF-YC11-1.

CsNF-YC gene family function analysis. A) RT-qPCR analysis of CsNF-YCs gene expression in wild-type (WT) cucumber seedlings grown under light and dark conditions for 5 d. Data represent mean ± Sd of 3 biological replicates. B)CsNF-YCs expression following TRSV-CsNF-YCs treatment. Error bars represent mean ± Sd (n = 3). C) The phenotype of TRSV-CsNF-YCs-treated cucumber at 30 d post-treatment. Scale bar = 1 cm. D) The chlorophyll contents of CsNF-YCs gene-silenced cucumber plants. Error bars represent mean ± Sd (n = 9). Student's t-test was performed and statistically significant differences were indicated by *P < 0.05, **P < 0.01 A, B and D).
To comprehensively analyze the function of cucumber NF-YC genes, we performed VIGS experiments for all 7 CsNF-YC genes. We collected at least 2 independent TRSV-treated seedlings for every gene, whose targeted gene silencing results were checked by RT-qPCR (Fig. 6B). Among the 7 genes, the down-regulation of CsNF-YC2, CsNF-YC9, CsNF-YC11-1, and CsNF-YC11-2 exhibited a chlorotic phenotype (Fig. 6C). Moreover, the chlorophyll contents (including total chlorophyll, chlorophyll a and b) were significantly lower in the 4 types of gene-silencing lines than in TRSV-CK (Fig. 6D).
CsNF-YC2 and CsNF-YC9 directly regulated CsTIC21 expression
Combined gene expression and silencing results revealed that CsNF-YC2 and CsNF-YC9 responded to light signals and regulated chloroplast photomorphogenesis and/or chlorophyll metabolism, which is consistent with the role of CsTIC21. In addition, in all the TRSV-CsNF-YC2 and TRSV-CsNF-YC9 lines, CsTIC21 was significantly down-regulated (Fig. 7, A and B). To test whether CsNF-YC2 and CsNF-YC9 influenced CsTIC21 expression directly, a heterologous transient expression analysis was performed using Nicotiana benthamiana. A 2-kb upstream sequence of the CsTIC21 translation start site was fused with a luciferase-encoding sequence (LUC) to construct a reporter. Meanwhile, two 35S promoter-driven effectors consisting of CsNF-YC2 and CsNF-YC9 CDSs (coding sequences) were developed (Fig. 7C). Each effector with the reporter was introduced into N. benthamiana leaves simultaneously, and the results revealed that both CsNF-YC2 and CsNF-YC9 induced the CsTIC21 promoter activity (Fig. 7D). A yeast 1-hybrid validation assay further revealed that CsNF-YC2 and CsNF-YC9 specifically bind the CsTIC21 promoter (Fig. 7E). The deduced cis-elements in the CsTIC21 promoter region had 5 light-responsive motifs, including 3 light response elements and 2 CCAAT boxes (with the same core sequence) (Fig. 7F). Therefore, 5 specific qPCR reactions (P1 to P5) were designed for chromatin co-immunoprecipitation (ChIP) analysis. Incubation of the cucumber protoplasts transformed by pro35S: CsNF-YC2-Flag and pro35S: CsNF-YC9-Flag with Flag antibodies revealed that the incubations had similar substantial enrichments on the P3 and P5 regions of the CsTIC21 promoter, respectively, containing the 2 CCAAT boxes (Fig. 7, G and H). Subsequently, the electrophoretic mobility shift assay (EMSA) validated that CsNF-YC2 and CsNF-YC9 physically bind at least one CCAAT box (in the P3 region) in the CsTIC21 promoter (Fig. 7, I and J). Overall, the findings reveal that CsNF-YC2 and CsNY-YC9 directly bind the CsTIC21 promoter and positively regulate CsTIC21 transcription independently.
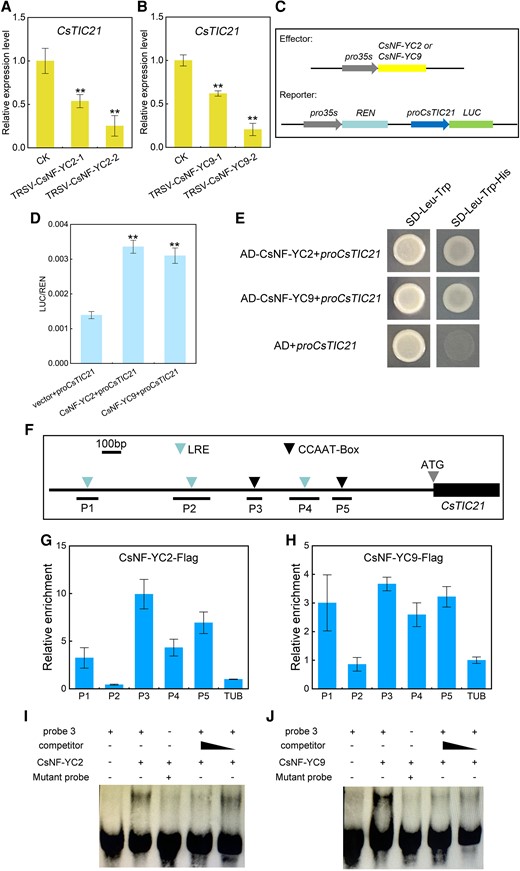
CsTIC21 regulation by CsNF-YC2 and CsNF-YC9. Expression analysis of CsTIC21 in TRSV-CsNF-YC2A) and TRSV-CsNF-YC9 plants B). Error bars represent mean ± Sd (n = 3). C) Schematic diagram of effector and reporter constructs using dual luciferase (LUC) analysis in an N. benthamiana transient expression system. D) Effects of CsNFYC2 and CsNF-YC9 on the CsTIC21 promoter activity. Error bars represent ± Sd (n = 9). E) Yeast 1-hybrid assay of CsNF-YC2 and CsNF-YC9 binding CsTIC21 promoter with 3-AT (30 mM) on SD/-His/-Trp/-Leu medium. F) The 2-kb CsTIC21 promoter sequence used in ChIP-qPCR. P1 to P5 indicate the 5 ChIP-qPCR regions with light-responsive element/CCAAT box. ChIP-qPCR assay of cucumber protoplasts transformed by pro35S: CsNF-YC2-FlagG) and pro35S: CsNF-YC9-FlagH). Data represent mean ± Sd of 3 biological and 3 technical replicates. I and J) EMSA of CsNF-YC2 and CsNF-YC9 binding the CsTIC21 promoter P3 region. LRE, light response element. Student's t-test was performed and statistically significant differences were indicated by **P < 0.01 A, B, and D).
Discussion
Light-sensitive CsTIC21 has a conserved function in chloroplast photomorphogenesis
Chloroplasts are the most important and common plastids containing green pigments (mainly chlorophyll a and b) in plant cells. They are semi-autonomous organelles carrying genetic information and are responsible for photosynthesis in plants (Cackett et al. 2021; Li et al. 2023). Chloroplasts originated from a cyanobacterium engulfed by a eukaryotic cell, where most of the genes in the cyanobacterial ancestor were transferred into the host nuclear genome during evolution (Dyall et al. 2004). Therefore, the expression of photosynthesis-associated nuclear genes encoding chloroplast proteins and the import of these proteins into chloroplasts are crucial for chloroplast formation. Most photosynthesis-associated nuclear genes are lowly expressed in the dark; however, their expression is induced rapidly when etiolated plants are exposed to light (Terzaghi and Cashmore 1995). Considering that TOC–TIC complexes mediate the import of numerous photosynthesis-associated proteins upon light illumination, the TOC and TIC genes should also respond to light signals. In Arabidopsis, at least 5 TOC (TOC33, TOC34, TOC75, TOC132, and TOC159) and TIC110 genes are induced rapidly by blue light, which is primarily mediated by cryptochrome 1 (CRY1) (Fukazawa et al. 2020). Therefore, identifying the light-regulated TOC/TIC pathway could enhance our understanding of chloroplast formation.
Given the etiolated lethality, albino mutants are precious in chloroplast developmental research. Herein, an albino cucumber mutant seedling from an M1 pedigree of an EMS-induced mutant population was identified. Genetic analysis, gene knock-out, and VIGS identified Cstic21 as the associated gene-driving mutation in the as locus. In Arabidopsis, tic21 mutant (cia5) was identified in 2006. Like the cucumber as mutant, the cia5 mutant was slightly green when the first leaves emerged but turned albino gradually as the leaves matured (Teng et al. 2006). In addition, the growth of the 2 mutant seedlings was completely limited on soil but produced some albino leaves on an artificial medium supplemented with sucrose. In previous studies, Arabidopsis TIC21 and its homologs in soybean, pea, medicago, maize, and rice had been shown to contain 4 conserved transmembrane domains (Teng et al. 2006). Here, we also found that CsTIC21 had these transmembrane domains (Supplemental Fig. S4). In Arabidopsis, the loss of function of TIC21 results in a pronounced albino phenotype and plastid protein transport defect (Teng et al. 2006). In addition, TIC21 can interact with other translocation components, such as TIC110 and TOC75, and is likely to act as part of the chloroplast inner membrane protein transport pathway (Teng et al. 2006). We found that the amino acid sequence similarity between CsTIC21 and AtTIC21 is up to 60%. All these findings indicate that CsTIC21 should perform the same function with AtTIC21, which transports the precursor proteins into chloroplast, and is associated with the cucumber chloroplast formation.
The etiolated cotyledon in the as mutant and the similar phenotype of normal seedlings grown in the dark implies that CsTIC21 may be nonfunctional without illumination. The low CsTIC21 expression level in dark-grown seedlings confirmed this, and the induction of CsTIC21 expression was observed when lighting was restored, adjusting its expression level to normal. Therefore, CsTIC21 is light-responsive, and its expression in cucumber is associated with plant de-etiolation.
CsNF-YC2/-YC9–CsTIC21 mediate the light-regulated chloroplast morphogenesis (photomorphogenesis) in cucumber
In most species, the NF-Y complex comprises NF-YA, NF-YB, and NF-YC subunits. In animals, each NF-Y subunit is encoded by a single gene. However, structural and functional diversification has occurred in plants, leading to enlarged gene families comprising 8 to 39 members in each subunit (Laloum et al. 2013). Compared to yeast and mammals, little functional information on the NF-Y subunit in plants is available. This is due to the overlapping functionality among the subunits; hence, very few NF-Y genes have been isolated via mutant genetic screening in plants. For example, in Arabidopsis, NF-YC3, NF-YC4, and NF-YC9 exhibit similar expression patterns, with no visible changes in any single or double mutants (Siefers et al. 2009). Only nf-yc3 nf-yc4 nf-yc9 triple mutant displayed an inhibited hypocotyl elongation via the COP1-dependent pathway (Myers et al. 2016). Therefore, studies on NF-Y function in the plant usually employ the overexpression system (Stephenson et al. 2010). In the present study, we have identified the NF-YC family in cucumber and report that the number of the family genes is lower than that in Arabidopsis. In addition, 4 out of the 7 genes identified have nonredundant functions regulating chlorophyll content. Therefore, cucumber could be more suitable for studying NF-YC gene family function.
There is a notable relationship between light, NF-Y, chlorophyll content, and chloroplast development (see the Introduction section). In the present study, VIGS and expression analysis revealed that CsNF-YC2, CsNF-YC9, and CsTIC21 responded to the light signals and that all played roles in chloroplast development. Furthermore, multiple interaction tests verified that CsNF-YC2 and CsNF-YC9 bind the CsTIC21 promoter independently. CsTIC21 promoter was activated in N. benthamiana after infiltration with Cs-NF-YC2 or Cs-NF-YC9 effectors; however, this result should be interpreted with caution due to the presence of endogenous N. benthamiana NF-Ys, which could interact with cucumber CsNF-YC to form a heterologous complex, inducing the CsTIC21 promoter activity. Although we have no evidence supporting that CsNF-YC2 or CsNF-YC9 alone directly induced CsTIC21 expression, it is possible that CsNF-YC2 or CsNF-YC9 recruited their specific CsNF-YA and CsNF-YB partners to form a complex, which directly induced CsTIC21 transcription. Overall, our findings present a CsNF-YC2/-YC9–CsTIC21 model of light-induced chloroplast photomorphogenesis in cucumber (Fig. 8).
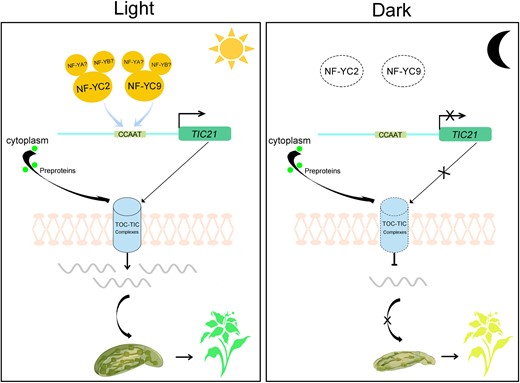
A working model depicting the role of CsNF-YCs promoting CsTIC pathway in light-regulated chloroplast photomorphogenesis. In the light, CsNF-YCs promote the CsTIC pathway by directly promoting CsTIC21 expression. CsTIC21 participates in the formation of TOC–TIC complex to transport the chloroplast proteins promoting chloroplast morphogenesis. In the dark, the NF-YCs–TIC21 module loss function resulting in chloroplast dysplasia. The dotted lines indicate the loss of function or malformation in TOC–TIC complex. The lines ending with arrows indicate promotion, and those ending with bars indicate repression.
Materials and methods
Plant materials
The North China cucumber (C. sativus L.) cultivar 649 was used to develop an EMS mutant library. The cucumber albino mutant seedling (as) was observed in the M1 pedigree. The heterologous mutant seedlings were selected to preserve the mutation. Two cucumber model inbred lines 9930 and Gy14 were used to develop the segregating populations. A South China cucumber line CU2 and an American cucumber line PS76 were used for VIGS and transformation assays, respectively. All mature plants were grown in a greenhouse at Northwest A&F University, Shaanxi, China.
Population development
Ten heterologous mutant seedlings with characteristic appearance were selected randomly from the same M1 pedigree and crossed with cucumber lines 9930 and Gy14. Fifty random hybrid seeds from each crossing were grown and separately self-pollinated to develop 100 populations. The populations with green/albino segregation were mixed to develop 9930 × 649 M F2 and Gy14 × 649 M F2 mapping populations, respectively.
Phenotyping of the cucumber albino mutant
The wild-type and as mutant cucumber samples were prepared and observed under the microscope as previously described by Fan et al. (2016). Briefly, the roots, hypocotyls, cotyledons, leaves, and petioles were separated, and their images were captured using an optical camera (Canon, Japan). The chloroplast structure in the leaves was observed under a transmission electron microscope.
Determination of the chlorophyll content and photosynthetic rate
Wild-type and as mutant leaf samples (0.1 g) were weighed for chlorophyll extraction, with 3 biological replicates per genotype. The chlorophyll was extracted in 10 mL of 95% ethanol under dark conditions at 4 °C for 12 h. The absorbance of chlorophyll extract was determined at 646 and 663 nm using a Uvikon 930 spectrophotometer (Kontron, Germany). Ethanol (95%, v/v) was used as the control. The chlorophyll content was calculated according to the formula described by Wang et al. (2016).
Bulked segregant analysis
The total DNA was extracted from 30 normal and 30 albino plants in the 9930 × 649 M F2 population and then equally pooled for BSA sequencing. The normalized bulk DNA samples were sent to Novogen (Tianjin, China) for library construction and sequencing on a HiSeq4000 Illumina platform (Illumina, USA).
SNP-index analysis
SNP calling was conducted as described by Li and Durbin (2009). The SNP index and ▵(SNP index) were calculated to identify the candidate interval for the as locus (Abe et al. 2012). Subsequently, the genome-wide simple sequence repeat markers were selected as previously described by Cavagnaro et al. (2010) and Yang et al. (2012), and fine-mapping was performed as described by Niu et al. (2018). The candidate genes in the final genomic interval were analyzed using the Cucumber Genome Database (http://cucurbitgenomics.org/). The candidate gene sequences were cloned and sequenced from the wild-type and as mutant plants. DNA sequence comparison and protein sequence deduction were performed using DNAMAN (v8.0) software (http://dnaman.software.informer.com/8.0/). The homologous gene in Arabidopsis was analyzed using the TAIR database (http://www.arabidopsis.org/). The primers used are listed in Supplemental Table S7.
RT-qPCR analysis
Total RNA was extracted from cotyledons or true young leaves from the wild type and as mutant using TRIzol reagent (Vazyme, China). The first-strand cDNA was synthesized through reverse transcription of the extracted total RNA using a HiScript II 1st Strand cDNA Synthesis Kit following the manufacturer's protocol (Vazyme). RT-qPCR was performed using the SYBR qPCR Master Mix (Vazyme) on an ABI 7500 Fast Real-Time PCR System (Applied Biosystems, USA). There were 3 biological and 3 technical replicates for each gene. The relative expression results were normalized to the level of expression of the cucumber CsActin2 gene. The relative expression level of each gene was calculated using the 2−ΔΔCT method (Livak and Schmittgen 2001). The primers used are listed in Supplemental Table S7.
Phylogenetic analysis
For phylogenetic analysis, Arabidopsis NF-YC protein sequences were downloaded from the TAIR and NCBI databases (https://www.ncbi.nlm.nih.gov/). To identify the NF-YC-related genes in cucumber, TBLASTN searches were performed in the cucumber genome database using the Arabidopsis NF-YC protein sequences as a reference. Next, MEME motif analysis (https://meme-suite.org/meme/index.html) was performed to identify the conserved motifs. Multiple sequence alignments and conserved domain analysis were performed using ClustalX, with default settings (Kohli and Bachhawat 2003). Based on the amino acid sequences, phylogenetic analysis was performed using the neighborhood joining algorithm with 1,000 repeats using MEGA 6.0 software (https://www.megasoftware.net/). The exon–intron distribution pattern was analyzed using a bioinformatics tool, Gene Structure Display Server software (http://gsds.gao-lab.org/).
VIGS assay
We adopted the TRSV-based VIGS in cucumber according to a previously developed protocol (Fang et al. 2021). Unique 200 to 300 bp CDS for each target gene derived from https://vigs.solgenomics.net/ were cloned into a pTRSV2 vector. The infected seedlings were planted in Hoagland solution and maintained for 20 d, and then the leaves were observed and recorded. The primers used are listed in Supplemental Table S7.
Cucumber transformation with CRISPR/Cas9 assay
CsTIC21 was edited using the CRISPR/Cas9 system, and the DNA targets were analyzed using CRISPR-P V2.0 software (Lei et al. 2014). Guide RNA sequences were synthesized and inserted into the pBSE402 vector (Xing et al. 2014). Agrobacterium tumefaciens strain GV3101 harboring the recombinant vector was used to transform the cucumber line PS76 according to previously described methods (Zhao et al. 2018; Xin et al. 2019). Finally, the genomic DNA was extracted from the wild-type PS76 and the putative transgenic lines, and the CsTIC21-edited fragments were amplified and sequenced to identify the positive knock-out plants. The primers used are listed in Supplemental Table S7.
Yeast 1-hybrid assay
Full-length CsNF-YC2 and CsNF-YC9 CDSs were cloned into the pGADT7 plasmid. The −1,835 to −347 sequence upstream of the translation initiation start site of CsTIC21 were cloned into the pHIS2 plasmid. Subsequently, pGADT7-CsNF-YC2/pGADT7-CsNF-YC9 and pHIS2-CsTIC21 were co-transformed into the yeast Y187 strain. After PCR clone and sequencing, the positive colony was cultured on a medium lacking Leu (leucine) and Trp (Simple Dropout/-Trp/-Leu) first and then on a medium without Trp, Leu, and His (histidine) (Simple Dropout/-Trp/-Leu/-His).
Dual-luciferase reporter assay
A 2.0-kb CsTIC21 promoter fragment was inserted into a transient expression vector, pGreenII 0800-Luc, creating a proCsTIC21: LUC reporter. Next, full-length CsNF-YC2 and CsNF-YC9 CDSs were cloned into pGreenII 62-SK, creating 2 effectors. The reporter and effectors were transformed into GV3101-pSoup, separately. Each effector and the reporter (effector:reporter = 9:1) were transiently co-infiltrated into N. benthamiana leaves, using the pGreenII 62-SK vector and proCsTIC21: LUC reporter as a negative control (Hellens et al. 2005). There were 3 biological replicates and 3 technical replicates per effector. Subsequently, the dual-luciferase reporter assay was performed, as previously described by Niu et al. (2018). The activities of firefly luciferase (LUC) and Renilla luciferase (REN) were detected using a Dual-Luciferase reporter gene detection kit (Yeasen Biotech, Shanghai, China), according to the manufacturer's instructions. The primers used are listed in Supplemental Table S7.
ChIP-qPCR assay
The ChIP-qPCR assay was performed according to a previously described protocol (Ding et al. 2015). The cucumber protoplasts transformed with pro35S: CsNF-YC2-FLAG and pro35S: CsNF-YC9-FLAG were incubated overnight at 23 °C. The incubated samples were treated with formaldehyde to crosslink the protein–DNA complexes. The crosslinked tissues were then used in ChIP experiments (Niu et al. 2018). ChIP reaction was assessed using anti-FLAG M2 affinity agarose beads (Smart-life Sciences, Changzhou, Jiangsu, China). The co-precipitated DNA samples were analyzed using RT-qPCR. The cucumber tubulin gene was used as an internal control. Three biological and 3 technical replicates were run per sample. The primers used are listed in Supplemental Table S7.
Electrophoretic mobility shift assay
Full-length CsNF-YC2 and CsNF-YC9 CDSs were fused with MBP-tag, introduced into Escherichia coli BL21, and then incubated with 0.5 mM IPTG (Isopropyl β-D-Thiogalactoside). Subsequently, CsNF-YC2-MBP and CsNF-YC9-MBP fusion proteins were purified using maltose in the EMSA assay. A probe (P3 in CsTIC21 promoter; Fig. 7F) was synthesized and labeled with the EMSA Probe Biotin Labeling Kit according to the manufacturer's protocol (Beyotime, China). The unlabeled DNA fragments were used as a competitor. EMSA assay was performed using Light-Shift Chemiluminescence EMSA Kit, according to the manufacturer's instructions (Beyotime, China). The primers used are listed in Supplemental Table S7.
Statistical analysis
All data were analyzed using Student's t-test in IBM SPSS Statistics 24 (IBM Corp., Armonk, NY, USA). Data are shown as mean ± Sd.
Accession numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers (Supplemental Tables S3, S5, and S6).
Acknowledgments
We are grateful to Prof. Xiangdong Li (Shandong Agricultural University) for kindly providing the plasmids in the VIGS assay.
Author contributions
J.S. and Z.L. planned and designed the research. X.K., Y.N., H.Z., Y.G., and H.W. performed experiments. P.S., T.Y., Y.J., B.Z., and Z.W. analyzed the data. T.W. supplied the plant materials. X.K., H.W., and Z.L. wrote the paper. All authors reviewed the manuscript.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Morphological characteristics of the wild-type and as mutant plants.
Supplemental Figure S2. The genomic-associated regions of the as mutation.
Supplemental Figure S3. Alignment of the nucleotide sequences of the CsTIC21 and Cstic21 alleles.
Supplemental Figure S4. Amino acid sequence alignment of CsTIC21 from 9 species and the mutant Cstic21.
Supplemental Figure S5. Phylogenetic analysis of CsTIC21 and its homologs.
Supplemental Figure S6. Analysis of VIGS efficiency in cucumber using CsPDS.
Supplemental Figure S7. Physical maps of the CRISPR/Cas9 vector (pBSE402) and guide RNAs.
Supplemental Figure S8. Schematic diagram of the light-responsive cis-elements in the 2-kb promoter regions of CsTIC21.
Supplemental Figure S9. Conserved motif, phylogenetic analysis, and gene structures of NF-YCs.
Supplemental Table S1. Segregation of the as trait in F1 and F2 populations of cucumber.
Supplemental Table S2. The annotated genes in the final 63.44 kb mapping genomic interval.
Supplemental Table S3. The TIC21 homologs used in the phylogenetic analysis.
Supplemental Table S4. Off-target analysis in the CsTIC21 knock-out lines.
Supplemental Table S5. The NF-YC gene family in cucumber.
Supplemental Table S6. The systematic presentation of Arabidopsis and cucumber NF-YC genes.
Supplemental Table S7. Primers used in this study.
Funding
This work was supported by grants from the National Key Research and Development Program of China (grant number 2022YFD1602000); the National Natural Science Foundation of China (grant numbers 32202519, U22A20498, 32072596); and the Science and Technology Innovation Team of Shaanxi (grant number 2021TD-32).
Data availability
All relevant data are included in the article and its supporting materials. Other relevant materials are available from the corresponding author upon reasonable request.
References
Author notes
Xubo Ke, Junjun Shen and Yuqian Niu contributed equally to this work.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://dbpia.nl.go.kr/plphys/pages/General-Instructions) are Huasen Wang and Zheng Li.
Conflict of interest statement. None declared.



