-
PDF
- Split View
-
Views
-
Cite
Cite
Jiawen Chen, A starch- and ROS-regulating heat shock protein helps maintain male fertility in heat-stressed rice plants, Plant Physiology, Volume 192, Issue 3, July 2023, Pages 2227–2229, https://doi.org/10.1093/plphys/kiad217
Close - Share Icon Share
To maintain crop yields in our warming global climate, there is an urgent need to breed crops that are resilient to high temperatures. Heat stress severely affects rice (Oryza sativa), especially its reproductive stages, and can lead to significant crop losses. Among its physiological consequences, heat stress can increase reactive oxygen species (ROS) levels (Chen et al. 2022) and decrease starch accumulation in male reproductive tissues of various plants (De Storme and Geelen 2014). In mature rice pollen, starch is needed to support pollen germination and pollen tube growth, and starchless mutants are male sterile (Lee et al. 2016). Conceivably, reduced starch levels in pollen under heat stress could lead directly to male sterility, or perhaps reduced levels of starch in response to heat stress is an indirect consequence of general pollen dysfunction at high temperatures.
Starch biosynthesis involves the orchestration of many enzymes and non-enzymatic proteins, and the mechanism of starch granule initiation is poorly understood. In nonphotosynthetic tissues such as pollen, starch is synthesized in plastids called amyloplasts. OsFLO6 (FLOURY ENDOSPERM 6) is a non-enzymatic protein with a carbohydrate binding domain that is important for proper starch biosynthesis in rice leaves, endosperm, and pollen (Peng et al. 2014; Zhang et al. 2022). The loss of OsFLO6 in pollen reduces male fertility. In Arabidopsis (Arabidopsis thaliana), an ortholog of FLO6 is needed for starch granule initiation in the leaves, possibly by its direction of glucan substrates to a glycosyltransferase enzyme (Seung et al. 2017).
Heat stress responses in plants involve myriad physiological and molecular processes, such as the induction of heat shock proteins (HSPs) that aid in protein folding and stability by acting as chaperonins. HSP60s are one of the 5 major families of HSPs, with one of the best studied HSP60s being GroEL from Escherichia coli, which functions as a large homooligomeric complex. In plants, the chloroplast HSP60s, also known as chaperonin60s (Cpn60s), have α and β subunits, likely forming heterooligomeric complexes. However, different species have different numbers of Cpn60α and Cpn60β isoforms, and the exact oligomeric assembly of these subunits is not known. In rice, 3 Cpn60α and 3 Cpn60β genes have been identified, of which OsCpn60α1, OsCpn60α2/TCD9, and OsCpn60β1 have been characterized (Kim et al. 2013; Jiang et al. 2014; Wu et al. 2020), all of which are crucial for chloroplast development.
In this issue of Plant Physiology, Lin et al. (2023) describe the previously uncharacterized gene OsHSP60-3B, which encodes a plastidial HSP and is important for pollen viability under heat stress. Using a radiation screen, the authors identified oshsp60-3b as a heat-sensitive, male sterile mutant, and its allelic identity was confirmed using map-based cloning and sequencing. As revealed by quantitative reverse transcription (qRT)-PCR and GUS staining, OsHSP60-3B is widely expressed across plant tissues. OsHSP60-3B expression increased over anther development and was induced by heat stress. However, in contrast to previously characterized OsHSP60s, the loss of OsHSP60-3B neither impacts the development of vegetative tissues nor influences the plant phenotype under ambient temperatures (22 to 28 °C). Instead, OsHSP60-3B had specific roles in regulating pollen starch synthesis and ROS under heat stress.
The oshsp60-3b mutant resembled the wild type under ambient temperatures but had pale anthers, inviable and shrunken pollen, and impaired pollen starch synthesis at high temperatures. This was seen under field conditions as well as in controlled environments. The early development of anther, tapetum, and microspore was not affected, suggesting heat stress specifically affected starch accumulation in the pollen at later stages. At pollen maturity, oshsp60-3b had smaller starch granules than the wild type at 28 °C and was missing starch entirely at 34 °C. Using a yeast two-hybrid screen for OsHSP60-3B interactors in the anther, the authors identified OsFLO6 as the only plastid-localized interaction candidate. They confirmed this interaction with yeast two-hybrid, split luciferase, and bimolecular fluorescence complementation. Also, an immunoblot showed that OsFLO6 protein abundance decreased at high temperatures in oshsp60-3b anthers compared with the wild type, supporting the role of OsHSP60-3B in maintaining starch synthesis by stabilizing OsFLO6. Also, OsHSP60-3B localized to the plastid stroma in a diffuse pattern, which resembled the known localization of OsFLO6 (Zhang et al. 2022). Based on these findings, the authors propose that OsHSP60-3B stabilizes OsFLO6 to maintain starch synthesis under high-temperature conditions (>34 °C).
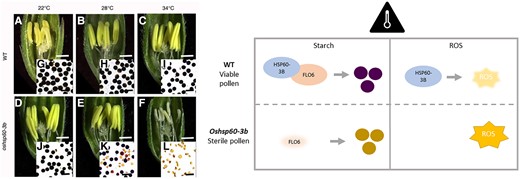
OsHSP60-3B ensures pollen viability under heat stress by regulating starch synthesis and ROS levels. Left: Rice anthers and pollen in oshsp60-3b mutants resemble wild type (WT) at ambient temperatures, but at high temperatures, anthers become pale and shrivelled and the pollen becomes inviable and loses starch accumulation. This image was taken from Lin et al. (2023). Right: Summary of the 2 functions of OsHSP6-3B under heat stress to ensure starch synthesis by stabilizing OsFLO6 and to prevent accumulation of ROS through a yet unknown mechanism.
Another interesting possibility raised by this work is that OsHSP60-3B may prevent the accumulation of ROS and cell death at high temperatures. The authors performed a comparative RNA-sequencing analysis of oshsp60-3b and the wild type at 3 different anther developmental stages at 34 °C and found 298 differentially expressed genes between the mutant and wild type common to all 3 stages. Gene Ontology analysis revealed that oxidation reduction was the most enriched biological process and oxidoreductase activity the most enriched molecular function at all 3 developmental stages. Trypan blue and 3,3′-diaminobenzidine staining confirmed that oshsp60-3b had increased H2O2 accumulation and cell death at high temperatures compared with the wild type.
The findings presented here suggest that OsHSP60-3B is a unique HSP involved in the rice male gametophyte heat stress response. Lin et al. (2023) have identified 2 pathways through which this response is regulated: starch synthesis and ROS levels (Fig. 1). Crucially, the authors also found that overexpression of OsHSP60 improves rice pollen heat tolerance. Further exploration of the molecular mechanisms could reveal the specificity of OsHSP60-3B regulation, such as whether other starch synthesis proteins are also stabilized by OsHSP60-3B, how OsHSP60-3B prevents excessive ROS accumulation, and whether there are additional OsHSP60-3B heat response pathways that have yet to be identified. Further studies should explore how the different subunits of Cpn60s function together to regulate protein folding under various environmental conditions.
References
Author notes
Conflict of interest statement. None declared.



