-
PDF
- Split View
-
Views
-
Cite
Cite
Zhen Yang, Yiwei Luo, Xiaoyu Xia, Jinzhi He, Jiajia Zhang, Qiwei Zeng, Dong Li, Bi Ma, Shaoyu Zhang, Changxin Zhai, Miao Chen, Ningjia He, Dehydrogenase MnGutB1 catalyzes 1-deoxynojirimycin biosynthesis in mulberry, Plant Physiology, Volume 192, Issue 2, June 2023, Pages 1307–1320, https://doi.org/10.1093/plphys/kiad065
Close - Share Icon Share
Abstract
As the prevalence of diabetes continues to increase, the number of individuals living with diabetes complications will reach an unprecedented magnitude. Continuous use of some synthetic agents to reduce blood glucose levels causes severe side effects, and thus, the demand for nontoxic, affordable drugs persists. Naturally occurring compounds, such as iminosugars derived from the mulberry (Morus spp.), have been shown to reduce blood glucose levels. In mulberry, 1-deoxynojirimycin (DNJ) is the predominant iminosugar. However, the mechanism underlying DNJ biosynthesis is not completely understood. Here, we showed that DNJ in mulberry is derived from sugar and catalyzed through 2-amino-2-deoxy-D-mannitol (ADM) dehydrogenase MnGutB1. Combining both targeted and nontargeted metabolite profiling methods, DNJ and its precursors ADM and nojirimycin (NJ) were quantified in mulberry samples from different tissues. Purified His-tagged MnGutB1 oxidized the hexose derivative ADM to form the 6-oxo compound DNJ. The mutant MnGutB1 D283N lost this remarkable capability. Furthermore, in contrast to virus-induced gene silencing of MnGutB1 in mulberry leaves that disrupted the biosynthesis of DNJ, overexpression of MnGutB1 in hairy roots and light-induced upregulation of MnGutB1 enhanced DNJ accumulation. Our results demonstrated that hexose derivative ADM, rather than lysine derivatives, is the precursor in DNJ biosynthesis, and it is catalyzed by MnGutB1 to form the 6-oxo compound. These results represent a breakthrough in producing DNJ and its analogs for medical use by metabolic engineering or synthetic biology.
Introduction
Diabetes is one of the fastest-growing global health emergencies of the 21st century. Approximately, 463 million people were reported to have diabetes in 2019, and this number is predicted to increase to 700 million by 2045. Type 2 diabetes accounts for the vast majority (around 90%) of diabetes worldwide (Federation 2019). Numerous antihyperglycemic drugs are currently available but many are limited by their adverse effects: these synthetic drugs are associated with a high incidence of gastrointestinal disorders, such as abdominal distension, abdominal pain, and diarrhea (Safavi et al. 2013; Qu et al. 2021). Therefore, the development of drugs to treat diabetes and to prevent associated complications is necessary and urgent (Choi et al. 2014). Plants produce a plethora of natural products that function as defensive compounds and are frequently used by humans for medicinal purposes (Nagel 2019). Some specialized plant metabolite such as montbretin A (MbA) was discovered as an improved treatment option for type 2 diabetes (Irmisch et al. 2019), the spatial and temporal patterns of metabolite accumulation limit its supply for drug development and application (Irmisch et al. 2020). Recently, Traditional Chinese Medicine Ramulus Mori (Sangzhi) alkaloid tablets (SZ-A) were approved by the China National Medical Products Administration for the treatment of type 2 diabetes (Liu et al. 2021b). SZ-A exhibited equivalent hypoglycemic effects to acarbose in patients with type 2 diabetes, but it caused fewer treatment-related adverse events and gastrointestinal disorders than acarbose. 1-deoxynojirimycin (DNJ) is the predominant iminosugar in SZ-A (Qu et al. 2021). In plants, iminosugars are widely present as secondary metabolites, and some iminosugars have previously been reported to inhibit seed germination (Stanley et al. 2011); their molecular structure resembles those of sugars, with the exception of the nitrogen atom that substitutes for the oxygen atom in the ring structure, thus giving rise to the terms “iminocyclitols,” “aza-sugars,” and “glycomimetics” to refer to these compounds (Parida et al. 2021). Due to their biological attributes, iminosugars have gained clinical importance in the treatment of type 2 diabetes (Dragutan et al. 2012).
There are many lines of evidence suggesting that α-glucosidase inhibitors could be useful for treating COVID-19 (Coronavirus disease 2019) (Williams and Goddard-Borger 2020). It is worth mentioning that the DNJ derivative N-Butyldeoxynojirimycin (NB-DNJ) leads to a marked decrease in viral proteins of SARS-CoV-2 (Severe acute respiratory syndrome coronavirus 2) and the release of infectious viruses (Rajasekharan et al. 2021). Another DNJ derivative, UV-4, also prevented SARS-CoV-2-induced death and reduced viral replication (Clarke et al. 2021). These results support the need to understand the mechanism of DNJ biosynthesis for potential applications in the medical field.
Thus far, DNJ has been found in a few plants, including mulberry (Morus spp.) (Yagi et al. 1976), Hyacinth (Hyacinthus orientalis) (Asano et al. 1998), dayflower (Commelina communis) (Kim et al. 1999), and Radix Adenophorae (Adenophora triphylla) (Asano et al. 2000). DNJ production in mulberry shows a higher abundance than other plant sources (Zhang et al. 2019; Parida et al. 2021). The DNJ content was in the range of 2.24 to 3.08 mg/g in mulberry buds, 0.62 to 1.61 mg/g in young leaves, and 0.47 to 0.96 mg/g in the mature leaves. However, the naturally available production of DNJ from mulberry is still insufficient to meet the demands of commercial manufacturing (Zhang et al. 2019). Traditionally, these bioactive compounds can be directly obtained by extraction from plant raw material or by chemical synthesis, but these processes often have poor or inconsistent yields (Pedreno and Almagro 2020). The availability of biosynthetic enzymes or variants with desired catalytic abilities from plant species can benefit metabolic engineering for natural plant products (Fossati et al. 2014; Galanie et al. 2015; Liu et al. 2017). A variety of strategies are being developed, including elicitation and metabolic engineering with single genes and transcription factors (Wilson and Roberts 2012).
For DNJ biosynthesis in mulberry plants, Wang et al. (2018a) provided a hypothesis that DNJ alkaloids are lysine-derived polyhydroxy piperidine alkaloids according to a theory of the mechanism of the phytochemical synthesis of certain alkaloids (Robinson 1917), subsequently forming rings through a series of enzymes, such as aspartic acid kinase and aspartate semialdehyde dehydrogenase, and finally generating DNJ under the action of cytochrome P450 and methyltransferase. Two short-chain dehydrogenases/reductases, MaSDR1 and MaSDR2, could catalyze the reaction of Δ1-piperideine to piperidine in vitro (Liu et al. 2020). This hypothesis of the DNJ carbon skeleton originating from amino acids was completely different from other plants in previous reports. A report on the synthesis precursor of DNJ in land plants used a 13C glucose feeding experiment in C. communis and employed 13C NMR spectroscopic analysis to determine the distribution of 13C in plant metabolites. From this experiment, the biosynthetic pathway of DNJ originating from sugar was proposed, starting with the C1 imination of the glucose molecule, followed by its reduction and oxidation to form nojirimycin (NJ), which would later undergo dehydration and reduction to yield DNJ (Shibano et al. 2004). In addition, another study revealed that increments in the concentrations of sugar in the medium were propitious to the synthesis and accumulation of DNJ in tissue culture with a mulberry seedling (Zhang et al. 2008). Different DNJ precursors were identified in plant, and thus further analysis is required to clarify the underlying mechanisms.
Plants and microorganisms have the capacity to synthesize an enormous repertoire of specialized compounds (Alseekh and Fernie 2018; Wang et al. 2019), or even produce the same functional compounds, providing scientific references for understanding the molecular mechanism of underlying biosynthesis of the same compounds (Kusari et al. 2011; Shi et al. 2012). In the microbial genus Bacillus, glucose is a precursor and NJ is an intermediate for DNJ biosynthesis, which is very similar to the results reported in land plants C. communis (Supplemental Fig. S1) (Wu et al. 2019). GutB1 was identified as a putative zinc-dependent dehydrogenase enzyme involved in DNJ synthesis in Bacillus subtilis (Clark et al. 2011), and oxidizes the preferred acyclic substrate 2-amino-2-deoxy-D-mannitol (ADM) to spontaneously cyclize to a 6-oxo compound as DNJ; GutB1 amino acid sequence alignment suggested that D283 was involved in the enzyme's specificity (Wu et al. 2014). Homology searches using the encoded sequences in microorganisms may provide insights into DNJ biosynthesis in plant species.
Considering the crucial importance of understanding the mechanism of DNJ biosynthesis, identifying the rate-limiting step provides a valuable opportunity for expanding the natural source of DNJ. The DNJ biosynthetic precursor ADM was first identified in mulberry (Morus notabilis), and integrated strategies, such as metabolite profiling analysis and homology cloning, were applied. A gene, MnGutB1, was identified and determined to be required for mulberry DNJ biosynthesis. In vitro functional analysis and in planta activity provided strong evidence that MnGutB1 is involved in DNJ biosynthesis in mulberry. The analyses of pathway precursors and end-products provided insights that the DNJ carbon skeleton originates from sugar rather than lysine in this plant species. This study provides a good model for biosynthetic research of the production of iminosugars in plant species.
Results
Identification of the precursor of DNJ biosynthesis in mulberry
DNJ of mulberry was extracted and identified using a widely targeted metabolomics approach based on high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) following Li et al. (2020b). To compare the content of DNJ and precursor in different tissues of mulberry, in the mulberry seedling, various tissues were collected for metabolite analysis, including the leaf bud, leaf, and major vein (MV) from different leaves, the stem, and callus cultured in vitro (Fig. 1A). The results showed that the DNJ content from leaves in different positions gradually decreased with the increase of leaf position (maturity). DNJ content was the highest in the bud and roots, but it was not detected in the callus. Additionally, the DNJ isomer, a metabolite identical to the mass spectrum of DNJ (Supplemental Fig. S2), was consistent with the DNJ variation regularity in different leaf positions. The results of this study showed that ADM had the highest content in callus. In contrast, the relative content of NJ in the callus was lowest compared to other tissues (Fig. 1B).
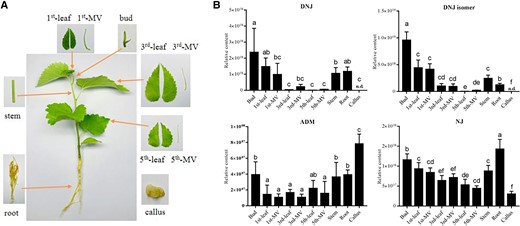
Identification of the precursor of DNJ biosynthesis. A) Mulberry tissues used for metabolite analysis. Callus cultured in vitro. B) DNJ and precursor in different tissues of mulberry. The n.d. indicates that the substance is not detected. DNJ isomer, a metabolite identical to the mass spectrum of DNJ. Data are presented as mean value and standard deviations of 3 independent biological replicates. Significance was analyzed with ANOVA (P < 0.05).
Using this technique, young leaves of H. orientalis and C. communis were collected separately from 2-mo-old greenhouse-grown seedlings. The fresh tissues were extracted by 70% methanol and analyzed by ultra-high-performance liquid chromatography-mass/mass spectrometry (UPLC-MS/MS; Supplemental Fig. S3). In addition, compounds related to ADM (m/z 182) and NJ (m/z 180) were isolated, detected, and further identified based on the UPLC-MS/MS data given for chemically synthesized ADM or literature containing NJ compound information (Wu et al. 2019).
Identification and cloning of mulberry GutB genes
Azasugar (iminosugar) biosynthesis involves a key dehydrogenase GutB1 that oxidizes ADM to the 6-oxo compound (Supplemental Fig. S1) (Clark et al. 2011; Kang et al. 2011; Wu et al. 2014). Three Bacillus amino polyol dehydrogenases GutB1 were used to screen for candidates, and a total of 13 MnGutBs were generated. The results of homology comparison with biosynthetic gene GutB1 of DNJ in microorganisms indicated that the sequence contained the same structural domain and possessed a highly conserved and important functional domain (Fig. 2A). Sequence alignments of MnGutB1 versus bacterial GutB from known DNJ producers revealed that the position of MnGutB1 302D homologous to either D or E for bacterial DNJ producers (Fig. 2B). In a recently constructed M. notabilis chromosome distribution map (Xuan et al. 2022), MnGutB1 was mapped to chromosome 3 (Chr3; Supplemental Fig. S4).
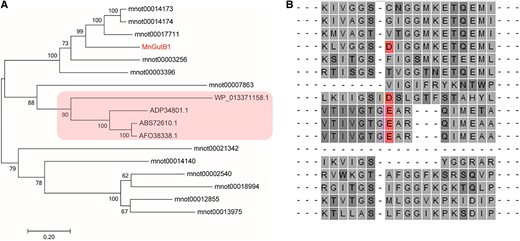
Phylogenetic relationship of GutB genes from different species. A) The phylogenetic tree was generated based on the multiple sequence alignment of the 17 GutB genes using the neighbor-joining method (bootstrap value = 1,000). The red rectangle indicates the proteins that catalyzed the formation of DNJ in B. amyloliquefaciens 140N (Genbank: AFO38338.1), B. amyloliquefaciens FZB42 (Genbank: ABS72610.1), B. atrophaeus 1942 (Genbank: ADP34801.1), and Paenibacillus polymyxa SC2 (Genbank: WP_013371158.1). B) The CLUSTAL sequence alignment presents putative aminopolyol dehydrogenases from DNJ producers and mulberry. The red position indicates that D or E residues from known or putative DNJ producers provide charge recognition with the positively charged ammonium group of an amino polyol substrate. The dashes indicated amino acid residues near the conserved domains.
Prokaryotic expression and functional analysis of MnGutBs
To identify catalytic activity, we prepared an ADM mixture solution for enzyme activity. N-terminal His-tagged MnGutBs were produced in Escherichia coli and then purified for enzymatic assays. In sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), the bands of purified proteins were consistent with their predicted molecular weight (∼99–120 kDa; Supplemental Fig. S5). Only mnot0000786 did not show protein expression, which was confirmed as a pseudogene by transcriptome data. Similarly, the expression construct of the D283N point mutation in MnGutB1 was transformed into E. coli, and recombinant MnGutB1 D283N was purified for enzymatic assays. When MnGutB proteins were provided to the enzymatic reaction system, only MnGutB1 catalyzed the formation of minute quantities of NJ in vitro, as indicated by HPLC-MS/MS (Supplemental Fig. S6A and S6D). The negative control (tag protein) and mutation MnGutB1 D283N did not produce NJ (Supplemental Fig. S6B and S6C). NJ kinetics were not analyzed due to the lack of commercially available ADM. Moreover, it is hard to confirm the roles of MnGutB1 in the formation of NJ to DNJ. Due to low purity, not enough NJ could be isolated for downstream enzymatic assays. We tried to detect the presence of DNJ in the MnGutB1 enzyme activity reaction with ADM as a substrate. Notably, a substance of high abundance was detected at a retention time of 1.30 min with the same mass spectrum consistent with the DNJ standard (Fig. 3). With the increase of substrate concentration, the yield of NADH increased gradually (Fig. 3C).
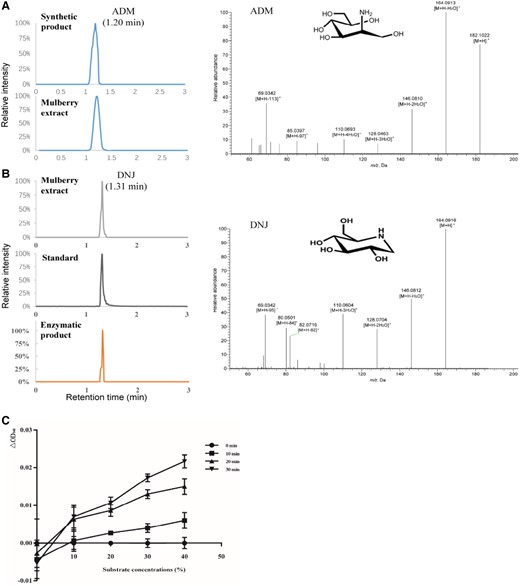
Enzyme activity assay of MnGutB1 in vitro. A) HPLC-MS/MS analyses of ADM and B) DNJ. Elution profile of ADM in the synthetic product and mulberry extract. Chromatograms of DNJ in the mulberry extract, standards, and enzymatic product are shown on the left. The chromatograms displayed in A) and B) are the intercepted segment of the first 3 min of the total chromatogram. Extracted fragment mass chromatograms of ADM and DNJ are shown on the right. C) Effect of substrate concentration on the activity of the enzyme. MnGutB1 enzymatic activity was measured in monitoring the NADH formation at OD340nm in the reaction system. Absorbance was recorded at 340 nm every 10 min in 3 parallel wells. Data represent mean ± SD (n = 3).
Expression of MnGutB1 in mulberry
The expression profiles of MnGutB1 in various tissues, namely the leaf bud, leaf, MV, stem, root, and callus, were investigated. As shown in Fig. 4B, MnGutB1 expression gradually decreased from top to bottom in different leaf positions, consistent with the DNJ content in mulberry leaves picked from upper leaves and their purified samples being higher than those from lower leaves in a previous report (Kimura et al. 2007). Roots have been reported to contain isolated DNJ (Yagi et al. 1976). The expression level of MnGutB1 was lower than that in young leaves, and the expression of MnGutB1 in the stem was also low. Interestingly, DNJ was not detected in mulberry callus cultured in vitro (Fig. 1B), and MnGutB1 was not expressed in callus. As presented in Supplemental Table S1, the MnGutB1 expression level was significantly positively correlated with the content of DNJ (R = 0.77, P < 0.05), DNJ isomer (R = 0.80, P < 0.05), and DNJ-O-hexoside (R = 0.85, P < 0.05).
Previous research clarified that MaSDR1 and MaSDR2 could catalyze 1-piperideine to generate piperidine in the proposed pathway from lysine to DNJ biosynthesis in mulberry leaves (Morus alba L.; Fig. 4), and expression levels of MaSDR1 and MaSDR2 were significantly and positively correlated with the content of DNJ (P < 0.05) in different seasons (Liu et al. 2020). We searched the homologous genes of MaSDR1 and MaSDR2 in M. notabilis, which were named MnSDR1 and MnSDR2, respectively. As shown in Fig. 4B, the expression level of MnSDR1 was the highest in the 3rd leaf, and very low in the roots. MnSDR1 expression was gradually decreased from top to bottom in different MV positions, and its expression level was not correlated with the content of DNJ (R = −0.03, P < 0.05, Supplemental Table S1). MnSDR2 was highly expressed in the 1st leaf and was also very low in the roots. There was no significant difference in the expression of MnSDR2 in the bud and main vein positions; its expression level was weakly correlated with the content of DNJ (R = 0.56, P < 0.05, Supplemental Table S1).

Tissue-specific expression of MnGutB1 in the mulberry plant. A) Proposed pathway of DNJ biosynthesis in mulberry. The blue dashed box represents the DNJ biosynthesis process derived from lysine; the red dashed box represents derived from hexose. B) Relative expression of MnSDR1, MnSDR2, and MnGutB1 in the tissues indicated in Fig. 1A, as quantified by qPCR. The relative expression in the bud was set as 1. MnRPL15 was the internal control. Callus cultured in vitro. The qPCR data are presented as mean value and standard deviations of 3 independent biological replicates. Significance was analyzed with ANOVA (P < 0.05).
DNJ is synthesized by MnGutB1 in the roots and leaves of mulberry
To further confirm the synthetic tissues of DNJ, an efficient Agrobacterium rhizogenes-mediated in vivo root transgenic system in mulberry was established, as described previously (Liu et al. 2021a). The promoter regions of the MnGutB1 gene were fused to the iGUS (β-glucuronidase gene with a cat1 intron) as reporters, with CaMV 35S::iGUS as effector constructs (Supplemental Fig. S7A). After GUS staining in transgenic mulberry hairy roots, the results of histochemical staining showed that the A. rhizogenes-mediated induction of transgenic roots exhibited obvious blue staining. The CaMV 35S promoter was used as a positive control and was also visibly blue in the transgenic hairy roots (Supplemental Fig. S7B).
Due to the lack of an efficient in vivo transgenic system for leaves in mulberry, a system for mulberry protoplast isolation (Supplemental Fig. S8) and transient expression was established to rapidly determine MnGutB1 localization in mulberry leaves. MnGutB1 fusion with enhanced green fluorescence protein (EGFP) was derived by the CaMV 35S promoter (Supplemental Fig. S7C). The MnGutB1 fusion protein produced green fluorescence in the chloroplast region, where chlorophyll exhibited red autofluorescence irradiated with 488 nm excitation light. The 35S::EGFP was used as a positive control protein and was detected in the nucleus and cytoplasm in mulberry protoplasts (Supplemental Fig. S7D).
MnGutB1 overexpression in mulberry roots enhances DNJ production
To further confirm the function of MnGutB1, overexpression of MnGutB1 was performed in the hairy root system. A vector of the MnGutB1 gene, pZYGC, that was fused to EGFP was constructed, and then the vector was transferred into A. rhizogenes K599 as described above. After 8 wk, transgenic roots with bright green fluorescence were obtained (Fig. 5A). Relative expression levels of MnGutB1 in transgenic root lines were significantly higher than that of the control line (Fig. 5B). When MnGutB1 was overexpressed in hairy roots, the DNJ level was approximately 1- to 2-fold higher than that of wild-type roots (Fig. 5C). Interestingly, the content of DNJ isomers also increased simultaneously (Fig. 5D). These results thus show that MnGutB1 overexpression enhances DNJ production in mulberry roots.
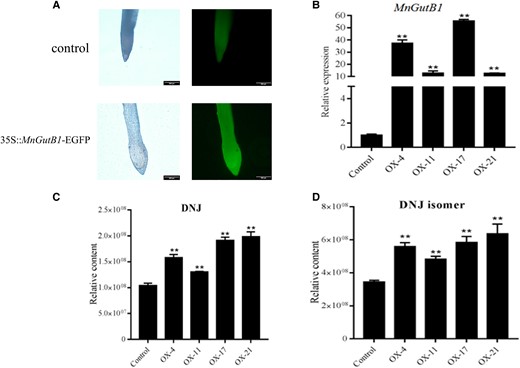
Overexpression of MnGutB1 in mulberry hairy roots promotes DNJ accumulation. A) Fluorescence microscopy detection of MnGutB1 in mulberry hairy roots induced by wild-type Agrobacterium rhizogenes K599 (control) and K599 containing the 35S::MnGutB1-EGFP vector. B) Relative expression of MnGutB1 in overexpression (OX) hairy root lines. C) Relative content of DNJ and D) its isomer in overexpression (OX) hairy root lines. In B), C), and D), metabolite data for each biological line are the mean ± SD of quantification made in 3 replicates. **P < 0.01 (as determined by a Student's t-test).
Virus-induced gene silencing of MnGutB1 impacts the DNJ content in mulberry leaves
Because of the high abundance of DNJ and the high expression levels of MnGutB1 in leaves (Fig. 1B and Fig. 4B), virus-induced gene silencing (VIGS) of MnGutB1 (Fig. 6A and 6B) in mulberry was conducted using the method previously reported by Xin et al. (2021) to explore how MnGutB1 influences DNJ accumulation in mulberry leaves. Phytoene desaturase (PDS) was used as a positive control. The colors of plants injected with MMDaV + 2mDNA1-MnPDS at 30 d after VIGS treatment were substantially paler than those of plants injected with the control construct MMDaV + 2mDNA1 (Supplemental Fig. S9). Twelve independent MnGutB1 gene-silenced lines were obtained (Fig. 6C) and then the leaves of 3 lines and control mulberry lines were collected and extracted by 70% methanol and analyzed by UPLC-MS/MS. Significant reductions in the transcript level of MnGutB1 gene-silenced lines i17, i18, and i26 were observed. DNJ quantitative analysis showed that, compared with the control, decreases in MnGutB1 expression led to the decline of DNJ and isomer content in the leaves of gene-silenced lines (Fig. 6D).
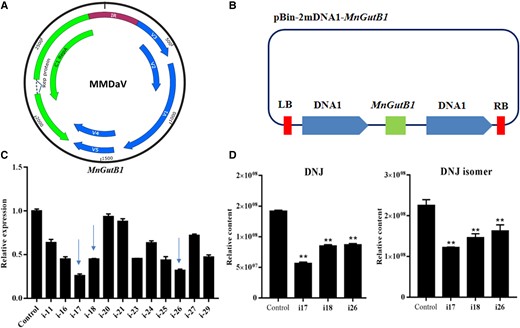
Effects of MnGutB1 suppression on the biosynthesis of DNJ in mulberry leaves. A) Genome organization of Mulberry mosaic dwarf-associated geminivirus (MMDaV). B) Schematic diagram of the vector. C) Expression of MnGutB1 in RNAi (i) mulberry lines. D) Relative content levels of DNJ and its isomer in RNAi (i) mulberry lines. Metabolite data for each biological line were assayed in triplicate; metabolite data for each biological line are the mean ± SD of quantification made in 3 replicates. **P < 0.01 (as determined by a Student's t-test).
MnGutB1 expression in mulberry is stimulated by light conditions
It has been shown that the DNJ content in mulberry leaves could significantly increase under longer light conditions (Li et al. 2014b). To verify whether MnGutB1 expression is regulated by light, the cis-acting elements of the MnGutB1 promoter were analyzed and predicted using PlantCare databases (Lescot et al. 2002). The results showed that the promoter contained multiple cis-acting elements associated with light responsiveness (Fig. 7A). Then, 2 kinds of mulberry cultivars, Guiyou12 and Chuisang, were grown in an incubation room treated with 16-h light condition per photoperiod (L) and compared with a 5-h photoperiod (S). MnGutB1 expression was only significantly affected after 3 photoperiods. MnGutB1 expression increased significantly when the length of the photoperiod was extended (Fig. 7B), although mulberry growth morphology was not obviously affected in such a short time. Furthermore, the detection of MnSDR1 and MnSDR2 under the above light conditions showed that a longer photoperiod suppressed expression of MnSDR1 and MnSDR2 (Fig. 7B). These results indicate that MnGutB1 was induced by the response to light by enhancing DNJ production; MnSDR1 and MnSDR2 did not play active roles in DNJ biosynthesis induced by light.
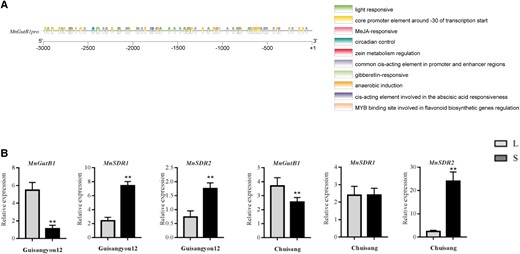
Expression of MnGutB1 in mulberry leaves was regulated by light duration. A) Transcriptional regulatory elements upstream of the MnGutB1 gene. B) Relative expression of MnGutB1, MnSDR1, and MnSDR2 in 2 kinds of mulberry resources grown in an incubation room treated with a 16-h photoperiod (L) compared with a 5-h photoperiod (S). The qPCR data are the mean ± SD of quantification made in 3 independent biological replicates. *P < 0.05 (Student's t-test).
Discussion
Improvement of DNJ production by substrate optimization suggests the same precursor in plants and microorganisms
The core primary metabolism of most plant species is highly similar to that of non-plant species (Wang et al. 2019). In some cases, the plant can metabolize products from the microorganisms and vice versa; these possibilities can concern only one, several, or all enzymatic steps for biochemical transformation (Ludwig-Muller 2015). DNJ was identified and isolated from the mulberry plant in 1976 (Yagi et al. 1976) and was then isolated from other plants, namely H. orientalis (Asano et al. 1998), C. communis (Kim et al. 1999), and A. triphylla (Asano et al. 2000) as well as microorganisms, including Streptomyces subrutilus (Hardick et al. 1992) and B. subtilis (Hardick and Hutchinson 1993). The carbon skeleton of DNJ in a biosynthetic pathway was discovered to have a similar sugar precursor in microorganisms and land plants C. communis, even though the position of the amino group in the amino polyol precursor is different. Glucose and fructose are common carbon skeletons for DNJ synthesis (Shibano et al. 2004). Increments of fructose concentrations in the medium were propitious to the synthesis and accumulation of DNJ in sterile mulberry seedlings (Zhang et al. 2008). These results suggest that hexose is the same precursor of DNJ in plants and microorganisms. In the in vitro system and under the conditions used, MnGutB1 catalyzes NJ and DNJ formation using ADM (Fig. 3 and Supplemental Fig. S6), a hexose derivative, that is accumulated in leaves and roots (Fig. 1B). With respect to DNJ precursors, these results provided direct evidence for the existence of ADM in sterile mulberry seedlings and for the importance of these compounds, especially ADM, in DNJ biosynthesis. In addition, sterile mulberry seedlings can independently synthesize DNJ without the need for endophytes to provide the substrate ADM.
DNJ absence in callus is due to repression of MnGutB1 expression
Several researchers have reported that the DNJ level is high in the upper leaves; these results demonstrate that the younger the leaf, the higher the DNJ concentration, suggesting the possibility that the young leaves had high enzymatic activity (Vichasilp et al. 2012; Hu et al. 2013; Sugiyama et al. 2016). However, the stem tissue specificity of DNJ biosynthesis-related enzymes remains largely unknown. Our study revealed DNJ synthesis and accumulation in photosynthetic tissues (leaves) and, to a certain extent, in some non-photosynthetic tissues (roots) (Fig. 1B). When comparing gene expression levels in different leaf positions and roots, MnGutB1 was significantly more expressed in younger leaves and roots. DNJ was isolated from the extract of mulberry root bark (Yagi et al. 1976). However, none of the metabolites isolated from the root bark were found in the callus (Ferrari et al. 1999), this may be related to the tissue specificity of mulberry metabolite synthesis genes. For example, the mulberry resveratrol gene is almost undetectable in young leaves, resulting in an extremely low resveratrol content in young leaves (Wang et al. 2017). In contrast to leaves and roots, DNJ was not detected in the callus, but ADM accumulated to the highest degree (Fig. 1B). Interestingly, the MnGutB1 gene showed a significantly low degree of expression levels in the callus (Fig. 4B). The expression of MnGutB1 was shown to be highly correlated with the content of DNJ and its analogs (isomer and DNJ-O-hexoside) in various mulberry tissues (Supplemental Table S1). Differences in the DNJ content of different stem tissues suggest that mulberry leaves have the potential for biotechnological applications. MnGutB1 plays a key role in DNJ biosynthesis and may explain the tissue-specific accumulation of DNJ. In particular, the expression of MnGutB1, a downstream gene of biosynthesis, was inhibited, resulting in no DNJ in the callus.
Functional analysis of a rate-limiting enzyme provides a basis for improving the source of medicinal DNJ
Plants are integral to human well-being. In addition to their obvious nutritional value, for centuries, they have also been used for their healing properties (Moses and Goossens 2017). Iminosugars have been considered key contributors to the purported health benefits of mulberry leaves. Most of the available literature on mulberry leaf iminosugars has focused on DNJ and its analogs (Parida et al. 2021). The safety and efficacy of DNJ in improving the blood glucose profile have also been frequently assessed in short- and long-term human trials. Kimura et al. (2007) reported the effectiveness of DNJ-enriched mulberry leaf powder in suppressing the sucrose-induced postprandial physiological glucose (PPG) level and lowering insulin secretion following a single oral dose. Hence, the DNJ content in mulberry leaves serves as an important indicator of quality. In our hypothetical DNJ biosynthetic pathway in mulberry (Fig. 4A), partially modifying that proposed by Shibano et al. (2004), ADM, which originated from the hexose derivative, undergoes carbon skeleton cyclization catalyzed by MnGutB1 to synthesize NJ and DNJ. We detected DNJ in the in vitro enzyme activity products, and overexpression or downregulation of MnGutB1 caused the DNJ content to change in the same trend (Figs. 5 and 6). NJ may undergo dehydration and reduction to yield DNJ (Inouye et al. 1968; Zhang et al. 2019). This hypothesis differs from that proposed by Wang et al. (2018b). In the DNJ biosynthesis hypothesis, with lysine as the precursor, the piperidine ring-forming reaction occurs by 5-aminopenttanal self-cyclization. There is a lack of evidence that piperidine is directly related to the process of DNJ biosynthesis. The expression levels of the piperidine synthase genes (MnSDR1 and MnSDR2) were not significantly or positively correlated with the content of DNJ in different mulberry tissues (Supplemental Table S1).
To further improve the yield of DNJ from mulberry leaves, strategies to optimize cultivation, harvesting, and post-harvest processing conditions have been proposed, but their effect on DNJ appeared to be limited (Zhang et al. 2019; Parida et al. 2021). Variations in the activity of DNJ biosynthetic enzymes or precursor molecules across different regions of the mulberry plant may be the underlying cause for the discrepancy in DNJ content (Parida et al. 2021). A previous study demonstrated that the DNJ content in mulberry leaves could significantly increase under longer light conditions (Li et al. 2014b); however, the mechanism underlying this response remains unclear. We found that MnGutB1 expression was significantly stimulated when the photoperiod was extended (Fig. 7B). Our results further prompted us to continue using the gene as a reporter to gain deeper knowledge about the light-induced mechanism that promotes DNJ production throughout the mulberry plant.
In summary, we identified MnGutB1, a gene required for mulberry DNJ biosynthesis. MnGutB1 can be used in molecular-assisted selection and provide a theoretical basis for the biofortification of DNJ in mulberry by breeding. This study provides valuable genes for producing DNJ through synthetic biology or metabolic engineering.
Materials and methods
Plant materials and growth conditions
Black mulberry (Morus nigra), Chuansang (M. notabilis), Hyacinth (Hyacinthus orientalis), and dayflower (Commelina communis) plants were grown in a greenhouse or a growth chamber under a 12-h light/12-h dark cycle and 75% humidity at 23 °C to 25 °C at the Mulberry Germplasm Nursery in Southwest University, Chongqing. The mulberry resources Guiyou12 and Chuisang and transgenic lines were grown in an incubation room and treated with a 16-h (L) or 5-h photoperiod (S). These plants were further cultured for metabolic analysis, qPCR, and gene cloning.
Metabolite extraction
Mulberry, H. orientalis, and C. communis tissues were ground to a fine powder in liquid nitrogen and used to obtain the dried plant material. A 30-min ultrasonic extraction procedure with 75% v/v methanol was then applied to isolate potential metabolites from dried plant material powder at 4 °C. After filtration through a 0.22-μm membrane filter, the solution was analyzed using a liquid chromatography-electrospray ionization tandem mass spectrometry system.
UHPLC-MS/MS system and analytical conditions
The extracts were analyzed using an LC-ESI-MS/MS system (UHPLC, Thermo Scientific Dionex UltiMate 3000; MS, Q Exactive hybrid quadrupole-orbitrap mass spectrometer; Thermo Fisher Scientific, Waltham, MA, USA). The UHPLC conditions were as follows: column, Acquity UPLC BEH C18 (1.7 μm particle size, length 2.1 × 150 mm) (Waters, Milford, MA, USA); solvent system, mobile phase A: water (0.04% acetic acid), mobile phase B: acetonitrile (0.04% acetic acid); gradient program, 95:5 VA/VB at 0 min, 5:95 VA/VB at 20.0 min, 5:95 VA/VB at 22.0 min, 95:5 VA/VB at 22.1 min, 95:5 VA/VB at 26.0 min; flow rate, 0.25 ml/min; column temperature, 40 °C; injection volume, 5 μl. The effluent was alternatively connected to a Q Exactive MS, which was operated in the positive ion mode and controlled by Thermo Scientific Xcalibur software v. 2.2. The analytical MS conditions were as follows: ESI source operation parameters: sheath gas, 35 arbitrary units; auxiliary gas, 10 arbitrary units; sweep gas, 0 arbitrary units; spray voltage, 3.5 kV; capillary temperature, 350 °C; and S-lens RF level, 50. The full MS parameters were as follows: MS scan range, 100–1,000 m/z; resolution, 70,000; microscans, 1; automatic gain control (AGC) target, 1 × 106; Max IT, 200 ms. The data-dependent MS2 (dd-MS2) quantification method parameters were as follows: resolution, 17,500; microscans, 1; AGC target, 2 × 104; Max IT, 100 ms; loop count, 5; topN, 5; isolation window, 1.0 m/z; (N)CE/stepped (N)CE, nce: 15, 30, 60; apex-trigger, 2–6 s. Instrument tuning and mass calibration were performed with Pierce LTQ Velos ESI positive ion calibration solution (Pierce, Rockford, IL, USA) (Li et al. 2020a). Metabolite identification was based on the accurate m/z, retention time (RT), and fragmentation patterns. Quantitative calculations were conducted using Thermo Scientific Xcalibur software v. 2.2. Each metabolite was quantified with accurate mass tolerance (units, 20 ppm) and precision (decimals, 0.0001) (Li et al. 2020b).
Bioinformatics analysis
Three predicted GutB1 sequences from Bacillus amyloliquefaciens 140N (Genbank: AFO38338.1), Bacillus amyloliquefaciens FZB42 (Genbank: ABS72610.1), and Bacillus atrophaeus 1942 (Genbank: ADP34801.1) were used to screen for candidates using the HMMER (Hidden Markov models) search against the M. notabilis genome database (http://morus.swu.edu.cn/morusdb/) (Seo et al. 2013; Li et al. 2014a). The expected value was 1e−9. Gene sequences of MaSDR1 (GenBank: MT989445) and MaSDR2 (GenBank: MT989446) were used to screen homologous genes in the M. notabilis genome database (Li et al. 2014a). Amino acid sequences, obtained from NCBI and other public databases, were aligned and the neighbor-joining trees were built using MEGA7 (Molecular Evolutionary Genetics Analysis version 7.0) with the following parameters: at least 1,000 bootstrap replications, Poisson model, uniform rates, and complete deletion. The 3,000-bp sequence upstream of MnGutB1 was extracted by TBtools software and submitted to the PlantCARE online database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) to predict the cis-elements in the promoter region (Lescot et al. 2002). TBtools software was used to draw the cis-elements of genes (Chen et al. 2020).
Protein expression constructs, purification, and enzymatic assays
Expression constructs for GutB1 and its homologs in Morus notabilis were in pMD19-T (Invitrogen), flanking the start and stop codons. The genes were subcloned into the pCold-TF vector for expression. Expression constructs of the MnGutB1 302N mutant were created using the pEASY-Basic Seamless Cloning and Assembly Kit (TransGen). The recombinant construct was confirmed by sequencing. The expression constructs were transformed into BL21 (DE3) E. coli. Expression of GutBs was induced by the addition of 0.5 mM IPTG when an optical density 600 nm (OD600) of 0.4 to 0.8 was reached. After 24 h of protein induction, the cells were harvested by centrifugation at 4 °C at 12,000 × g for 5 min. The cell pellets were each resuspended in lysis buffer (20 mM Tris-HCl, pH = 8.0, 250 mM NaCl, 5 mM imidazole). After lysis by sonication, the lysate was centrifuged at 12,000 × g for 10 min at 4 °C. The clarified lysate was applied to Ni-NTA 6FF Sefinose Resin Kit (Sangon Biotech), and the column was washed with a step gradient of 10, 50, and 100 mM imidazole. GutB proteins were eluted with 250 mM imidazole-containing buffer (20 mM Tris-HCl, pH = 8.0, 250 mM NaCl, 250 mM imidazole). The eluted enzyme was dialyzed at 4 °C against 20 mM Tris buffer (50 mM NaCl, pH = 7.0). The enzymatic reaction mixture (200 ml) of MnGutB1 and another 13 MnGutB genes contained 100 mM Tris-HCl buffer (pH 8.5), 25 mM NaCl, 0.25 mM ZnCl2, 2 mM NAD+, 1 mg purified GutB1, and the MnGutB enzyme; ADM was used as the substrate. This substrate was synthesized according to previous reports (Wu et al. 2014), with some modifications: the NaBH4 (572 mg in 5 ml of 0.05 M NaOH) catalyzed the reduction of D-mannosamine (300 mg) to ADM at room temperature for 4 h. Instead of acetic acid, phosphoric acid was added to destroy the residual NaBH4 in the present study. Methanol was added to the above reaction mixture followed by rotary evaporation. This evaporation process was repeated for 4 times. The resultant product was dissolved directly in 3.5 ml water and the pH was adjusted to 8.5 using phosphoric acid for enzyme activity assay. The reactions were initiated by the addition of NAD+ and incubated at 30 °C for 30 min (Wu et al. 2014). The concentration of ADM was adjusted to about 0 to 100 mM. All reactions were initiated by the addition of 2 mM NAD+ and measured in triplicate. The enzymatic solution was filtered through a 0.45-μm syringe filter and analyzed by a liquid chromatography-electrospray ionization tandem mass spectrometry system in positive ion mode (Ultra High-performance liquid chromatography, Thermo Scientific Dionex UltiMate 3000; MS, Q Exactive hybrid quadrupole-Orbitrap mass spectrometer; Thermo Fisher Scientific, Waltham, MA, USA). The sequence primers are listed in Supplemental Table S2.
RNA isolation and qPCR
CTAB-pBIOZOL reagent (Bioer, Hangzhou, China) was used to purify the total RNA from plant tissues in accordance with the manufacturer's instructions. The mRNA was purified using magnetic beads with attached oligo(dT)s. First-strand complementary DNA (cDNA) was synthesized using a PrimeScript RT reagent Kit with gDNA Eraser (Perfect Real Time) (Takara), followed by second-strand cDNA synthesis. PCR was performed using an SYBR Green PCR Master Mix on the Step One and Step OnePlus real-time PCR system (Applied Biosystems). The mulberry ribosomal protein L15 (RPL15) gene was used as a control to normalize the relative expression of target genes (Qi et al. 2014). In all experiments, 3 technical replicates were performed. Relative gene expression was performed using the comparative 2−△△Ct method.
VIGS and transient overexpression
VIGS was performed using the method of our laboratory (Xin et al. 2021), with minor changes. Fragments of MnGutB1 (300 bp) and MnPDS (188 bp) were PCR amplified from M. notabilis cDNA and cloned independently into the 2mDNA1 vector to generate the plasmids 2mDNA1-MnGutB1 and 2mDNA1-MnPDS, respectively. Mulberry mosaic dwarf-associated virus (MMDaV) was the auxiliary plasmid. MMDaV, 2mDNA1, 2mDNA1-MnGutB1, and 2mDNA1-MnPDS were introduced independently into Agrobacterium tumefaciens strain GV3101 (Weidi). MMDaV and 2mDNA1 or their derivatives were mixed at 1:1 (v/v) ratios and infiltrated into the cotyledons of 1-wk-old mulberry seedlings using a 1-ml syringe without a needle. After 24 h in the dark, the mulberry seedlings were transferred to a greenhouse. At 30 d post agroinfiltration, the second true leaf was analyzed by qPCR.
The full-length MnGutB1 coding sequence was cloned into the pZYGC vector. Then, pZYGC and pZYGC-MnGutB1 were introduced independently into A. rhizogenes K599 (Weidi), which was then injected into the junction of true leaves and cotyledons as described previously (Meng et al. 2019; Liu et al. 2021a). After growing in soil for 8 wk, the roots showing green fluorescence in mulberry seedlings were collected, washed, and analyzed. Mulberry protoplast isolation and polyethylene glycol (PEG)-mediated transformation were performed using the method according to a previous report (Umate 2010). After 48 h, fluorescence was detected with an Olympus IX73 fluorescence microscope. Excitation and emission wavelengths for GFP were 488/510–540 nm, and for capturing chlorophyll autofluorescence were 544/660–690 nm.
Statistical analyses
The numbers of samples and replicates are indicated in the figure legends. Statistically significant differences between control and experimental groups were determined by 1-way ANOVA (Student's t-test; P < 0.05).
Accession numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers. Three predicted GutB1 sequences from Bacillus amyloliquefaciens 140N (AFO38338.1), Bacillus amyloliquefaciens FZB42 (ABS72610.1), Bacillus atrophaeus 1942 (ADP34801.1), MaSDR1 (MT989445), MaSDR2 (MT989446), and MnGutB1 (XM_024173098.1).
Acknowledgments
We would like to thank the analytical and testing facility in Chongqing Engineering Research Center for Rapeseed, College of Agronomy and Biotechnology, and Academy of Agricultural Sciences, Southwest University, for their assistance with the HPLC-MS/MS analysis. We also thank Dr. Nengwen Yin for providing technical support.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Proposed biosynthetic route for the production of 1-deoxynojirimycin (DNJ).
Supplemental Figure S2. Ultra-high-performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) analysis of 1-deoxynojirimycin (DNJ) and its isomers in mulberry leaves.
Supplemental Figure S3. HPLC chromatograms of DNJ identified in different plants.
Supplemental Figure S4. Chromosomal distribution map of MnGutB genes in Morus notabilis.
Supplemental Figure S5. SDS-PAGE of recombinant protein expression.
Supplemental Figure S6. Products of MnGutB1 and MnGutB1 D302N enzymatic reactions detected by UPLC-MS analysis.
Supplemental Figure S7. MnGutB1 localization in mulberry hairy roots and mesophyll protoplasts.
Supplemental Figure S8. Fluorescence microscopy detection of Morus nigra callus and mesophyll protoplasts.
Supplemental Figure S9. PDS silencing phenotypes in mulberry leaves.
Supplemental Table S1. Correlation between expression levels of MnGutB1 and DNJ analog content in different tissues in Fig. 5.
Supplemental Table S2. Primer sequences of GutBs.
Funding
This project was funded by the National Key Research and Development Program (No. 2022YFD1201602), the Chongqing Research Program of Basic Research and Frontier Technology (cstc2021yszx-jcyj0004), and Fundamental Research Funds for the Central Universities (SWU120028).
References
Sun H, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ.
Author notes
Z.Y. and N.H. conceived and designed the research. Z.Y. conducted experiments. Y.L., X.X., J.H., J.Z., Q.Z., S.Z., C.Z., and M.C. provided technical assistance to Z.Y. in the transgenic experiments. B.M. and D.L. analyzed the data. Z.Y. wrote the paper, and N.H. revised the manuscript. All authors read and approved the manuscript.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://dbpia.nl.go.kr/plphys/pages/General-Instructions) is Ningjia He ([email protected]).
Conflict of interest statement. The authors declare that there is no conflict of interest.



