-
PDF
- Split View
-
Views
-
Cite
Cite
Priya Ramakrishna, Igor Cesarino, Loosen up! How lignin manipulations affect biomass molecular assembly and deconstruction, Plant Physiology, Volume 191, Issue 1, January 2023, Pages 3–5, https://doi.org/10.1093/plphys/kiac503
Close - Share Icon Share
In a bio-based economy, fossil resources are replaced by plant biomass as a renewable feedstock to produce high-value-added products, including chemicals, biofuels, and advanced materials (Ning et al., 2021). Plant biomass has a rich diversity in cell wall chemistry and is composed of an array of polysaccharides that form a rich source of sugars for fermentation to bioproducts and the phenolic polymer lignin (de Vries et al., 2021). Lignin makes plant biomass recalcitrant, hampering biomass deconstruction by blocking polysaccharide hydrolysis (Vermaas et al., 2015). Lignin can also be a potential treasure trove of high-value aromatics upon depolymerization (Lin and Eudes, 2020). The genetic manipulation of lignin content and structure holds promise to tailor plants for optimized biomass utilization in both polysaccharide- and lignin-oriented contexts.
Lignin derives from the phenylpropanoid pathway (Vanholme et al., 2019; Figure 1A). Misregulation of lignin biosynthetic genes frequently results in altered lignin content and composition, allowing the production of plants with improved biomass characteristics (Saleme et al., 2017; Umezawa, 2018; Halpin, 2019; Mahon and Mansfield, 2019). Two of the most representative bioengineering targets for improving lignin characteristics are the lignin biosynthetic genes 5-HYDROXYCONIFERALDEHYDE O-METHYLTRANSFERASE (CAldOMT) and CINNAMYL ALCOHOL DEHYDROGENASE (CAD). CAldOMT is a bifunctional enzyme that catalyzes O-methylation reactions in the syringyl (S) lignin-specific pathway and in the parallel flavonoid pathway dedicated to the production of tricin (T), a lignin monomer in grasses (Lan et al., 2016). Decreased CAldOMT activity leads to concomitant decreases of S and T units in lignin polymers and to the incorporation of the atypical 5-hydroxyguaiacyl (5H) units (Fornalé et al., 2017; Lam et al., 2019; Figure 1A). CAD catalyzes the reduction of hydroxycinnamaldehydes into their corresponding monolignols, and its downregulation leads to the incorporation of its aldehyde substrates into the polymer (Anderson et al., 2015; Ferreira et al., 2022; Figure 1A). Targeting both genes often leads to improved biomass deconstruction characteristics. However, the mechanisms underlying the effects of altered lignin chemistry on the supramolecular assembly of plant biomass and, consequently, its deconstruction are not completely understood.
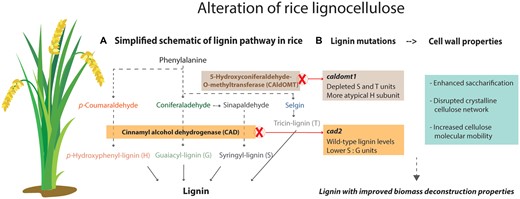
Lignin manipulation strategies to improve plant biomass deconstruction characteristics. A, Simplified scheme of the phenylpropanoid pathway for lignin production in rice. B, Lignin mutants show enhanced saccharification efficiency and alterations in the supramolecular structure of cell wall. caldomt1 shows depletion of S and T units and augmentation of 5H units; cad2 mutants show reduced incorporation of canonical monolignols and augmentation of hydroxycinnamaldehydes units into the lignin polymer. Both mutants show disruption to their crystalline cellulose network. caldomt1 in particular shows a prominent increase in cellulose molecular mobility suggestive of a loosened lignocellulose. Both strategies result in improved biomass processability. Figure created with Illustrator.
In this issue of Plant Physiology, Martin et al. report on a comparative and integrative analysis of cell wall chemical structures, supramolecular structures, and digestibility in CAldOMT- and CAD-deficient rice (Oryza sativa) plants to better understand the relationship between lignin modifications and improvement in biomass processing. The authors used a series of rice mutant lines deficient in either or both OsCAldOMT1 and OsCAD2, the major CAldOMT and CAD genes responsible for cell wall lignification in rice (Lam et al., 2019; Martin et al., 2019). Chemical analyses confirmed the cell wall chemotypes previously reported for the caldomt1 and cad2 single-knockout lines: the former showed lower lignin content and polymers heavily depleted in S and T units but augmented with the atypical 5H units, whereas the latter showed wild-type (WT) lignin levels and a lower ratio of S to guaiacyl (G) units (Figure 1B). Cell wall polysaccharide profiles, based on neutral sugar compositions, were generally similar between the mutants and WT plants. Moreover, the cell wall chemotype of the caldomt1 cad2 double mutants was similar to that of the caldomt1 single mutant. These results confirm major shifts in lignin structure upon decreased CAldOMT and CAD activities in rice.
Next, the authors analyzed how these lignin manipulations affected biomass processing by evaluating the enzymatic saccharification efficiency of the knockout mutant cell walls. Both single mutants showed enhanced glucose release when compared with the control, but caldomt1 clearly surpassed cad2 in terms of saccharification efficiency. Interestingly, the glucose release profiles of the double mutants were higher than those of control plants and the cad2 mutant but were similar to or even lower than that of caldomt1. These data indicate that targeting OsCAldOMT1 improved biomass processing better than targeting OsCAD2 alone or combined with OsCAldOMT1.
How might the cell walls be modified in these mutants? To address this issue, the authors performed a comparative analysis of cellulose molecular assembly in the mutant cell wall samples. They observed that the relative abundance of crystalline over amorphous cellulose regions decreased in all mutants compared with the WT, suggesting disruption in the crystalline cellulose network in these genotypes. Moreover, the molecular mobility of cellulose was also affected in the cell walls of both caldomt1 and cad2 mutants, whereas stacking OsCAldOMT1 and OsCAD2 mutations further increased cellulose mobility. Conversely, no drastic alterations occurred in the porosity and physical accessibility of cellulose in the cell walls of the mutants. Altogether, these results indicate that both CAldOMT- and CAD-deficient rice cell walls had notably altered molecular assemblies that may contribute to their improved biomass processability.
The work of Martin et al. adds to the notion that manipulating lignin content and composition can substantially alter the associations among the different cell wall polymers, affecting the overall assembly of plant biomass. The distinct lignin compositions and functionalities arising from bioengineering approaches might disrupt some lignin-hemicellulose associations, resulting in a looser cell wall assembly that is more easily processed in biorefineries. A better knowledge of the structural consequences of lignin manipulation on the overall biomass assembly will give us more effective strategies toward optimized biomass feedstocks for the bioeconomy.
Conflict of interest statement. None declared.



