-
PDF
- Split View
-
Views
-
Cite
Cite
Trinh-Don Nguyen, Plant and pest: The art of (cyanide) war, Plant Physiology, Volume 189, Issue 4, August 2022, Pages 1896–1897, https://doi.org/10.1093/plphys/kiac243
Close - Share Icon Share
Cyanide is a deadly poison and an integral part in the defensive arsenals of many plants. Understanding how cyanide is deployed and neutralized provides valuable insights into the co-evolution of plants and herbivores with critical crop protection implications. As a product of ethylene metabolism, hydrogen cyanide (HCN) naturally occurs at minute amounts in all plants. However, certain plants can set off powerful “cyanide bombs” by hydrolyzing their stored cyanogenic glycosides to cyanohydrins and eventually HCN when their tissues are damaged (Gleadow and Møller, 2014).
In Arabidopsis (Arabidopsis thaliana), instead of cyanogenic glycosides, the camalexin and the recently discovered 4-hydroxyindole-3-carbonyl nitrile (4-OH-ICN) pathways are the major sources for cyanohydrins and HCN (Rajniak et al., 2015; Figure 1). In many plants, cyanohydrin lyases, also known as hydroxynitrile lyases (HNLs), catalyze the reversible cleavage of cyanohydrins to the corresponding aldehydes or ketones and HCN. An HNL homolog underlies the cyanohydrin reaction in A. thaliana; however, its ecophysiological role has never been established (Andexer et al., 2007). Based on the observation that the A. thaliana HNL (AtHNL) homolog expresses differently between mite-susceptible and mite-resistant A. thaliana accessions (Zhurov et al., 2014), in this issue of Plant Physiology, Arnaiz et al. (2022) set out to investigate the gene’s role in HCN biosynthesis. The intricate evolutionary arms race between A. thaliana and the notoriously polyphagous two-spotted spider mite (Tetranychus urticae) was also investigated in the context of cyanide metabolism.
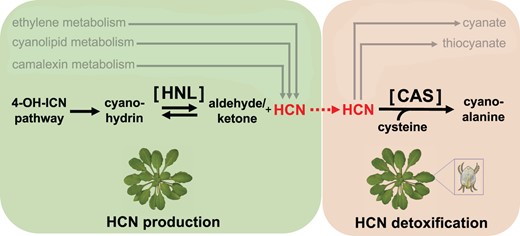
HCN production (left, green box) and detoxification (right, orange box) in plants and herbivorous arthropods. Plants employ several pathways to produce and detoxify their own HCN. Herbivores share the same HCN detoxification pathways as those in plants. Arnaiz et al. (2022) showed that HNL and CAS in the highlighted pathway confer mite resistance in Arabidopsis thaliana and HCN tolerance in the polyphagous two-spotted spider mites (Tetranychus urticae), respectively. Figure adapted from Arnaiz et al. (2022), Figure 1 with the A. thaliana image by Charles Andrès and two-spotted spider mite illustration by Thai-An D. Nguyen.
Using reverse transcription quantitative PCR (RT-qPCR), the team confirmed lower expression of AtHNL in mite-susceptible accessions than in mite-resistant accessions, which includes the wild-type ecotype Col-0. When the plants were exposed to mites, AtHNL expression increased, as did expression of genes in the 4-OH-ICN pathway (Figure 1). In addition, AtHNL was expressed constitutively across tissues, including the main targets of two-spotted spider mites, such as cauline and rosette leaves, strongly suggesting a defensive role. To unambiguously verify such a role, the authors generated two AtHNL-overexpressing lines using the strong constitutive CaMV 35S promoter and compared their response to mite infestation with that of the two publicly available AtHNL knockdown lines. As expected, the knockdown lines showed more leaf damage, on the order of two-fold, than that of the overexpressing lines. The average number of eggs per mite on AtHNL knockdown plants was also more than double that of mites on AtHNL overexpressing plants. These results confirm an association between AtHNL expression and resistance to herbivory by the mites.
Two-spotted spider mites have a wide host range of more than 1,000 plant species and are thus a major agricultural concern (Dixit et al., 2022). To gauge how these mites survive and reproduce while under cyanide attacks by A. thaliana, Arnaiz et al. (2022) monitored several mite genes that have putative roles in HCN detoxification. RT-qPCR data showed tight association between the HCN levels and the expression of a pair of genes encoding enzymes that turnover HCN in mites as well as plants (Machingura et al., 2016; Dixit et al., 2022). Suppressing one of the two genes in mites that encodes β-cyanoalanine synthase (CAS) using RNA interference resulted in substantially lower fecundity in the mites. However, the reproduction-lowering effect of CAS silencing was lessened when the mites were fed on AtHNL knockdown plants, revealing a direct relation between mite CAS and AtHNL in the arms race between the two species.
The functional characterization of AtHNL by Arnaiz et al. (2022) fills in another crucial piece in the chemical defense puzzle in A. thaliana and again exemplifies this plant as an indispensable model to understand plant–environment interactions via bioactive small molecules. Due to its limited known set of natural products, A. thaliana has for some time been under-appreciated when studying specialized (secondary) metabolism. However, research in the past two decades has uncovered hundreds of specialized metabolites in A. thaliana (D’Auria and Gershenzon, 2005; Field and Osbourn, 2008; Tohge et al., 2018). In particular, recently gained knowledge of HCN biosynthesis has revised the classic view of A. thaliana as a non-cyanogenic plant, showing a more complete picture of the continuous cyanide battles between the plant and its pests (Zhurov et al., 2014; Rajniak et al., 2015; Arnaiz et al., 2022; Dixit et al., 2022; Figure 1). Many questions remain, such as why the association between CAS and HNL in A. thaliana is not always apparent, how the interconnected HCN pathways are coordinated in plants, how non-indole cyanohydrins are biosynthesized in A. thaliana, and how different detoxification pathways in mites are activated. Nevertheless, it is clear that while A. thaliana uses AtHNL and other enzymes to launch and evade its own cyanide attacks, herbivorous arthropods are armed with several countering biochemical pathways. Such is the art of war and its further study may help develop effective plant protection strategies.
Conflict of interest statement. None declared.



