-
PDF
- Split View
-
Views
-
Cite
Cite
Charles Copeland, The feeling is mutual: Increased host putrescine biosynthesis promotes both plant and endophyte growth, Plant Physiology, Volume 188, Issue 4, April 2022, Pages 1939–1941, https://doi.org/10.1093/plphys/kiac001
Close - Share Icon Share
Plants interact with a variety of microbes in their environment, and the outcome of these interactions for the plant can range from detrimental to beneficial. While many microbes are known to promote plant growth, the mechanisms behind the growth promotion are often unknown, and multiple mechanisms are likely involved (Vacheron et al., 2013; Vandenkoornhuyse et al., 2015).
The fungus Piriformospora indica colonizes a wide range of plants, including Arabidopsis (Arabidopsis thaliana) and barley (Hordeum vulgare), growing as an endophyte within the roots (Kundu et al., 2022). Piriformospora indica promotes growth of its host plant, possibly by increasing biotic or abiotic stress tolerance. Plants respond to P. indica colonization with major transcriptional, hormonal, and metabolic reprogramming, and many questions about the signaling pathways and mechanisms responsible for the growth promotion remain to be addressed. In this issue of Plant Physiology, Kundu et al. (2022) characterize P. indica-mediated growth promotion in tomato (Solanum lycopersicum) and analyze the associated metabolic reprogramming.
The authors first confirmed that inoculation of P. indica on tomato roots leads to increased plant growth. They then used untargeted gas chromatography–mass spectrometry (GC–MS) to determine how P. indica colonization affects the root and shoot metabolome. As expected, the colonized plants showed altered levels of many metabolites; however, many of these metabolites showed opposite patterns of regulation in roots compared to shoots. Since P. indica is a root endophyte, the authors focused on root metabolites using a statistical model to identify the metabolites that contribute most significantly to the P. indica-mediated metabolome changes. From this model, they found putrescine to be the most important variable driving the metabolome changes. Putrescine is a polyamine, a low-molecular weight molecule derived from the decarboxylation of amino acids, often arginine (Alcázar et al., 2010). The GC–MS data showed that the arginine metabolic pathway was also affected by P. indica (Kundu et al., 2022). In addition, P. indica-colonized roots showed increased transcript abundance of ARGININE DECARBOXYLASE 1 (SlADC1), which encodes an arginine decarboxylase in the putrescine biosynthetic pathway. The metabolomic and gene expression data suggest that P. indica specifically induces putrescine production in its tomato host (Figure 1A).
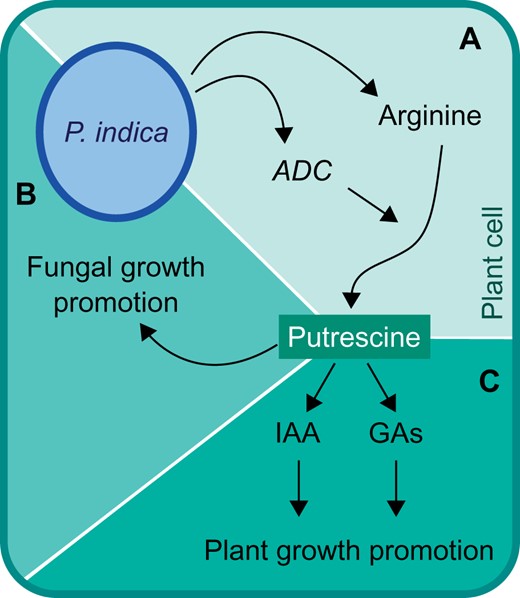
Working model for the role of putrescine in root colonization and plant growth promotion by P. indica. A, Piriformospora indica alters host metabolism, including increasing ADC1 expression, resulting in increased putrescine biosynthesis. B, Putrescine increases growth of P. indica and is required for robust root colonization. C, Putrescine increases abundance of the plant hormones auxin (IAA) and GAs, leading to plant growth promotion. Adapted from Kundu et al. (2021).
The authors next tested the effect of exogenous putrescine treatment on each species individually. Piriformospora indica showed increased mycelium growth on media containing putrescine, and putrescine treatment similarly triggered growth promotion in tomato without P. indica. The authors therefore hypothesized that increased putrescine biosynthesis in P. indica-colonized roots could be important for both fungal growth within the root and for the observed plant growth promotion. To confirm this hypothesis, virus-induced gene silencing was used to generate tomato SlADC1 knockdown lines that produce less putrescine. Piriformospora indica showed a reduced ability to colonize the silenced lines, and the growth promotion effect was abolished. Host-derived putrescine is thus required for optimal P. indica colonization (Figure 1B) and the associated plant growth promotion.
Kundu et al. (2022) further examined the involvement of putrescine in the interactions between P. indica and another host, A. thaliana. Similar to tomato, they found that P. indica inoculation and putrescine treatment both cause plant growth promotion in A. thaliana. Likewise, biosynthesis of putrescine by the host is important in this interaction, as Atadc1 and Atadc2 mutants supported less P. indica growth and did not show growth promotion. Notably, P. indica inoculation and putrescine treatment both promoted plant growth to a similar extent, and the combined treatments did not show additive effects, suggesting that P. indica does not cause growth promotion through additional putrescine-independent pathways.
As many growth-promoting microbes, including P. indica, are known to influence host hormone signaling pathways (Schäfer et al., 2009; Vacheron et al., 2013), the authors examined how putrescine treatment affects hormone levels. Tomato seedlings treated with putrescine showed increased abundance of auxin (indole-3-acetic acid, IAA) and gibberellins (GAs). The authors suggest a model in which P. indica-induced putrescine triggers an increase in IAA and GA levels, which then leads to plant growth promotion (Figure 1C).
Polyamines and auxin have both been previously implicated as important molecules in plant–microbe interactions, contributing to successful microbial colonization of the plant and to plant growth promotion. The genomes of plant-associated bacteria often contain putrescine uptake transport genes, and a Pseudomonas fluorescens mutant that cannot metabolize putrescine is impaired in its ability to colonize A. thaliana roots (Liu et al., 2018; de Souza et al., 2019). The plant growth promotion activity of a Bacillus subtilis strain also requires bacterial production of spermidine, another polyamine (Xie et al., 2014). In addition, many plant growth-promoting bacteria produce auxin or modulate auxin production and signaling in the host (Vacheron et al., 2013; Pieterse et al., 2020). The model proposed by Kundu et al. (2022) connects polyamine and hormone signaling in plant growth promotion. Diverse microbes may trigger plant growth promotion through different nodes in this pathway, either by producing the signaling molecules themselves or, as with P. indica, by influencing host metabolism.
Many exciting questions remain to be answered about the role of putrescine in interactions between P. indica and tomato: How is SlADC1 expression induced by fungal colonization, and how does increased putrescine abundance lead to changes in hormone levels? The role of putrescine in plant cell signaling is complex as polyamine biosynthesis pathways are upregulated under many different biotic and abiotic stress conditions, and polyamines can potentially regulate the activities of proteins, such as ion channels and protein kinases (Alcázar et al., 2010; Gerlin et al., 2021). It is similarly unclear what function putrescine performs for P. indica. Although it is required for fungal growth, putrescine may also serve as a messenger molecule between plants and microbes, perhaps informing the microbe of the presence of the host or modulating the plant immune system to permit microbial colonization (Liu et al., 2018; Gerlin et al., 2021). The work of Kundu et al. (2022) reveals a mechanism by which a microbe can modify plant metabolism to the mutual benefit of both microbe and host.
Conflict of interest statement. None declared.



