-
PDF
- Split View
-
Views
-
Cite
Cite
Zaki Ahmad, Martin Balcerowicz, Shining light on molecular details of SPA2, a repressor of photomorphogenesis, Plant Physiology, Volume 187, Issue 1, September 2021, Pages 17–18, https://doi.org/10.1093/plphys/kiab233
Close - Share Icon Share
Light either directly or indirectly fuels all life on earth and acts as a critical signal for plant growth and development. Photomorphogenesis refers to the development plants undergo when exposed to light; at the seedling stage, this includes inhibition of hypocotyl elongation, opening and greening of cotyledons, and development of true leaves, processes collectively known as de-etiolation (Nemhauser and Chory, 2002). In contrast, etiolated or dark-grown seedlings possess elongated hypocotyls, closed cotyledons, and poorly developed shoot and root systems (Nemhauser and Chory, 2002). Hypocotyl length is typically used as a phenotypic read-out for etiolation and de-etiolation responses.
At the molecular level, negative regulators of photomorphogenesis are suppressed in the light through signaling pathways that mostly center around phytochrome (phy) and cryptochrome (cry) photoreceptors, which respond to red/far-red and blue light, respectively (Chen et al., 2004). When seedlings experience dark, positive regulators of the light response are actively repressed by CONSTITUTIVE PHOTOMORPHOGENESIS 1 (COP1) and SUPPRESSOR OF PHYTOCHROME A-105 (SPA) proteins. COP1 as well as SPAs feature C-terminal tryptophan-aspartic acid (WD) repeats and a central coiled-coil domain, all of which are involved in multiple protein–protein interactions, but the proteins differ at their N termini: COP1 harbors a RING domain that confers E3 ligase activity, whereas the SPA N-terminus contains a domain with similarity to a serine/threonine kinase. The E3 ligase activity of COP1 depends on forming a stable complex with SPAs. This complex ubiquitinates photomorphogenesis-promoting proteins and thereby marks them for proteasomal degradation in darkness; its targets include the photoreceptors cry1, cry2, phyA, and phyB as well as a host of transcription factors, such as ELONGATED HYPOCOTYL 5 (HY5) and LONG HYPOCOTYL IN FAR-RED 1 (HFR1; Ponnu and Hoecker, 2021). The COP1-SPA2 complex also has auto-ubiquitination activity, which is potentiated in the light and is thought to contribute to COP1-SPA2 inactivation (Chen et al., 2015).
In this issue of Plant Physiology, Schenk and co-workers investigated the molecular details that underpin the repression of SPA2 in de-etiolating Arabidopsis (Arabidopsis thaliana) plants (Schenk et al., 2021). Among the four Arabidopsis SPAs, the authors found SPA2 to be most efficiently degraded in response to light, confirming previous findings (Balcerowicz et al., 2011), while SPA3 and SPA4 remained stable upon light exposure. The varying degree of SPA protein stability implies functional diversification among the SPAs, with SPA2 playing a dominant role in darkness. More specifically, Schenk et al. (2021) found the kinase domain of SPA2 to be required for inactivating SPA2 during de-etiolation as deletion of the N-terminus containing the kinase domain prevented the protein’s degradation. Deletion of the WD-repeat domain also stabilized SPA2 when dark-grown seedlings were exposed to light, suggesting multiple sites contribute to the regulation of SPA2 turnover.
A spa1 spa2 spa3 mutant undergoes de-etiolation even in darkness (Fittinghoff et al., 2006). Schenk et al. (2021) found that while introducing full-length SPA2 in the spa triple mutant restored the long-hypocotyl phenotype in the dark, expressing an SPA2 version lacking the N-terminus prevented de-etiolation under high intensities of far-red light (Figure 1). This observation highlights the critical role of the SPA2 N-terminus in the control of photomorphogenesis. Far-red light is predominantly sensed by phyA, which is responsible for triggering light-induced degradation of SPA2 (Chen et al., 2015). To determine whether specific domains of SPA2 are required for phyA-SPA2 interaction, Schenk and colleagues carried out yeast-2-hybrid and co-immunoprecipitation experiments. They detected interaction of phyA with SPA2 constructs lacking either its N-terminus or its WD-repeat domain, suggesting the presence of at least two phyA-interacting sites in the SPA2 protein.
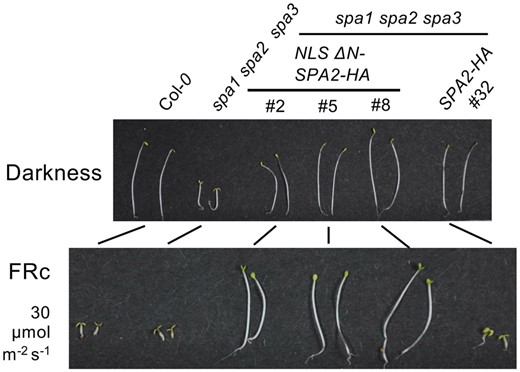
Deletion of the SPA2 N-terminus prevents de-etiolation in light-grown seedlings. While expressing a full-length SPA2 protein in a spa1 spa2 spa3 triple mutant background does not alter the seedlings’ light response, an SPA2 protein lacking the protein’s N-terminus (NLS-ΔN-SPA2) prevents de-etiolation in far-red light (FRc).
In short, the study by Schenk et al. (2021) revealed multiple domains act cooperatively in the light regulation of SPA2 protein abundance, with a prominent role for its N-terminal kinase domain. To what extent deletions of either the N-terminal or WD-repeat domain of SPA2 compromise the ability of the COP1-SPA2 complex to auto-ubiquitinate remains to be established. Similarly, it remains unclear whether the putative kinase activity of the SPA2 N-terminus is involved in regulating the protein’s stability. Future proteomics work may shed light on specific sites within the N-terminal and WD-repeat domains of SPA2 that determine its regulatory activity in photomorphogenesis. Moving beyond its role in controlling developmental responses, it is largely unknown whether SPA2 and other SPAs interact with additional signaling processes in the dark. For instance, COP1 inhibits the nutrient-sensing TARGET OF RAPAMYCIN (TOR) pathway in etiolated seedlings (Chen et al., 2018); future work could investigate if and how phys and SPAs are involved in TOR-COP1 crosstalk, uncovering the degree of coordination that occurs between metabolic and light signaling pathways to fine-tune events underpinning etiolation and de-etiolation.



