-
PDF
- Split View
-
Views
-
Cite
Cite
Deepanksha Arora, Daniёl Van Damme, Motif-based endomembrane trafficking, Plant Physiology, Volume 186, Issue 1, May 2021, Pages 221–238, https://doi.org/10.1093/plphys/kiab077
Close - Share Icon Share
Abstract
Endomembrane trafficking, which allows proteins and lipids to flow between the different endomembrane compartments, largely occurs by vesicle-mediated transport. Transmembrane proteins intended for transport are concentrated into a vesicle or carrier by undulation of a donor membrane. This is followed by vesicle scission, uncoating, and finally, fusion at the target membrane. Three major trafficking pathways operate inside eukaryotic cells: anterograde, retrograde, and endocytic. Each pathway involves a unique set of machinery and coat proteins that pack the transmembrane proteins, along with their associated lipids, into specific carriers. Adaptor and coatomer complexes are major facilitators that function in anterograde transport and in endocytosis. These complexes recognize the transmembrane cargoes destined for transport and recruit the coat proteins that help form the carriers. These complexes use either linear motifs or posttranslational modifications to recognize the cargoes, which are then packaged and delivered along the trafficking pathways. In this review, we focus on the different trafficking complexes that share a common evolutionary branch in Arabidopsis (Arabidopsis thaliana), and we discuss up-to-date knowledge about the cargo recognition motifs they use.
Plant research is slowly gaining insight into the linear trafficking motifs used by the various AP complexes.Recent observations point out that steady-state accumulation of cargo proteins at the plasma membrane is not necessarily caused by to impaired internalization.
TSET/TPC, the most recently identified member of the heterotetrameric adaptor complex-containing coat (HTAC-CC) family, and the identification of an endocytic-autophagosomal degradation pathway operating between the contact sites of the endoplasmic reticulum with the plasma membrane and the vacuole provide previously undiscovered additional layers of complexity to endomembrane trafficking in plants.
Endomembrane trafficking
Protein trafficking via the endomembrane system is an essential mechanism by which eukaryotic cells maintain homeostasis of their membrane proteome. It is tightly regulated and essential for physiological responses to stimuli (Inada and Ueda, 2014), cell survival (Tanaka et al., 2013; Bozkurt et al., 2015), and development (Dhonukshe et al., 2007; Zhou et al., 2018). Trafficking begins following protein synthesis in the Endoplasmic Reticulum (ER); transmembrane proteins, as well as associated soluble cargo proteins, are then transported to the Golgi for sorting to their respective destinations via the Trans Golgi Network (TGN). The proteins are packed into vesicles formed by the deformation of their respective donor membrane. Six essential types of multimeric protein complexes function in vesicle formation at specific donor membranes in plants. These are Adaptor complexes (AP and the TSET/TPLATE complex [TPC]), Coatomer complexes (COPI and COPII), the Endosomal Complex Required for Transport (ESCRT), and the Retromer complex (Reyes et al., 2011; Lee and Hwang, 2014; Elkin et al., 2016). Proteins can reach their destination target membrane only after these protein complexes are removed from the vesicles by uncoating and following the subsequent fusion of the vesicles with the target membrane.
Three types of trafficking pathways, anterograde, retrograde, and endocytic, are responsible for transporting transmembrane proteins in eukaryotic cells. The anterograde pathway uses the COPII machinery to bring proteins from the ER to the Golgi. From there, proteins move via the TGN to the plasma membrane (PM) or the forming cell plate, to the apoplast (in secretion), or to the vacuole with the help of AP complexes. The retrograde pathway transports proteins from endosomes back to the TGN and from the TGN back to the ER (Sannerud et al., 2003). This pathway is involved in lipid transport, retrieval of ER-resident proteins, and recycling of receptors, such as Vacuolar Sorting Receptors (VSRs; Früholz et al., 2018; Heucken and Ivanov, 2018), and relies on Retromer (from endosomes to TGN) and COPI machinery (Intra Golgi and from Golgi to ER). Endocytic trafficking selectively internalizes PM proteins, lipids, and other extracellular molecules (Dhonukshe et al., 2007; Kitakura et al., 2011; Fan et al., 2015; Zhang et al., 2015), and eventually uses ESCRT to deliver the internalized cargo into intraluminal vesicles (ILVs) at the late endosomes (Hwang, 2008; Hwang and Robinson, 2009; Gao et al., 2017). This process can either rely on a specific type of scaffolding protein, clathrin, and hence is called clathrin-mediated endocytosis (CME; McMahon and Boucrot, 2011; Kirchhausen et al., 2014), or can be clathrin-independent (Mayor et al., 2014). CME functions with the aid of AP2 and the TSET/TPCs. The current view in plants and other model systems is that clathrin-independent pathways do not efficiently contribute to the bulk flux of membrane proteins (Bitsikas et al., 2014; Sandvig et al., 2018). In plants, the three trafficking pathways intersect at the TGN, making it the hub of endosomal trafficking (Viotti et al., 2010). The TGN is also the place where the major decisions of protein sorting, that is, to recycle or to degrade, are usually performed (Uemura and Nakano, 2013; Rosquete et al., 2018).
The process of transmembrane protein trafficking begins with the recognition of the cargo proteins by cytoplasmic multimeric protein complexes (trafficking complexes) or by monomeric adaptors associated with these complexes. A direct interaction between the trafficking complexes and the cargo occurs via sorting motifs or signals such as posttranslational modifications present in the cytoplasmic domains of the cargo (Traub, 2009). Scaffolding molecules, such as clathrin, do not bind the cargo directly (Kirchhausen et al., 2014). Hence, they require adaptor complexes to pack the transmembrane cargo proteins into vesicles and recruit the clathrin scaffold onto the donor membrane (Boehm and Bonifacino, 2001; Traub and Bonifacino, 2013). Clathrin forms a second protein layer, next to the first layer, which contains the cargo and AP complexes, and is connected to the cargoes via these AP complexes. Similarly, the COPII machinery is also organized into an inner and an outer layer (Gomez-Navarro and Miller, 2016; Dacks and Robinson, 2017). COPI, on the other hand, forms a more heterogeneous coat, without making the continuous cage that occurs for COPII and clathrin. COPI organization to some extent does resemble that of clathrin with AP2, with its propeller domains facing the vesicles. This is in contrast to COPII where these propeller domains are involved in linking the cage-forming subunits (Yip and Walz, 2011; Faini et al., 2012; Dodonova et al., 2015; Dacks and Robinson, 2017).
In this review, we will look into sorting of transmembrane proteins by focusing on motif-based recognition by the multimeric protein complexes in Arabidopsis that belong to the heterotetrameric adaptor complex-containing coat (HTAC-CC) family (Dacks and Robinson, 2017). This family consists of COPI, the TSET/TPC, and the AP complexes (Figure 1A). We provide a comprehensive list of adaptor complex subunits, their associated mutant phenotypes, as well as their currently known transmembrane cargoes and mechanisms of recognition. The APs were initially discovered and have been extensively studied in yeast and animal models. Five specific adaptor complexes (AP 1–5) can be identified in all eukaryotic systems. They share a common evolutionary origin with the COPI complex and with the TSET/TPC (Figure 1A), which is partially present in several eukaryotic super groups and was therefore likely a constituent of the last eukaryotic common ancestor (LECA). However, TSET has not been evolutionarily retained in the most studied model systems, the Opisthokonts (Gadeyne et al., 2014; Hirst et al., 2014).
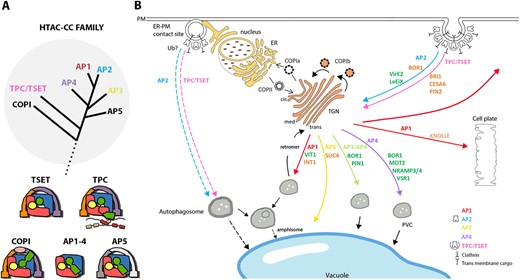
Trafficking complexes from the HTAC-CC family and some of their associated cargoes. (A) Schematic phylogenetic tree (upper) showing the common origin of the members of the HTAC-CC (grey circle) and schematic models of the different complexes and their subunits (lower). Subunits of the different complexes that are related to each other are depicted in a similar color. Lines represent flexible linkers between subunit domains. The dotted line refers to the connection with the other branches of the tree of life. The tree and the models are based on (Hirst et al., 2011; 2014; Yperman et al., 2021). (B) The localization where the different trafficking complexes AP1-4, COPI, COPII, TPC and retromer complex operate in plants is shown (arrows colored according to the complex involved). We highlight here the roles of the AP complexes in endomembrane trafficking using some selected cargoes. A more complete list of cargoes, although likely not exhaustive, is compiled in Table 1. Cargoes highlighted in dark green have been associated with an adaptor complex through a known recognition motif, while for those depicted in orange, the adaptor complexes are known, but the recognition motif has not yet been identified. The red arrow from the TGN to PM represents secretion in interphase cells. The recently identified autophagosomal degradation pathway from the ER–PM contact sites to the vacuole, is also depicted, although no cargoes for this pathway have been identified so far. The exact function of AP2 and TPC/TSET in this autophagosomal pathway remains to be discovered (dashed lines). Brown speckles in the nucleus represent nuclear pores. cis/med/trans, cis/medial/trans Golgi; PVC, prevacuolar compartment; CESA6, cellulose synthase A catalytic subunit 6; PIN1/2, Pin-formed 1/2.
Endomembrane trafficking relies on multi-subunit protein complexes
The Arabidopsis genome encodes different protein complexes involved in endomembrane trafficking. These include the five adaptor complexes (AP 1 to −5), the TSET/ TPC, both COP complexes (COPI and COPII), the ESCRT complex, and the Retromer complex. Each of these complexes has a specific role, localization, and different physiological implications when its functions are impaired.
APs are heterotetrameric complexes consisting of four subunits called adaptins. These include two large subunits (∼90–130 kDa), one medium (∼50 kDa), and one small subunit (∼20 kDa; Aguilar et al., 2001; Robinson, 2015). These complexes are highly evolutionarily conserved across eukaryotic kingdoms (Boehm and Bonifacino, 2001; Traub and Bonifacino, 2013; Hirst et al., 2013b). Among the five complexes, AP1 and AP2 were discovered in purified clathrin-coated vesicles (CCVs; Keen, 1987); AP3, AP4, and AP5 were discovered as homologs of AP1 and AP2 via database searches (Dell'Angelica et al., 1997; Dell’Angelica et al., 1999; Hirst et al., 2011). AP3 and AP4 are generally considered to work without clathrin in animals (Simpson et al., 1996; Hirst et al., 1999) and yeast (Cowles et al., 1997). For AP3, however, there is some discrepancy in the literature because in some animal and plant models, AP3 has been linked to clathrin (Drake et al., 2000; Zwiewka et al., 2011; Kural et al., 2012). AP5, the last discovered adaptor complex, is reportedly absent from some major model organisms like Drosophila melanogaster, Caenorhabditis elegans, Schizosaccharomyces pombe, or Saccharomyces cerevisiae, as is AP4, hinting at a possible functional relationship between these two complexes (Hirst et al., 2013b).
The adaptor complexes share an evolutionary origin with two other multiprotein trafficking complexes, COPI and the TSET/TPC. Sensitive homology searches and structural studies show the occurrence of similar folds between adaptor complexes and the core subcomplex of COPI, termed F-COPI, dating back to LECA (Schledzewski et al., 1999; Hirst et al., 2011). In animals and yeast, structural homology is seen between βCOP and β adaptin (Duden et al., 1991), between δCOP and µ adaptin (Faulstich et al., 1996), and between ζCOP and σ adaptin (Kuge et al., 1993).
COPI vesicles are responsible for retrograde intra-Golgi and Golgi to ER transport (Pimpl et al., 2000; Donohoe et al., 2007). COPI is a roughly 600kDa hetero-heptamer complex, which is conceptually divided into two subcomplexes, the F-subcomplex, and the B-subcomplex (Jackson, 2014). The F-subcomplex (F-COPI) consists of βCOP, δCOP, γCOP and ζCOP subunits, while the B-subcomplex (B-COPI) consists of αCOP, βCOP, and εCOP.
When the founding member of the TSET/TPC, TPLATE, was discovered in plants, its relationship with coat proteins, such as βCOP, was already recognized (Van Damme et al., 2006). Later on, the TSET/TPC was simultaneously discovered in plants and slime moulds, either by using a proteomics approach starting from its founding member in plants (Gadeyne et al., 2014), or by a structure-based bioinformatics approach, which identified the core subunits of this complex as the missing link between the AP complexes and COPI. The ancestor of TSET, COPI, and the AP complexes, that is, the protocoatomer complex, was likely a heterohexamer consisting of a tetrameric core and two scaffolding proteins. These scaffolding proteins reportedly evolved independently in COPI (α- and β′-COP) and TSET (TTRAY/TWD40-1 and -2), and were lost in most AP complexes, except for AP5 (Hirst et al., 2011; 2013b; 2014; Figure 1A). It is noteworthy that the two scaffolding subunits in COPI and TSET (and most likely also those from AP5—although this is not yet experimentally shown) form a 3D structure with the β-propeller domains facing away from each other, resembling the organization of clathrin (Dodonova et al., 2015; Yperman et al., 2021). In plants, the TPC is a hetero-octameric complex of roughly 900 kDa. It acts an early modulator of CME and is likely recruited en bloc to the PM to work both together, as well as independently of, AP2 (Gadeyne et al., 2014; Bashline et al., 2015; Wang et al., 2020). Quite surprisingly, although this ancient complex was already present in LECA and therefore represents primal eukaryotic endocytic machinery, it became functionally redundant in slime moulds and was even lost from animal and yeast genomes (Hirst et al., 2014).
Next to those protein complexes, which share a clearly recognizable common origin and are the focus of this review, eukaryotic genomes also encode for COPII, ESCRT, and Retromer complexes. COPII facilitates anterograde trafficking from ER to Golgi (Hwang and Robinson, 2009). The COPII machinery consists of a SAR1 GTPase that, together with SEC23 and SEC24, forms the inner coat that connects to the cargo proteins, and the cage complex, consisting of SEC13 and SEC31 that assemble into a polyhedral cage (Barlowe et al., 1994). ESCRT is a multi-subunit evolutionarily conserved machinery responsible for sorting ubiquitinated cargo proteins into ILVs of late endosomal Multi Vesicular Bodies (MVBs; Cui et al., 2016; Gao et al., 2017; Kalinowska and Isono, 2018). In budding yeast and mammals, ESCRT machinery consists of five subcomplexes (ESCRT 0-III and VPS4) along with accessory proteins, and is involved in MVB biogenesis (Raiborg and Stenmark, 2009; Henne et al., 2011). Plant genomes encode most of the ESCRT machinery with the exception of ESCRT-0 and some components of ESCRT-I (Gao et al., 2017; Otegui, 2018; Mosesso et al., 2019). However, proteins sharing some aspects of the domain structure of the ESCRT-0 like TOM1-like proteins (TOLs) are found in the Arabidopsis genome (Korbei et al., 2013; Mosesso et al., 2019). Finally, the Retromer complex is involved in retrograde transport, which includes recycling proteins from the TGN as well as from the early stages of the MVB formation (Niemes et al., 2010; Robinson and Neuhaus, 2016). It consists of two evolutionarily conserved subcomplexes, the core Retromer, and the sorting nexin (SNX) subcomplex. Higher-order mutants in the SNX subcomplex, however, do not mimic the developmental defects observed for core Retromer mutants, and lack of physical interaction data between them fuels the hypothesis that these subcomplexes might act separately (Robinson et al., 2012; Heucken and Ivanov, 2018).
Comprehensive knowledge of membrane trafficking complexes, their cargo proteins, and the mode of interaction with these cargoes exists in yeast and animal model organisms (Traub and Bonifacino, 2013; Feyder et al., 2015; Robinson, 2015; Sanger et al., 2019). Our current understanding of the role of these complexes in endomembrane trafficking in plants is, however, still minimal. Hereafter, we discuss the different membrane trafficking complexes that are part of the COPI family that evolved from the ancestral protocoatomer complex (Rout and Field, 2017), that is, the AP complexes, TSET/TPC, and COPI, in Arabidopsis. The other multimeric trafficking complexes COPII, ESCRT, and Retromer, which also share a common origin with those mentioned above but are part of a different branch, are not addressed in detail here but were recently reviewed (Brandizzi and Barlowe, 2013; Chung et al., 2016; Gao et al., 2017; Heucken and Ivanov, 2018; Mosesso et al., 2019). An overview of different adaptor complexes in Arabidopsis, with their component subunits and observed mutant phenotypes, is summarized in Supplemental Table S1. A schematic view of where the different adaptor complexes function inside the cell, and some of their known cargoes, is represented in Figure 1B.
COPI maintains Golgi homeostasis
According to the current model of COPI assembly in animals and yeast, the COPI complex is recruited to the Golgi membrane by the activation of the GTPase ADP-ribosylation factor 1 (ARF1), which generates a conformational change, exposing hydrophobic residues that facilitate ARF1 membrane insertion (Serafini et al., 1991). A characteristic feature of the COPI complex is its en bloc recruitment to the membrane, that is, the B and F subcomplexes remain associated in the cytoplasm (Hara-Kuge et al., 1994). The COPI complex associates with the Golgi membrane through the FCOPI subunits, βCOP and γCOP (Yu et al., 2012). The BCOPI subunits, αCOP and β′COP, and the FCOPI subunit γCOP have been shown to function in cargo recognition (Bethune et al., 2006; Jackson et al., 2012).
The Arabidopsis genome encodes multiple paralogs of each COPI component except for γCOP and εCOP (Robinson et al., 2007). Moreover, in Arabidopsis, based on several morphological criteria, two different subpopulations of COPI vesicles exist. COPIa is involved in trafficking from the cis-Golgi to the ER, whereas COPIb is involved in intra-Golgi trafficking from medial and trans-Golgi compartments to retain Golgi resident proteins (Donohoe et al., 2007). Although not experimentally addressed yet, the presence of different paralogs of COPI subunits in Arabidopsis is likely causal to the observed formation of the different subpopulations of COPI vesicles, which act at different locations in retrograde transport and recognize different cargoes (Gao et al., 2014).
Mutants of several COPI genes such as βCOP, αCOP, and εCOP, have been identified in the literature and are summarized in Supplemental Table S1. Although no phenotypic defects are seen in β1cop and β2cop mutants, amiR-β1/β2-COP mutants exhibited a dwarf phenotype with increased sensitivity to salt stress (Sánchez-Simarro et al., 2020). In the case of αCOPI, the two isoforms, α1COP and α2COP, show differential expression and do not compensate for each other. While α1cop1 mutants are phenotypically wild-type, α2cop mutant plants exhibit a dwarf phenotype. Furthermore, a direct correlation between anterograde and retrograde trafficking machinery was also seen in these mutants, as they exhibited increased expression of the COPII subunit AtSEC31A (Gimeno-Ferrer et al., 2017). The amiR-β1/β2-COP mutants, α2cop mutants, plants with viral-induced gene silencing of δCOP, and plants with RNAi silencing of εCOP, all exhibit morphological changes of the Golgi apparatus (Hee-Kyung et al., 2015; Woo et al., 2015). The above observations, therefore, highlight the importance of COPI-based trafficking in maintaining Golgi homeostasis. Additionally, RNAi silencing of β′COP results in defective cell plate formation and induction of programmed cell death in Nicotiana benthamiana (Hee-Kyung et al., 2015).
AP1 controls transport from the TGN
The Arabidopsis genome contains two genes for each of the four AP1 subunits, AP1ϒ, AP1β, AP1µ, and AP1σ (Teh et al., 2013). The presence of a double copy of each subunit results in functional redundancy, as was reported for the homologs of the large (ϒ) and medium (µ) subunits (Park et al., 2013; Wang et al., 2013). The β subunit appears not to be specific for AP1 as it is also part of the AP2 complex (Di Rubbo et al., 2013; Teh et al., 2013). AP1 localizes to the TGN and shares a role in vacuolar trafficking that is evolutionarily conserved between yeast and plants (Park et al., 2013; Wang et al., 2013).
AP1 plays an essential role in plant survival, as a double mutant (for AP1γ1 and AP1γ2) could not be generated (Wang et al., 2014). Of the two medium subunits, AP1µ2 is the major isoform. In line with this, the AP1µ2 mutant (hap13-1) shows pleiotropic growth defects and compromised auxin signaling, suggesting a vital role for AP1 in maintaining cell polarity (Park et al., 2013). AP1 is also required for the efficient secretion of cell wall components to the apoplast as AP1µ2 mutants show mislocalization of mucilage in the seed coat (Shimada et al., 2018). Cell wall stubs in the cotyledon and root meristem are also reported in ap1µ2, highlighting a role for AP1 in directing secretion to the forming cell plate during cytokinesis (Teh et al., 2013). Therefore, AP1 is so far the only adaptor complex shown to be involved in two independent trafficking pathways, that is, vacuolar sorting as well as secretion to the PM, the plane of cell division, and the apoplast (Figure 1B).
AP2 operates exclusively at the PM
There are six genes in the Arabidopsis genome encoding for AP2 complex subunits, two each for the large subunits, AP2α and AP2β, and one each for AP2µ and AP2σ (Di Rubbo et al., 2013). AP2 localizes at the PM and is involved in CME (Di Rubbo et al., 2013; Yamaoka et al., 2013). In CME, the AP2 complex is required for the initiation, assembly, and maturation of CCVs (McMahon and Boucrot, 2011). AP2 is involved in hormone signaling (Di Rubbo et al., 2013) and immunity (Hatsugai et al., 2016) as well as plant growth and development, as ap2 mutants (ap2µ and ap2σ) showed abnormal cotyledon and vascular patterning (Fan et al., 2013), and multiple reproductive abnormalities, culminating in highly reduced seed setting (Kim et al., 2013; Yamaoka et al., 2013).
Single subunit mutants of AP2 are viable in Arabidopsis, which is also the case in yeast and worms (Huang et al., 1999; Yeung et al., 1999; Gu et al., 2013), but contrasts with the situation in vertebrates (Mitsunari et al., 2005). In C. elegans, single subunit mutants of AP2 (ap2α and ap2µ) retain some residual function while mutants lacking both AP2α and AP2µ show severe phenotypes and are sub-viable (Gu et al., 2013). Additionally, AP2α and AP2σ still localize to the PM in the worm ap2µ mutant background, suggesting the possibility of AP2 hemicomplexes that are partially stable and can function in vivo in the absence of the complete AP2 complex (Gu et al., 2013). As AP2µ and AP2σ subunits still localize to the PM in the respective ap2σ and ap2µ Arabidopsis single mutants (Wang et al., 2016), the ability of AP2 hemicomplexes to function may also be conserved in plants, and this could explain the relatively weak mutant phenotypes reported. A phenotypical analysis of a double mutant combination of AP2, lacking both AP2µ and aP2σ, would allow verification of this hypothesis, but such a mutant has not yet been reported in Arabidopsis.
AP3 mediates vacuolar transport
The AP3 complex subunits are each coded by a single copy gene in Arabidopsis (Feraru et al., 2010). This complex localizes at the TGN and plays an evolutionarily conserved role in vacuolar trafficking, which bypasses the traditional route involving late endosomes (Feraru et al., 2010). The role of this complex is not essential in Arabidopsis as ap3 mutants do not show any abnormal macroscopic mutant phenotypes under standard growth conditions. Defective shoot gravitropism, however, occurs under sucrose deficient conditions, indicating that ap3 mutant backgrounds are sensitized to certain stress conditions (Feraru et al., 2010; Zwiewka et al., 2011; Kansup et al., 2013). Additionally, ap3β mutants, such as pat2 (lines expressing a dominant-negative form of AP3μ) and pat4-1 (a recessive loss of function mutation of AP3δ), show altered morphology of their lytic and protein storage vacuoles without altered sorting of storage proteins (Feraru et al., 2010; Zwiewka et al., 2011). AP3 thus plays a role in the biogenesis and function of vacuolar compartments.
AP4 mediates vacuolar function
The AP4 complex consists of AP4ε, β, µ, and σ, each coded by a single copy gene in Arabidopsis (Fuji et al., 2016). Like AP1 and AP3, this complex also localizes and functions at the TGN (Figure 1), but at different subdomains and with limited colocalization with AP1 (Fuji et al., 2016). Similar to AP1 and AP3, this complex also plays an evolutionarily conserved role in anterograde trafficking from TGN to the vacuole (Hirst et al., 2011) and in plants, it has been implicated in fusion events between the vacuole and the PM in the context of hypersensitive cell death (Hatsugai et al., 2018). Next to the above-mentioned role in immunity, single mutants in each of the AP4 subunits show missorting of storage proteins and accumulate high levels of the VSR1 in seeds (Fuji et al., 2016). Single subunit ap4 mutants also show pleiotropic macroscopic phenotypical defects, such as significantly shorter roots and supernumerary trichome branching, as well as reproductive defects affecting male fertility. Mutant phenotypes were not enhanced in an ap4β-2/ap4µ double mutant combination (Fuji et al., 2016; Müdsam et al., 2018), arguing against a role for AP4 hemicomplexes, in contrast to AP2 (Gu et al., 2013).
AP5 function in plants remains to be determined
This AP complex was the last one to be identified. AP5 represents an ancestral adaptor complex, frequently lost throughout the eukaryotic lineage. In Hela cells, this complex localizes to the late endosomal compartment and lysosomes (Hirst et al., 2013b) and is involved in a late endosomal retrieval pathway leading back to the TGN (Hirst et al., 2018). Proteomics experiments showed that this complex interacts in a 1:1 ratio with SPG11 and SPG15, two proteins that are mutated in patients with hereditary spastic paraplegia, a disease affecting primary motor neurons. SPG11 and SPG15 have a predicted structure resembling clathrin or COPI subunits and they are hypothesized to form a coat-like complex together with the other four AP5 subunits (Hirst et al., 2013a).
Database homology searches suggest that the AP5 complex in Arabidopsis is a trimeric complex consisting of AP5ζ, AP5β, and AP5µ, as AP5σ seems to be absent (Hirst et al., 2011). AP5 complex composition, including whether or which other sigma subunit substitutes for the lack of AP5σ, its localization, and functions, remain unknown in plants. In animals, the crucial cargo binding residues found in other µ subunits are altered in AP5µ, which suggests that unconventional motifs are recognized by this subunit (Hirst et al., 2018). Moreover, this complex is ubiquitous, expressed in low amounts, and non-essential as it only shows a subtle null phenotype in animal cells. The fact that Arabidopsis does not contain a small AP5 subunit is enigmatic and might indicate that this complex is no longer functional or that it has functionally diversified in plants. The composition, the role, and mode-of-action of AP5 in plants, therefore, represents an interesting future challenge.
The TSET/TPC is a jotnarlog
The TSET/(TPC is an evolutionarily ancient pan-eukaryotic trafficking complex. The late discovery of this complex is likely caused by its absence from yeast and animal model systems. Such proteins and complexes, with an ancient evolutionary pattern hidden from view, were recently termed ‘jotnarlogs’ after the dark and hidden world in Norse mythology (More et al., 2020). In plants, TPC contains eight subunits, TPLATE, TML, TASH3, TWD40-1, TWD40-2, LOLITA, AtEH1/Pan1, and AtEH2/Pan1. The homologous TSET complex in Dictyostelium is a hexamer that lacks the latter two subunits, which appear to be plant-specific adaptations (Hirst et al., 2014; Zhang et al., 2015). These two subunits function in an actin-dependent autophagosomal degradation pathway between the ER–PM contact sites and the vacuole under stress conditions, in concert with several other endocytic players (Wang et al., 2019). Attempts to visualize the recruitment of these two AtEH/Pan1 subunits to the PM, with respect to the other subunits, by lowering the temperature to enhance the temporal resolution of endocytosis could not reveal any differential behavior. It, therefore, appears that similar to COPI, the octameric TPC is also simultaneously recruited to the membrane (Wang et al., 2020).
TPC subunits interact with multiple conserved CME regulators including clathrin, AP2, Dynamin-related proteins (DRPs), and AP180 N-terminal homology (ANTH) -domain containing monomeric adaptors. TPC is recruited to the PM along with AP2 and prior to clathrin, which implies its role as an early adaptor complex (Van Damme et al., 2011; Gadeyne et al., 2014; Bashline et al., 2015; Narasimhan et al., 2020). Although TPC subunits have no counterparts in animal cells, several conserved domains are shared between TPC subunits and mammalian endocytic machinery such as Eps15, intersectin, and muniscin proteins, which are involved in membrane interaction, cargo recognition, and recruitment of accessory proteins (Reider and Wendland, 2011; Gadeyne et al., 2014).
The TPC is essential for plant survival and is involved in male gametogenesis, as all analyzed TPC subunit null mutants are male sterile. In addition, reduced expression of single subunits using constitutive and conditional amiR-TPLATE and amiR-TML expression strategies are seedling lethal (Van Damme et al., 2006; Gadeyne et al., 2014). TPC, however, appears to be essential only in plants, as a knock out mutant of the smallest TSET subunit in Dictyostelium only shows some defects in CME and a mild overall mutant phenotype (Hirst et al., 2014). Plants are, therefore, the only kingdom where TSET/TPC has been shown to be essential. No other TSET subunits have been mutated so far and a mutant in the smallest TPC subunit has not been experimentally tested in plants. Therefore, we cannot currently conclude that this specific subunit is exceptionally non-essential, either in slime moulds or in plants. In Arabidopsis, TPC and AP2 function largely, but not exclusively, together in CME at the PM. TPC plays an important role in stabilizing AP2 at the PM, as silencing TML significantly reduces the PM recruitment of AP2, and mild impairment of TPC function caused by lowering the levels of TWD40-2 in the weak twd40-2-3 mutant strongly affects AP2 dynamics (Gadeyne et al., 2014; Bashline et al., 2015).
Membrane trafficking complexes recognize cargo via specific motifs
Cargo recognition by endomembrane trafficking complexes occurs via sorting motifs, found in the cytoplasmic domains of the transmembrane cargo proteins. There are two categories of sorting motifs. The first group consists of linear recognition motifs that are 4–7 amino acids long. These motifs are flexible concerning their amino acid constitution and contain amino acids with bulky hydrophobic groups as critical residues. The second group comprises post-translational modifications such as ubiquitination and phosphorylation (Bonifacino and Traub, 2003).
The first sorting motif was discovered in a patient with familial hypercholesterolemia. In this case, a recessive base-pair mutation (tyrosine to cytosine) led to a failure in internalization of the LDL receptor (Anderson et al., 1977). Furthermore, in vitro mutagenesis of the LDL receptor revealed that the tetrapeptide NPVY, more specifically, its tyrosine amino acid, is required for endocytosis (Davis et al., 1986). This analysis enabled the discovery of NPxY as a sorting motif for various transmembrane proteins across the endomembrane system. In the following section, we will discuss the current knowledge on different cargo recognition motifs present in Arabidopsis by highlighting various examples for each case from the list summarized in Table 1.
| Cargo . | Adaptor . | Mode of Interaction . | Motif . | Mutant Phenotype . | References . |
|---|---|---|---|---|---|
| 12S GP | AP4 | IEM | UKN | Accumulation of precursor protein in ap4 seeds | (Fuji et al., 2016) |
| AALP | AP1 | LCCM, WB | UKN | Unprocessed protein mislocalizing to ER in ap1µ2-1 | (Park et al., 2013) |
| AGB-1 | AP3 | Y2H, CoIP, BiFC | UKN | – | (Kansup et al., 2013) |
| AtβFructosidase4 | AP1 | WB | UKN | Unprocessed protein in ap1µ2-1. | (Park et al., 2013) |
| BOR1 | AP3/AP4 | Y2H, CoIP | Tyr | Y to A mutations cause apolar localization. | (Takano et al., 2010; Yoshinari et al., 2016; 2019) |
| AP2 | Y2H, LCCM | Ct tail | Polar localization is disturbed in ap2 mutants | ||
| BRI1 | AP1 | LCCM | UKN | Recycling is compromised in hap13-1. | (Wang et al., 2013) |
| AP2 | LCCM, Co-IP | Ub | Reduced BFA accumulation in AP2µδC. 25 K to R shows excessive accumulation at the PM. | (Di Rubbo et al., 2013; Martins et al., 2015; Zhou et al., 2018) | |
| AP2 | CoIP | Tyr | Y to F of 898YKAI reduces BRI1 internalization and causes hypersensitivity to BR | (Liu et al., 2020) | |
| AP3 | LCCM | UKN | Aggregation in intracellular compartment in pat4 | (Zwiewka et al., 2011) | |
| TPC | LCCM | UKN | Enhanced PM accumulation in amiR-TML | (Gadeyne et al., 2014) | |
| CESA6 | AP2 | Y2H,CoIP, LCCM | UKN | Enhanced PM accumulation in ap2µ-1 mutant | (Bashline et al., 2013) |
| TPC | CoIP, BiFC | UKN | Enhanced PM accumulation in amiR-TML lines. | (Bashline et al., 2015; Sánchez-Rodríguez et al., 2018) | |
| EFR | UKN | LCCM | UKN | – | (Mbengue et al., 2016) |
| ESL1 | UKN | LCCM | LL | L to A mutation results in mislocalization to PM. | (Yamada et al., 2010; Wolfenstetter et al., 2012) |
| FLS2 | UKN | LCCM | Phos | T867V mutation causes flg22 insensitivity and reduced endocytosis | (Robatzek et al., 2006; Mbengue et al., 2016) |
| UKN | CoIP, WB | Ub | E3 ligase mutant reduces endocytosis. | (Lu et al., 2011) | |
| INT1 | AP1 | LCCM | LL | Deletion of LL results in ER accumulation. Accumulation of INT1 in AP1γ deficient cells. | (Wolfenstetter et al., 2012; Wang et al., 2014) |
| IRT1 | UKN | CoIP, LCCM | Ub | K to R mutation increases IRT1 on PM. | (Barberon et al., 2011) |
| UKN | CoIP, LCCM, WB | Phos | Cipk23 shows vacuolar sorting defects | (Dubeaux et al., 2018) | |
| Invertase | AP1 | LCCM, WB | UKN | Unprocessed protein mislocalizing to ER ap1µ2-1 | (Park et al., 2013) |
| KNOLLE (KN) | AP1 | LCCM | UKN | Mislocalization in ap1µ2-1 mutant. | (Park et al., 2013; Teh et al., 2013) |
| TPC | LCCM | UKN | Ectopic localization in amiR-TML | (Gadeyne et al., 2014) | |
| KOR1 | UKN | LCCM | Tyr & LL | Y to A and LL to AA results in PM accumulation | (Zuo et al., 2000) |
| LeEiX2 | AP2 | BiFC | Tyr | Y993A abolishes hyper-sensitivity response | (Ron and Avni, 2004; Bar et al., 2009) |
| LYK5 | UKN | LCCM, WB | Ub | Reduced internalization in pub13 mutant | (Liao et al., 2017) |
| UKN | CoIP, LCCM, WB | Phos | Kinase inhibitors show lower endocytosis | (Erwig et al., 2017) | |
| MOT2 | AP4 | LCCM | LL | L to A resulted in mislocalization to PM. Missorting in ap4β-1 mutant protoplasts. | (Gasber et al., 2011; Müdsam et al., 2018) |
| NIP5 | AP2 | LCCM | Phos | TPG to APG causes compromised polar distribution and reduces endocytosis. Low endocytosis in ap2µ. | (Wang et al., 2017) |
| NRAMP3, NRAMP4 | AP4 | LCCM | LL | Missorting to PM in β4-1 mutant. PM accumulation upon LL to A mutation. | (Müdsam et al., 2018) |
| PAT10 | AP3 | LCCM | UKN | Relocalization to cis-Golgi stacks in ap3. | (Feng et al., 2017) |
| PEPR1 | UKN | LCCM | UKN | No vesicles in fls2 and bak3-1. | (Mbengue et al., 2016; Ortiz-Morea et al., 2016) |
| PIN1 | AP2 | LCCM | UKN | Disruption of subcellular localization and internalization in ap2σ. | (Fan et al., 2013) |
| AP3 | CoIP, LCCM | MEQFP | F165A cannot bind µ3 and accumulates at the ER. | (Feraru et al., 2010; Zwiewka et al., 2011; Sancho-Andrés et al., 2016) | |
| TPC | LCCM | UKN | Reduced accumulation in BFA bodies in amiR-TML | (Gadeyne et al., 2014) | |
| PIN2 | AP1 | LCCM | UKN | Compromised BFA recycling in hap13-1. Delivery to vacuole severely impaired in ap1µ2-1 | (Park et al., 2013; Wang et al., 2013) |
| AP2 | LCCM | UKN | Reduced BFA accumulation in ap2µδC. | (Di Rubbo et al., 2013; Wang et al., 2016) | |
| AP3 | LCCM | UKN | Aggregation in intracellular compartment in ap3 | (Feraru et al., 2010; Zwiewka et al., 2011) | |
| TPC | LCCM | UKN | Reduced BFA accumulation in amiR-TML | (Gadeyne et al., 2014) | |
| UKN | CoIP, LCCM | Ub | MG132 treatment causes accumulation at the PM. | (Abas et al., 2006; Leitner et al., 2012a) | |
| PIP2 | UKN | LCCM | Ub | Increased levels in rma | (Lee et al., 2009) |
| AP3 | LCCM | UKN | Aggregation in internal compartments in ap3 | (Feraru et al., 2010; Zwiewka et al., 2011) | |
| AP4 | LCCM | UKN | Aggregation in internal compartments in ap4 | (Pertl-Obermeyer et al., 2016) | |
| PTR2, PTR4, PTR6 | UKN | LCCM | UKN | Mutation leads to failure in TP targeting. | (Komarova et al., 2012) |
| Sporamin | AP1 | WB | UKN | Unprocessed protein in ap1µ2-1. | (Park et al., 2013) |
| SUB | CME | CoIP, LCCM, WB | UKN | chc mutants show hyper accumulation of SUB at the PM | (Gao et al., 2019) |
| UKN | LCCM, WB | Ub | – | ||
| SUC4 | AP3 | LCCM | UKN | Accumulation in Golgi in ap3 | (Wolfenstetter et al., 2012) |
| TPC1 | UKN | LCCM | LL | LL to A results in mislocalization to PM | (Larisch et al., 2012) |
| VAMP711 | AP3 | LCCM | UKN | Mis targeting to PM in pat4-2. | (Feng et al., 2017) |
| VAMP713 | AP3 | LCCM | UKN | Mis localization to PM in ap3δ | (Ebine et al., 2014) |
| VirE1 | AP2 | CoIP, LCCM | Tyr | (Y488A/Y494A) eliminates interaction with ap2µ and decreases internalization | (Li and Pan, 2017) |
| VIT1 | AP1 | LCCM, Co-IP | LL | LL to AA mutation blocks tonoplast trafficking | (Wang et al., 2014) |
| VSR1 | AP1 | COIP, LCCM | UKN | N/A | (Park et al., 2013) |
| AP4 | Y2H,LCCM, CoIP | Tyr | Y606A mutation does not interact with ap4µ | (Fuji et al., 2016) | |
| VSR-BP80 | UKN | LCCM, CoIP | Tyr | Y612A mutation results in mistargeting to PM and inefficient progress to the PVC. | (daSilva et al., 2006) |
| PsVSR-PS1 | AP2 | Co-IP | Tyr | Y to A mutation no longer binds ap2µ | (Happel et al., 2004) |
| VAMP721 | PICALM1a PICALM1b | LCCM, Y2H, CoIP | UNKN | PM accumulation of VAMP721 in picalm1a/1b | (Fujimoto et al., 2020) |
| Cargo . | Adaptor . | Mode of Interaction . | Motif . | Mutant Phenotype . | References . |
|---|---|---|---|---|---|
| 12S GP | AP4 | IEM | UKN | Accumulation of precursor protein in ap4 seeds | (Fuji et al., 2016) |
| AALP | AP1 | LCCM, WB | UKN | Unprocessed protein mislocalizing to ER in ap1µ2-1 | (Park et al., 2013) |
| AGB-1 | AP3 | Y2H, CoIP, BiFC | UKN | – | (Kansup et al., 2013) |
| AtβFructosidase4 | AP1 | WB | UKN | Unprocessed protein in ap1µ2-1. | (Park et al., 2013) |
| BOR1 | AP3/AP4 | Y2H, CoIP | Tyr | Y to A mutations cause apolar localization. | (Takano et al., 2010; Yoshinari et al., 2016; 2019) |
| AP2 | Y2H, LCCM | Ct tail | Polar localization is disturbed in ap2 mutants | ||
| BRI1 | AP1 | LCCM | UKN | Recycling is compromised in hap13-1. | (Wang et al., 2013) |
| AP2 | LCCM, Co-IP | Ub | Reduced BFA accumulation in AP2µδC. 25 K to R shows excessive accumulation at the PM. | (Di Rubbo et al., 2013; Martins et al., 2015; Zhou et al., 2018) | |
| AP2 | CoIP | Tyr | Y to F of 898YKAI reduces BRI1 internalization and causes hypersensitivity to BR | (Liu et al., 2020) | |
| AP3 | LCCM | UKN | Aggregation in intracellular compartment in pat4 | (Zwiewka et al., 2011) | |
| TPC | LCCM | UKN | Enhanced PM accumulation in amiR-TML | (Gadeyne et al., 2014) | |
| CESA6 | AP2 | Y2H,CoIP, LCCM | UKN | Enhanced PM accumulation in ap2µ-1 mutant | (Bashline et al., 2013) |
| TPC | CoIP, BiFC | UKN | Enhanced PM accumulation in amiR-TML lines. | (Bashline et al., 2015; Sánchez-Rodríguez et al., 2018) | |
| EFR | UKN | LCCM | UKN | – | (Mbengue et al., 2016) |
| ESL1 | UKN | LCCM | LL | L to A mutation results in mislocalization to PM. | (Yamada et al., 2010; Wolfenstetter et al., 2012) |
| FLS2 | UKN | LCCM | Phos | T867V mutation causes flg22 insensitivity and reduced endocytosis | (Robatzek et al., 2006; Mbengue et al., 2016) |
| UKN | CoIP, WB | Ub | E3 ligase mutant reduces endocytosis. | (Lu et al., 2011) | |
| INT1 | AP1 | LCCM | LL | Deletion of LL results in ER accumulation. Accumulation of INT1 in AP1γ deficient cells. | (Wolfenstetter et al., 2012; Wang et al., 2014) |
| IRT1 | UKN | CoIP, LCCM | Ub | K to R mutation increases IRT1 on PM. | (Barberon et al., 2011) |
| UKN | CoIP, LCCM, WB | Phos | Cipk23 shows vacuolar sorting defects | (Dubeaux et al., 2018) | |
| Invertase | AP1 | LCCM, WB | UKN | Unprocessed protein mislocalizing to ER ap1µ2-1 | (Park et al., 2013) |
| KNOLLE (KN) | AP1 | LCCM | UKN | Mislocalization in ap1µ2-1 mutant. | (Park et al., 2013; Teh et al., 2013) |
| TPC | LCCM | UKN | Ectopic localization in amiR-TML | (Gadeyne et al., 2014) | |
| KOR1 | UKN | LCCM | Tyr & LL | Y to A and LL to AA results in PM accumulation | (Zuo et al., 2000) |
| LeEiX2 | AP2 | BiFC | Tyr | Y993A abolishes hyper-sensitivity response | (Ron and Avni, 2004; Bar et al., 2009) |
| LYK5 | UKN | LCCM, WB | Ub | Reduced internalization in pub13 mutant | (Liao et al., 2017) |
| UKN | CoIP, LCCM, WB | Phos | Kinase inhibitors show lower endocytosis | (Erwig et al., 2017) | |
| MOT2 | AP4 | LCCM | LL | L to A resulted in mislocalization to PM. Missorting in ap4β-1 mutant protoplasts. | (Gasber et al., 2011; Müdsam et al., 2018) |
| NIP5 | AP2 | LCCM | Phos | TPG to APG causes compromised polar distribution and reduces endocytosis. Low endocytosis in ap2µ. | (Wang et al., 2017) |
| NRAMP3, NRAMP4 | AP4 | LCCM | LL | Missorting to PM in β4-1 mutant. PM accumulation upon LL to A mutation. | (Müdsam et al., 2018) |
| PAT10 | AP3 | LCCM | UKN | Relocalization to cis-Golgi stacks in ap3. | (Feng et al., 2017) |
| PEPR1 | UKN | LCCM | UKN | No vesicles in fls2 and bak3-1. | (Mbengue et al., 2016; Ortiz-Morea et al., 2016) |
| PIN1 | AP2 | LCCM | UKN | Disruption of subcellular localization and internalization in ap2σ. | (Fan et al., 2013) |
| AP3 | CoIP, LCCM | MEQFP | F165A cannot bind µ3 and accumulates at the ER. | (Feraru et al., 2010; Zwiewka et al., 2011; Sancho-Andrés et al., 2016) | |
| TPC | LCCM | UKN | Reduced accumulation in BFA bodies in amiR-TML | (Gadeyne et al., 2014) | |
| PIN2 | AP1 | LCCM | UKN | Compromised BFA recycling in hap13-1. Delivery to vacuole severely impaired in ap1µ2-1 | (Park et al., 2013; Wang et al., 2013) |
| AP2 | LCCM | UKN | Reduced BFA accumulation in ap2µδC. | (Di Rubbo et al., 2013; Wang et al., 2016) | |
| AP3 | LCCM | UKN | Aggregation in intracellular compartment in ap3 | (Feraru et al., 2010; Zwiewka et al., 2011) | |
| TPC | LCCM | UKN | Reduced BFA accumulation in amiR-TML | (Gadeyne et al., 2014) | |
| UKN | CoIP, LCCM | Ub | MG132 treatment causes accumulation at the PM. | (Abas et al., 2006; Leitner et al., 2012a) | |
| PIP2 | UKN | LCCM | Ub | Increased levels in rma | (Lee et al., 2009) |
| AP3 | LCCM | UKN | Aggregation in internal compartments in ap3 | (Feraru et al., 2010; Zwiewka et al., 2011) | |
| AP4 | LCCM | UKN | Aggregation in internal compartments in ap4 | (Pertl-Obermeyer et al., 2016) | |
| PTR2, PTR4, PTR6 | UKN | LCCM | UKN | Mutation leads to failure in TP targeting. | (Komarova et al., 2012) |
| Sporamin | AP1 | WB | UKN | Unprocessed protein in ap1µ2-1. | (Park et al., 2013) |
| SUB | CME | CoIP, LCCM, WB | UKN | chc mutants show hyper accumulation of SUB at the PM | (Gao et al., 2019) |
| UKN | LCCM, WB | Ub | – | ||
| SUC4 | AP3 | LCCM | UKN | Accumulation in Golgi in ap3 | (Wolfenstetter et al., 2012) |
| TPC1 | UKN | LCCM | LL | LL to A results in mislocalization to PM | (Larisch et al., 2012) |
| VAMP711 | AP3 | LCCM | UKN | Mis targeting to PM in pat4-2. | (Feng et al., 2017) |
| VAMP713 | AP3 | LCCM | UKN | Mis localization to PM in ap3δ | (Ebine et al., 2014) |
| VirE1 | AP2 | CoIP, LCCM | Tyr | (Y488A/Y494A) eliminates interaction with ap2µ and decreases internalization | (Li and Pan, 2017) |
| VIT1 | AP1 | LCCM, Co-IP | LL | LL to AA mutation blocks tonoplast trafficking | (Wang et al., 2014) |
| VSR1 | AP1 | COIP, LCCM | UKN | N/A | (Park et al., 2013) |
| AP4 | Y2H,LCCM, CoIP | Tyr | Y606A mutation does not interact with ap4µ | (Fuji et al., 2016) | |
| VSR-BP80 | UKN | LCCM, CoIP | Tyr | Y612A mutation results in mistargeting to PM and inefficient progress to the PVC. | (daSilva et al., 2006) |
| PsVSR-PS1 | AP2 | Co-IP | Tyr | Y to A mutation no longer binds ap2µ | (Happel et al., 2004) |
| VAMP721 | PICALM1a PICALM1b | LCCM, Y2H, CoIP | UNKN | PM accumulation of VAMP721 in picalm1a/1b | (Fujimoto et al., 2020) |
Ps, Pisum sativum; LCCM, live cell confocal microscopy; UKN, unknown; LL, dileucine motif; Tyr,tyrosine motif; Ub, ubiquitin ; BiFC, bimolecular fluorescence complementation; WB, Western blot; IEM, immuno-electron microscopy; GP, globulin precursor; Phos, phosphorylation; TPC1, two pore channel-1; Ct, C-terminal; CoIP, Co-immuno precipitation.
| Cargo . | Adaptor . | Mode of Interaction . | Motif . | Mutant Phenotype . | References . |
|---|---|---|---|---|---|
| 12S GP | AP4 | IEM | UKN | Accumulation of precursor protein in ap4 seeds | (Fuji et al., 2016) |
| AALP | AP1 | LCCM, WB | UKN | Unprocessed protein mislocalizing to ER in ap1µ2-1 | (Park et al., 2013) |
| AGB-1 | AP3 | Y2H, CoIP, BiFC | UKN | – | (Kansup et al., 2013) |
| AtβFructosidase4 | AP1 | WB | UKN | Unprocessed protein in ap1µ2-1. | (Park et al., 2013) |
| BOR1 | AP3/AP4 | Y2H, CoIP | Tyr | Y to A mutations cause apolar localization. | (Takano et al., 2010; Yoshinari et al., 2016; 2019) |
| AP2 | Y2H, LCCM | Ct tail | Polar localization is disturbed in ap2 mutants | ||
| BRI1 | AP1 | LCCM | UKN | Recycling is compromised in hap13-1. | (Wang et al., 2013) |
| AP2 | LCCM, Co-IP | Ub | Reduced BFA accumulation in AP2µδC. 25 K to R shows excessive accumulation at the PM. | (Di Rubbo et al., 2013; Martins et al., 2015; Zhou et al., 2018) | |
| AP2 | CoIP | Tyr | Y to F of 898YKAI reduces BRI1 internalization and causes hypersensitivity to BR | (Liu et al., 2020) | |
| AP3 | LCCM | UKN | Aggregation in intracellular compartment in pat4 | (Zwiewka et al., 2011) | |
| TPC | LCCM | UKN | Enhanced PM accumulation in amiR-TML | (Gadeyne et al., 2014) | |
| CESA6 | AP2 | Y2H,CoIP, LCCM | UKN | Enhanced PM accumulation in ap2µ-1 mutant | (Bashline et al., 2013) |
| TPC | CoIP, BiFC | UKN | Enhanced PM accumulation in amiR-TML lines. | (Bashline et al., 2015; Sánchez-Rodríguez et al., 2018) | |
| EFR | UKN | LCCM | UKN | – | (Mbengue et al., 2016) |
| ESL1 | UKN | LCCM | LL | L to A mutation results in mislocalization to PM. | (Yamada et al., 2010; Wolfenstetter et al., 2012) |
| FLS2 | UKN | LCCM | Phos | T867V mutation causes flg22 insensitivity and reduced endocytosis | (Robatzek et al., 2006; Mbengue et al., 2016) |
| UKN | CoIP, WB | Ub | E3 ligase mutant reduces endocytosis. | (Lu et al., 2011) | |
| INT1 | AP1 | LCCM | LL | Deletion of LL results in ER accumulation. Accumulation of INT1 in AP1γ deficient cells. | (Wolfenstetter et al., 2012; Wang et al., 2014) |
| IRT1 | UKN | CoIP, LCCM | Ub | K to R mutation increases IRT1 on PM. | (Barberon et al., 2011) |
| UKN | CoIP, LCCM, WB | Phos | Cipk23 shows vacuolar sorting defects | (Dubeaux et al., 2018) | |
| Invertase | AP1 | LCCM, WB | UKN | Unprocessed protein mislocalizing to ER ap1µ2-1 | (Park et al., 2013) |
| KNOLLE (KN) | AP1 | LCCM | UKN | Mislocalization in ap1µ2-1 mutant. | (Park et al., 2013; Teh et al., 2013) |
| TPC | LCCM | UKN | Ectopic localization in amiR-TML | (Gadeyne et al., 2014) | |
| KOR1 | UKN | LCCM | Tyr & LL | Y to A and LL to AA results in PM accumulation | (Zuo et al., 2000) |
| LeEiX2 | AP2 | BiFC | Tyr | Y993A abolishes hyper-sensitivity response | (Ron and Avni, 2004; Bar et al., 2009) |
| LYK5 | UKN | LCCM, WB | Ub | Reduced internalization in pub13 mutant | (Liao et al., 2017) |
| UKN | CoIP, LCCM, WB | Phos | Kinase inhibitors show lower endocytosis | (Erwig et al., 2017) | |
| MOT2 | AP4 | LCCM | LL | L to A resulted in mislocalization to PM. Missorting in ap4β-1 mutant protoplasts. | (Gasber et al., 2011; Müdsam et al., 2018) |
| NIP5 | AP2 | LCCM | Phos | TPG to APG causes compromised polar distribution and reduces endocytosis. Low endocytosis in ap2µ. | (Wang et al., 2017) |
| NRAMP3, NRAMP4 | AP4 | LCCM | LL | Missorting to PM in β4-1 mutant. PM accumulation upon LL to A mutation. | (Müdsam et al., 2018) |
| PAT10 | AP3 | LCCM | UKN | Relocalization to cis-Golgi stacks in ap3. | (Feng et al., 2017) |
| PEPR1 | UKN | LCCM | UKN | No vesicles in fls2 and bak3-1. | (Mbengue et al., 2016; Ortiz-Morea et al., 2016) |
| PIN1 | AP2 | LCCM | UKN | Disruption of subcellular localization and internalization in ap2σ. | (Fan et al., 2013) |
| AP3 | CoIP, LCCM | MEQFP | F165A cannot bind µ3 and accumulates at the ER. | (Feraru et al., 2010; Zwiewka et al., 2011; Sancho-Andrés et al., 2016) | |
| TPC | LCCM | UKN | Reduced accumulation in BFA bodies in amiR-TML | (Gadeyne et al., 2014) | |
| PIN2 | AP1 | LCCM | UKN | Compromised BFA recycling in hap13-1. Delivery to vacuole severely impaired in ap1µ2-1 | (Park et al., 2013; Wang et al., 2013) |
| AP2 | LCCM | UKN | Reduced BFA accumulation in ap2µδC. | (Di Rubbo et al., 2013; Wang et al., 2016) | |
| AP3 | LCCM | UKN | Aggregation in intracellular compartment in ap3 | (Feraru et al., 2010; Zwiewka et al., 2011) | |
| TPC | LCCM | UKN | Reduced BFA accumulation in amiR-TML | (Gadeyne et al., 2014) | |
| UKN | CoIP, LCCM | Ub | MG132 treatment causes accumulation at the PM. | (Abas et al., 2006; Leitner et al., 2012a) | |
| PIP2 | UKN | LCCM | Ub | Increased levels in rma | (Lee et al., 2009) |
| AP3 | LCCM | UKN | Aggregation in internal compartments in ap3 | (Feraru et al., 2010; Zwiewka et al., 2011) | |
| AP4 | LCCM | UKN | Aggregation in internal compartments in ap4 | (Pertl-Obermeyer et al., 2016) | |
| PTR2, PTR4, PTR6 | UKN | LCCM | UKN | Mutation leads to failure in TP targeting. | (Komarova et al., 2012) |
| Sporamin | AP1 | WB | UKN | Unprocessed protein in ap1µ2-1. | (Park et al., 2013) |
| SUB | CME | CoIP, LCCM, WB | UKN | chc mutants show hyper accumulation of SUB at the PM | (Gao et al., 2019) |
| UKN | LCCM, WB | Ub | – | ||
| SUC4 | AP3 | LCCM | UKN | Accumulation in Golgi in ap3 | (Wolfenstetter et al., 2012) |
| TPC1 | UKN | LCCM | LL | LL to A results in mislocalization to PM | (Larisch et al., 2012) |
| VAMP711 | AP3 | LCCM | UKN | Mis targeting to PM in pat4-2. | (Feng et al., 2017) |
| VAMP713 | AP3 | LCCM | UKN | Mis localization to PM in ap3δ | (Ebine et al., 2014) |
| VirE1 | AP2 | CoIP, LCCM | Tyr | (Y488A/Y494A) eliminates interaction with ap2µ and decreases internalization | (Li and Pan, 2017) |
| VIT1 | AP1 | LCCM, Co-IP | LL | LL to AA mutation blocks tonoplast trafficking | (Wang et al., 2014) |
| VSR1 | AP1 | COIP, LCCM | UKN | N/A | (Park et al., 2013) |
| AP4 | Y2H,LCCM, CoIP | Tyr | Y606A mutation does not interact with ap4µ | (Fuji et al., 2016) | |
| VSR-BP80 | UKN | LCCM, CoIP | Tyr | Y612A mutation results in mistargeting to PM and inefficient progress to the PVC. | (daSilva et al., 2006) |
| PsVSR-PS1 | AP2 | Co-IP | Tyr | Y to A mutation no longer binds ap2µ | (Happel et al., 2004) |
| VAMP721 | PICALM1a PICALM1b | LCCM, Y2H, CoIP | UNKN | PM accumulation of VAMP721 in picalm1a/1b | (Fujimoto et al., 2020) |
| Cargo . | Adaptor . | Mode of Interaction . | Motif . | Mutant Phenotype . | References . |
|---|---|---|---|---|---|
| 12S GP | AP4 | IEM | UKN | Accumulation of precursor protein in ap4 seeds | (Fuji et al., 2016) |
| AALP | AP1 | LCCM, WB | UKN | Unprocessed protein mislocalizing to ER in ap1µ2-1 | (Park et al., 2013) |
| AGB-1 | AP3 | Y2H, CoIP, BiFC | UKN | – | (Kansup et al., 2013) |
| AtβFructosidase4 | AP1 | WB | UKN | Unprocessed protein in ap1µ2-1. | (Park et al., 2013) |
| BOR1 | AP3/AP4 | Y2H, CoIP | Tyr | Y to A mutations cause apolar localization. | (Takano et al., 2010; Yoshinari et al., 2016; 2019) |
| AP2 | Y2H, LCCM | Ct tail | Polar localization is disturbed in ap2 mutants | ||
| BRI1 | AP1 | LCCM | UKN | Recycling is compromised in hap13-1. | (Wang et al., 2013) |
| AP2 | LCCM, Co-IP | Ub | Reduced BFA accumulation in AP2µδC. 25 K to R shows excessive accumulation at the PM. | (Di Rubbo et al., 2013; Martins et al., 2015; Zhou et al., 2018) | |
| AP2 | CoIP | Tyr | Y to F of 898YKAI reduces BRI1 internalization and causes hypersensitivity to BR | (Liu et al., 2020) | |
| AP3 | LCCM | UKN | Aggregation in intracellular compartment in pat4 | (Zwiewka et al., 2011) | |
| TPC | LCCM | UKN | Enhanced PM accumulation in amiR-TML | (Gadeyne et al., 2014) | |
| CESA6 | AP2 | Y2H,CoIP, LCCM | UKN | Enhanced PM accumulation in ap2µ-1 mutant | (Bashline et al., 2013) |
| TPC | CoIP, BiFC | UKN | Enhanced PM accumulation in amiR-TML lines. | (Bashline et al., 2015; Sánchez-Rodríguez et al., 2018) | |
| EFR | UKN | LCCM | UKN | – | (Mbengue et al., 2016) |
| ESL1 | UKN | LCCM | LL | L to A mutation results in mislocalization to PM. | (Yamada et al., 2010; Wolfenstetter et al., 2012) |
| FLS2 | UKN | LCCM | Phos | T867V mutation causes flg22 insensitivity and reduced endocytosis | (Robatzek et al., 2006; Mbengue et al., 2016) |
| UKN | CoIP, WB | Ub | E3 ligase mutant reduces endocytosis. | (Lu et al., 2011) | |
| INT1 | AP1 | LCCM | LL | Deletion of LL results in ER accumulation. Accumulation of INT1 in AP1γ deficient cells. | (Wolfenstetter et al., 2012; Wang et al., 2014) |
| IRT1 | UKN | CoIP, LCCM | Ub | K to R mutation increases IRT1 on PM. | (Barberon et al., 2011) |
| UKN | CoIP, LCCM, WB | Phos | Cipk23 shows vacuolar sorting defects | (Dubeaux et al., 2018) | |
| Invertase | AP1 | LCCM, WB | UKN | Unprocessed protein mislocalizing to ER ap1µ2-1 | (Park et al., 2013) |
| KNOLLE (KN) | AP1 | LCCM | UKN | Mislocalization in ap1µ2-1 mutant. | (Park et al., 2013; Teh et al., 2013) |
| TPC | LCCM | UKN | Ectopic localization in amiR-TML | (Gadeyne et al., 2014) | |
| KOR1 | UKN | LCCM | Tyr & LL | Y to A and LL to AA results in PM accumulation | (Zuo et al., 2000) |
| LeEiX2 | AP2 | BiFC | Tyr | Y993A abolishes hyper-sensitivity response | (Ron and Avni, 2004; Bar et al., 2009) |
| LYK5 | UKN | LCCM, WB | Ub | Reduced internalization in pub13 mutant | (Liao et al., 2017) |
| UKN | CoIP, LCCM, WB | Phos | Kinase inhibitors show lower endocytosis | (Erwig et al., 2017) | |
| MOT2 | AP4 | LCCM | LL | L to A resulted in mislocalization to PM. Missorting in ap4β-1 mutant protoplasts. | (Gasber et al., 2011; Müdsam et al., 2018) |
| NIP5 | AP2 | LCCM | Phos | TPG to APG causes compromised polar distribution and reduces endocytosis. Low endocytosis in ap2µ. | (Wang et al., 2017) |
| NRAMP3, NRAMP4 | AP4 | LCCM | LL | Missorting to PM in β4-1 mutant. PM accumulation upon LL to A mutation. | (Müdsam et al., 2018) |
| PAT10 | AP3 | LCCM | UKN | Relocalization to cis-Golgi stacks in ap3. | (Feng et al., 2017) |
| PEPR1 | UKN | LCCM | UKN | No vesicles in fls2 and bak3-1. | (Mbengue et al., 2016; Ortiz-Morea et al., 2016) |
| PIN1 | AP2 | LCCM | UKN | Disruption of subcellular localization and internalization in ap2σ. | (Fan et al., 2013) |
| AP3 | CoIP, LCCM | MEQFP | F165A cannot bind µ3 and accumulates at the ER. | (Feraru et al., 2010; Zwiewka et al., 2011; Sancho-Andrés et al., 2016) | |
| TPC | LCCM | UKN | Reduced accumulation in BFA bodies in amiR-TML | (Gadeyne et al., 2014) | |
| PIN2 | AP1 | LCCM | UKN | Compromised BFA recycling in hap13-1. Delivery to vacuole severely impaired in ap1µ2-1 | (Park et al., 2013; Wang et al., 2013) |
| AP2 | LCCM | UKN | Reduced BFA accumulation in ap2µδC. | (Di Rubbo et al., 2013; Wang et al., 2016) | |
| AP3 | LCCM | UKN | Aggregation in intracellular compartment in ap3 | (Feraru et al., 2010; Zwiewka et al., 2011) | |
| TPC | LCCM | UKN | Reduced BFA accumulation in amiR-TML | (Gadeyne et al., 2014) | |
| UKN | CoIP, LCCM | Ub | MG132 treatment causes accumulation at the PM. | (Abas et al., 2006; Leitner et al., 2012a) | |
| PIP2 | UKN | LCCM | Ub | Increased levels in rma | (Lee et al., 2009) |
| AP3 | LCCM | UKN | Aggregation in internal compartments in ap3 | (Feraru et al., 2010; Zwiewka et al., 2011) | |
| AP4 | LCCM | UKN | Aggregation in internal compartments in ap4 | (Pertl-Obermeyer et al., 2016) | |
| PTR2, PTR4, PTR6 | UKN | LCCM | UKN | Mutation leads to failure in TP targeting. | (Komarova et al., 2012) |
| Sporamin | AP1 | WB | UKN | Unprocessed protein in ap1µ2-1. | (Park et al., 2013) |
| SUB | CME | CoIP, LCCM, WB | UKN | chc mutants show hyper accumulation of SUB at the PM | (Gao et al., 2019) |
| UKN | LCCM, WB | Ub | – | ||
| SUC4 | AP3 | LCCM | UKN | Accumulation in Golgi in ap3 | (Wolfenstetter et al., 2012) |
| TPC1 | UKN | LCCM | LL | LL to A results in mislocalization to PM | (Larisch et al., 2012) |
| VAMP711 | AP3 | LCCM | UKN | Mis targeting to PM in pat4-2. | (Feng et al., 2017) |
| VAMP713 | AP3 | LCCM | UKN | Mis localization to PM in ap3δ | (Ebine et al., 2014) |
| VirE1 | AP2 | CoIP, LCCM | Tyr | (Y488A/Y494A) eliminates interaction with ap2µ and decreases internalization | (Li and Pan, 2017) |
| VIT1 | AP1 | LCCM, Co-IP | LL | LL to AA mutation blocks tonoplast trafficking | (Wang et al., 2014) |
| VSR1 | AP1 | COIP, LCCM | UKN | N/A | (Park et al., 2013) |
| AP4 | Y2H,LCCM, CoIP | Tyr | Y606A mutation does not interact with ap4µ | (Fuji et al., 2016) | |
| VSR-BP80 | UKN | LCCM, CoIP | Tyr | Y612A mutation results in mistargeting to PM and inefficient progress to the PVC. | (daSilva et al., 2006) |
| PsVSR-PS1 | AP2 | Co-IP | Tyr | Y to A mutation no longer binds ap2µ | (Happel et al., 2004) |
| VAMP721 | PICALM1a PICALM1b | LCCM, Y2H, CoIP | UNKN | PM accumulation of VAMP721 in picalm1a/1b | (Fujimoto et al., 2020) |
Ps, Pisum sativum; LCCM, live cell confocal microscopy; UKN, unknown; LL, dileucine motif; Tyr,tyrosine motif; Ub, ubiquitin ; BiFC, bimolecular fluorescence complementation; WB, Western blot; IEM, immuno-electron microscopy; GP, globulin precursor; Phos, phosphorylation; TPC1, two pore channel-1; Ct, C-terminal; CoIP, Co-immuno precipitation.
Linear Recognition motifs
This group comprises some of the most widely studied motifs involved in transmembrane protein trafficking. Linear recognition motifs in eukaryotes are annotated based on their critical residues, namely, tyrosine-, lysine-, or dileucine-based, and to a minor extent also phenylalanine-based motifs. The interactions mediated by these short motifs are of low affinity. Overexpression of chimeric proteins containing linear motifs leads to the accumulation on the cell surface of endogenous proteins containing these motifs, indicating that they saturate the system by competing for adaptor molecules for their sorting (Marks et al., 1996). How transmembrane proteins utilize these motifs under physiological conditions to compete with each other for adaptor molecules to allow their efficient trafficking remains an open question. We next discuss different types of linear recognition motifs found in Arabidopsis by focusing on several known cargoes that rely on those motifs for their trafficking.
Tyrosine motifs
In eukaryotes, two types of tyrosine-based motifs, represented by NPxY and YXXϕ are present. Between these two, the YXXϕ motifs have been mapped onto several receptor-like kinases (Geldner and Robatzek, 2008). Some of these motifs have been so far been implicated in the sorting of transmembrane proteins in plants; therefore, we focus on this motif here. The presence of tyrosine motifs in membrane proteins is unfortunately not necessarily correlated with a role as a recognition signal for trafficking (Yamamoto et al., 2018).
In YXXϕ, tyrosine (Y) is the essential amino acid, which highlights the importance of the phenolic hydroxyl group in recognition of this motif. Moreover, X and other amino acids neighboring this motif do not affect the functionality, but they can contribute to the strength and specificity of the interaction with different AP complexes (Ohno et al., 1998). At the same time, ϕ can be any bulky hydrophobic amino acid. In animals, µ subunits interact with tyrosine-based motifs with an affinity ranging between 10 and 70 µM (Rapoport et al., 1997; Stephens et al., 1997). YXXϕ motifs are responsible for transmembrane protein trafficking via AP2, AP3, and AP4 in animal cells.
The first tyrosine motif-based interaction in plants was discovered when the pea (Pisum sativum) VSR-PS1 was shown to interact with AP2µ of Arabidopsis (Happel et al., 2004). VSR-PS1 is a transmembrane protein containing a tyrosine motif (606YMPL) in its cytoplasmic C-terminal tail. The tyrosine motif, along with the whole cytoplasmic tail of VSR-PS1, interacts in vitro with the receptor-binding domain of AP2µ. Since VSRs traffic between the ER and the vacuole, and AP2 functions at the PM, the only physiological relevance for this interaction would lie in the unlikely possibility of AP2 functioning in the retrieval of missorted VSRs from the PM. The physiological relevance of this interaction, therefore, remains unclear. Nevertheless, it exemplifies the capacity of AP2µ to interact with tyrosine-based motifs.
Virulence protein E2 (VirE2) is a secretory, virulent protein of Agrobacterium that uses the host endocytic machinery for its internalization (Ward and Zambryski, 2001; Li and Pan, 2017). In vitro studies have shown that VirE2 can form voltage-gated transmembrane channels capable of transporting ssDNA through membranes (Dumas et al., 2001). VirE2 is internalized and colocalizes with early endosome markers, but not with late ones, suggesting that the protein escapes from the endosomes to carry out its transformation function as an ssDNA binding protein. The C-terminal tail of VirE2 has two tyrosine motifs (488YTSV and 494YERL), which interact with AP2µ. Mutating one or both of these tyrosine motifs significantly reduces the efficiency of Agrobacterium to transform Arabidopsis in comparison to wild-type (Li and Pan, 2017), indicating that AP2 dependent CME of VirE2 mediates its virulence. VirE2 represents one of the few examples where tyrosine motif recognition by AP2µ has been shown to be important for protein trafficking.
Another example of tyrosine motif recognition for AP2 dependent internalization was recently published (Liu et al., 2020). The Brassinosteroid hormone receptor Brassinosteroid Insensitive 1 (BRI1) is a transmembrane PM localized Leucine-Rich Repeat Receptor-Like Kinase (LRR-RLK). BRI1 undergoes ligand-independent, AP2 mediated endocytosis (Geldner et al., 2007; Irani et al., 2012; Di Rubbo et al., 2013). The C-terminal cytoplasmic tail of BRI1 contains five putative canonical and surface-exposed tyrosine motifs. Combining motif mutations with functionality assays such as autophosphorylation and transphosphorylation, and mutant complementation allowed the identification of a single motif (898YKAI) that affected BRI1 endocytosis without affecting its kinase activity. In vitro, a recombinant AP2µ-homology domain interacts with a peptide containing a triple repeat of this motif (3xDVYKAI), and this interaction is reduced by substituting the three tyrosine residues with alanine. Together, this highlights the partial importance of the 898YKAI motif in the AP2 dependent internalization of BRI1. The mild increase in BR hypersensitivity, as well as the fact that BRI1 internalization was not abolished in the 898YKAI motif mutant, does imply that AP2-dependent tyrosine motif recognition works in concert with other signals to regulate BRI1 endocytosis (Liu et al., 2020).
Boron transporter 1 (BOR1), a borate efflux transporter, depends on AP2 mediated endocytosis for maintaining its polar PM localization (Yoshinari et al., 2019). BOR1 constitutively undergoes endocytosis and recycling under low boron conditions, and undergoes transport to the vacuole via MVBs under high boron conditions (Takano et al., 2005). An initial hypothesis suggested that CME of BOR1 was mediated via the tyrosine motifs (Y373/Y398/Y405) present in its cytoplasmic loop. Mutating one or more of these three tyrosine residues showed significantly slower BOR1 degradation (i.e. a longer PM residence time) than the wild-type BOR1, initially implying that this hypothesis was valid (Takano et al., 2010). Later on, however, yeast two-hybrid (Y2H) and pull-down experiments showed that the BOR1 tyrosine motifs interacted with AP3µ and AP4µ rather than AP2µ. Moreover, BOR1 interacted with AP2 via its cytoplasmic C-terminal tail, which does not contain tyrosine motifs (Yoshinari et al., 2019). Therefore, the failure of BOR1 (containing mutated tyrosine motifs) to be removed from the PM does not indicate failed internalization by AP2, but actually represents induced recycling caused by a failure in vacuolar trafficking. Hence, the three tyrosine motifs in the cytoplasmic loop BOR1 serve to designate it for vacuolar trafficking, while its internalization by AP2 occurs via an unknown motif present in its C-terminal tail.
In tomato, Lycopersicon esculentum ethylene-inducing xylanase 2 (LeEiX2) is an LRR-RLP protein involved in signaling and defense responses to the fungal microbe-associated molecular pattern EIX (Ron and Avni, 2004) . Interaction between the fungal elicitor EIX and LeEIX2 causes the receptor to undergo ligand-mediated internalization, and induces a hypersensitive response with electrolyte leakage and ethylene biosynthesis (Ron et al., 2000; Bar and Avni, 2009). The internalization and hypersensitivity response of LeEIX2 are dependent on an intact tyrosine motif (993YFTF) present in its short cytoplasmic domain, and LeEIX2 interacts with AP2µ (Ron and Avni, 2004; Bar et al., 2009). Whether the tyrosine motif is actually required for internalization or degradation remains, however, to be determined. As seen in the case of BOR1, decreased internalization of LeEiX2 in the case of tyrosine mutation could also be explained by blocked degradation rather than blocked internalization. Therefore, whether the interaction between the receptor and AP2µ requires this tyrosine motif needs to be tested directly. The same holds true for the tyrosine motifs in the endo-1,4-b-D-glucanase KORRIGAN. Mutating these motifs caused KORRIGAN to accumulate at the PM, yet so far there is no established link between these motifs and a specific AP complex (Zuo et al., 2000).
Tyrosine motifs have also been investigated in the context of the PIN auxin efflux carriers. A conserved tyrosine motif in the cytoplasmic loop of PIN2 is required for its polar localization (Kleine-Vehn et al., 2011), and mutating a similar tyrosine motif in PIN1 (NPNSY to NSLSL) retained this protein in the ER, similar to the PIN proteins of the PIN5 clade, where this motif is not present (Mravec et al., 2009). Several Tyrosine motifs present in the cytoplasmic loop of PIN1 have been shown to possess some degree of differential affinity toward specific APµ subunits via in vitro interaction assays using truncated constructs lacking certain motifs, and the NPNSY motif mentioned above was linked to AP1µ and AP4µ, but not to AP2µ or AP3µ. Several single tyrosine motif mutants, however, showed no difference in steady-state localization of PIN1 and rescued the pin1 mutant phenotype. This indicates that none of those motifs is absolutely required for PIN1 function, although higher-order combinations might be (Marcote et al., 2016; Sancho-Andrés et al., 2016). Tyrosine motifs also mediate the sorting of VSR1, a transmembrane VSR, from TGN to the vacuole. AP4µ interacts with the tyrosine motif (606YMPL) present in the C-terminus cytosolic tail of VSR1. Mutational studies highlight the necessity of this tyrosine motif for the function and localization VSR1 (Fuji et al., 2016).
In conclusion, the evidence accumulated so far linking tyrosine motifs to the AP-dependent trafficking of Arabidopsis proteins remains scarce. Moreover, the evidence for tyrosine motifs functioning in trafficking pathways downstream of CME, such as vacuolar sorting for degradation (i.e. AP3 and AP4-dependent), outcompetes the evidence implicating tyrosine motif-mediated, AP2-dependent CME. We basically lack clear examples of transmembrane PM localized proteins whose internalization has been unambiguously shown to rely on the interaction between a tyrosine motif and AP2.
Dileucine motifs
The dileucine motif was discovered and shown to be functional in the sorting of the CD3γ chain of the human T-cell antigen receptor (Letourneur and Klausner, 1992). There are two types of dileucine motifs present in animals, represented by [D/E]xxxL[L/I] and DxxLL. The [D/E]xxxL[L/I] motif interacts with AP1 (γ-σ), AP2 (α-σ), and AP3 (δ-σ) hemicomplexes (Bonifacino, 2004; Mattera et al., 2011). Moreover, crystallization experiments have shown that the two leucines of the [D/E]xxxL[L/I] motif occupy two adjacent hydrophobic pockets in the small subunit of the AP complex, which is highly conserved in AP 1–4 complexes in animals. At the same time, the negatively charged amino acids present at its L-4 position (D/E) are also important, as this position was shown to bind to the positively charged hydrophilic patches of the AP2 (α-σ) hemicomplex (Kelly et al., 2008).
Vacuolar iron transporter 1 (VIT1), an iron transporter, and inositol transporter 1 (INT1), an inositol-proton symporter, are tonoplast localized transmembrane proteins of Arabidopsis. The N-terminally located cytoplasmic dileucine motif 16EKQTLL21 of VIT1 and the C-terminally located cytoplasmic dileucine motif 499NMEGLLEQ506 of INT1 are essential for their tonoplast sorting (Wang et al., 2014). Furthermore, these dileucine motifs have also been shown to be sufficient for ectopic tonoplast targeting of PM proteins like SCAMP1 and INT4 (Wang et al., 2014). Both VIT1 and INT1, specifically interact with AP1, as they mislocalize in functionally disabled AP1δ lines (Wang et al., 2014). In addition, there are examples of other AP complexes sorting transmembrane cargo proteins to the tonoplast via dileucine motifs. The tonoplast localized, divalent cation transporters natural resistance macrophage protein 3 and 4 (NRAMP3/NRAMP4) and the molybdate exporter 2 (MOT2) contain N-terminally localized dileucine motifs that interact with AP4 for their sorting (Gasber et al., 2011; Müdsam et al., 2018), while dileucine-dependent tonoplast sorting of sucrose transport protein 4 (SUC4), a sucrose transporter, is mediated by AP3 (Wolfenstetter et al., 2012).
In conclusion, the dileucine motifs have been convincingly shown to be essential and sufficient for the trafficking of transmembrane cargoes to the tonoplast in plants. While VIT1 contains a dileucine motif that fits the consensus representation [D/E]xxxL[L/I] (16EKQTLL21), motifs in other plant proteins often deviate from the consensus motif. Various cargoes with functional dileucine residues, such as NRAMPs, MOT2, and INT1, do not contain an acidic amino acid at the −4 position. For example, the C-terminal dileucine motif of INT1 (499NMEGLLEQ506) contains an asparagine. Although the dileucine motif, which is essential for trafficking of early-responsive to dehydration6-like1 (ESL1; LEAGLLL; Table 1), fits the conventional dileucine representation, three of the four leucines are actually essential for its localization (Yamada et al., 2010). Hence, this dileucine motif is also unconventional, and represented by LxxxLL. The dileucine peptide (5EEEARPLI12), which is sufficient for targeting the peptide-/nitrate- transporter PTR2 to the tonoplast (Table 1), requires LI and glutamate at its −6 position for its trafficking. The dileucine motifs for sorting of cargoes in plants deviate from the canonical motif proposed in animals, and the plant consensus dileucine motif is better represented by [D/E]x3-5L[L/I], containing 3–5 amino acids between the acidic amino acid and the two essential dileucine/isoleucine residues (Komarova et al., 2012).
Dileucine motifs are recognized by AP1, AP2, AP3, and AP4 complexes in animal cells (Kelly et al., 2008). In plants, so far, we only have examples of dileucine motifs mediating the trafficking of proteins recognized by AP1, AP3, and AP4. The above-mentioned cargoes, trafficked via recognition of their dileucine motif, have the tonoplast as the destination membrane.
Similar to the scarce evidence linking AP2 to tyrosine-based motifs, there are so far no examples of dileucine motifs that are recognized by AP2.
Lysine motifs
Lysine motifs are used for COPI-mediated retention of proteins in the Golgi and ER. KKXX and KXKXX are two types of canonical dilysine motifs functioning in eukaryotes. Among these two, the dilysine motif represented by KKXX functions in plants (Gao et al., 2014). The identity of the two amino acids following the dilysine motif (XX) has been shown to affect the ER targeting efficiency of the dilysine motif in animals (Itin et al., 1995; Zerangue et al., 2001). These motifs are present in the cytoplasmic C-terminal tail and function in ER retention of ER-resident proteins, for example, the Type-I membrane proteins, such as the p24 family in Arabidopsis (Langhans et al., 2008). The dilysine motif 213KKLI present at the C-terminus is sufficient for ER localization of p24δ5, as K to S mutation, leads to vacuolar missorting and less efficient binding with COPI (Contreras et al., 2004; Langhans et al., 2008). Dilysine motifs present in the p24 family proteins, as well as other plant transmembrane proteins, are summarized in (Gao et al., 2014).
Another lysine-based motif (KXD/E) is involved in Golgi retention of Golgi-resident proteins in eukaryotes. In plants, Endomembrane protein 12 (EMP12) is a Golgi localized integral protein that contains a 589KCD motif in its cytoplasmic C-terminal domain. The lysine motif is important and sufficient for Golgi retention, as K to A mutation results in vacuolar targeting of EMP12 (Gao et al., 2012). The lysine motif-dependent Golgi retention of EMP12 protein depends on its interaction with COPI, as a knockdown mutant of a COPI subunit, εCOP, results in mislocalization of EMP12 to vacuole. The experimental analysis of lysine motifs present in EMP proteins across different species shows the conserved function of this motif in COPI-mediated Golgi retention across kingdoms (Woo et al., 2015).
In conclusion, from the evidence gathered so far, one can speculate that COPIa mediated retrograde transport from Golgi to ER is dependent on the dilysine based KKXX motif, while COPIb mediated intra-Golgi transport is dependent on KXD/E motif-based interaction. However, further investigation is required for a better understanding of lysine motif-mediated COPI transport.
Phenylalanine motifs
The human mannose-6-phosphate receptor contains three partially redundant motifs necessary for its internalization and sorting (Johnson et al., 1990; Höning et al., 1997). Two are canonical tyrosine and dileucine-based sorting motifs. The third internalization motif is a non-canonical one, containing phenylalanine as its critical residue (Denzer et al., 1997). The sequence is a heptapeptide (7SKGMEQF) in its cytoplasmic tail, and which is essential for binding AP1 and AP2 (Höning et al., 1997). Similarly, a phenylalanine-based motif (around Phe165) is essential for the correct localization of Arabidopsis PIN1 and its trafficking along the secretory pathway. The trafficking of PIN1 in Arabidopsis is mediated via the interaction of the phenylalanine motif with AP2µ and AP3µ. In Arabidopsis, PIN1 is so far the only example shown to use a phenylalanine motif. Mutating this motif, however, does not impair PIN1 internalization, indicating the presence of other, so far unknown, motifs. These could be tyrosine-based, given that AP2µ could bind to three tyrosine motifs in the cytoplasmic loop of PIN1 in in vitro pull-down assays (Marcote et al., 2016; Sancho-Andrés et al., 2016).
Post-translational modifications
Reversible post-translational modifications on specific amino acids enable the conditional recognition of different proteins by adaptor complexes and have been shown to contribute to the endosomal trafficking of transmembrane proteins (Traub and Bonifacino, 2013). The most common modifications are ubiquitination and phosphorylation, which are tightly coupled to ubiquitination.
Ubiquitination
Ubiquitination is a dynamic, reversible, evolutionarily conserved post-translational modification (Husnjak and Dikic, 2012). The ubiquitin (Ub) polypeptide has a compact globular structure with an exposed C-terminal glycine that can be covalently linked to the primary ε-amino group of a lysine residue. Activating and transferring Ub to the target proteins requires the coordinated activity of three enzymes, a Ub-activating enzyme, a Ub-conjugating enzyme, and a Ub-ligase (Husnjak and Dikic, 2012; Dubeaux and Vert, 2017). An analysis using an artificial ubiquitinated transmembrane protein shows that Ub is a sufficient primary sorting signal (Scheuring et al., 2012), and mono-, di-ubiquitination and K63-linked Ub chains drive the endosomal sorting of PM cargoes to TGN (Fan et al., 2015). De-ubiquitination by de-ubiquitinating enzymes enables the recycling of PM cargoes (Weinberg and Drubin, 2014; Nagel et al., 2017). An additional control occurs at the TGN when recognition of ubiquitinated cargoes by the ESCRT pathway takes place, enabling vacuolar sorting of the PM proteins (Dubeaux and Vert, 2017).
The LRR-RLK Flagellin-sensing 2 (FLS2), the Brassinosteroid receptor BRI1 and the lysine motif-containing RLK (LYK5) all undergo Ub-dependent internalization mediated by the E3 ligases PUB12/13 (Lu et al., 2011; Liao et al., 2017; Zhou et al., 2018). The PUB12/13 E3 ligases might therefore act to control the levels of various receptor kinases at the PM. Next to those, the Arabidopsis E3 Ub ligases RING-H2 FINGER A3A (RHA3A) and RHA3B mediate the mono-ubiquitination of the receptor-like cytoplasmic kinase (RLCK) BOTRYTIS-INDUCED KINASE 1 (BIK1), which contributes to its internalization from the PM and activates immune signaling (Ma et al., 2020). Other PM proteins such as the atypical LRR-RLK STRUBBELIG (SUB; a member of the LRR-V family of RKs) and PIP2 (an Arabidopsis PM localized water channel protein or aquaporin) are also ubiquitinated, but there is less evidence available with respect to the effect of ubiquitination on their endocytosis (Lee et al., 2009; Gao et al., 2019).
Iron regulated transporter (IRT1) represents a prime example of the complex regulation that can be exerted by ubiquitination. This transmembrane protein localizes to early endosomes and continuously cycles to the PM and traffics to the vacuole for constant turnover (Vert et al., 2002; Barberon et al., 2011). Monoubiquitination of IRT at two lysine residues (K154 and K179) triggers its internalization by endocytosis (Barberon et al., 2011). Under non-iron excess metal conditions, IRT1 is phosphorylated by CIPK23 and undergoes IDF1 dependent extension of the monoubiquitination to K63 linked polyubiquitination, which causes IRT1 degradation (Dubeaux and Vert, 2017; Dubeaux et al., 2018). K63-linked polyubiquitination represents a general degradation cue, as this has also been reported to cause vacuolar degradation of BRI1 (Martins et al., 2015; Zhou et al., 2018) and to trigger gravity response-mediated endocytosis and vacuolar targeting of PIN2 (Abas et al., 2006; Leitner et al., 2012a; 2012b).
The boron exporter BOR1 represents another clear example of Ub-dependent cargo trafficking. Under high B conditions, BOR1 undergoes rapid ubiquitination-dependent vacuolar degradation via MVBs (Kasai et al., 2011). Ubiquitination of lysine-590 present in the cytoplasmic tail is necessary for boron-induced BOR1 degradation (Yoshinari et al., 2019). Interestingly, boron-induced BOR1 degradation is AP2 independent and can be blocked via dominant-negative DRP1A (DN DRP1A) expression (Yoshinari et al., 2016; 2019). Beta-estradiol-induced overexpression of the DN DRP1A targets this GTPase to the PM, as well as ectopically to the TGN. Accumulation of BOR1 at the PM upon DN DRP1A expression strongly suggests that the boron-induced degradation is blocked at the PM. However, we cannot exclude that ectopic DN DRP1A accumulation at the TGN interferes with TGN-dependent vacuolar degradation of BOR1. BOR1 trafficking is thus regulated by tyrosine-based and Ub-based recognition. The interplay between both, or whether one is responsible for internalization and the other for degradation, or whether their role is dependent on boron levels, remains unclear.
Boron-induced BOR1 vacuolar sorting is largely impaired in the quintuple tol mutant (tol23569; Yoshinari et al., 2018). The Arabidopsis TOL (Target of Myb 1-like) protein family consists of nine members that bind Ub in vitro. The TOLs act as Ub receptors for cargo delivery to the vacuole via the conserved ESCRT pathway (Korbei et al., 2013; Moulinier-Anzola et al., 2020). Possibly, ubiquitinated BOR1 is recognized by the TOL proteins and degraded via the ESCRT pathway, similarly as is suggested for PIN2 (Korbei et al., 2013) and FLS2 (Spallek et al., 2013). TOL proteins show various intracellular localizations, with TOL6 and TOL9 mainly accumulating at the PM (Korbei et al., 2013; Moulinier-Anzola et al., 2020). Furthermore, TOLs have clathrin binding motifs (Korbei et al., 2013), and TOL2, TOL6, and TOL9 were identified as proximity-based interactors of the TPC (Arora et al., 2020), and two members of the TPC, AtEH1/Pan1 and AtEH2/Pan1, have been recently identified as key players in an autophagy-dependent degradation pathway operating between the ER–PM contact sites and the vacuole. The endocytic machinery, including TPC, AP2 subunits and clathrin, is recruited to this pathway, and TPLATE has been shown to be degraded under nutrient stress conditions (Wang et al., 2019). Given the connection between TOLs, TPC, AP2, clathrin, and autophagosomal degradation, an exciting hypothesis to test would be whether the degradation of BOR1, IRT1, and other polyubiquitinated cargoes, under the above-mentioned adverse conditions, depends on the recently discovered endocytic AtEH/Pan1-dependent autophagy pathway.
Phosphorylation events regulate signal recognition
Phosphorylation is the second post-translational modification that acts as a cue for endomembrane trafficking, largely by functioning as a signal for ubiquitination. Phosphorylation has been shown to play a crucial role in regulating endocytosis of PM proteins in plants (Bonifacino and Traub, 2003).
Along with ubiquitination, phosphorylation of IRT1 acts as a sorting control mechanism under non-iron high metal conditions. The IRT1 phosphorylation that is responsible for its degradation is mediated by the CIPK23 kinase, as ubiquitinated IRT1 accumulates at the PM in the cipk23 mutant (Dubeaux et al., 2018). Therefore, efficient vacuolar sorting and degradation of IRT1 under non-iron high metal conditions requires a combination of two post-translational modifications, phosphorylation and K63-linked polyubiquitination (Dubeaux and Vert, 2017). Similarly, for FLS2, flg22 mediated endocytosis also depends on the phosphorylation and phosphorylation-dependent monoubiquitination of the RLCK BIK1. The phosphorylation-dependent monoubiquitination of BIK1 releases it from the FLS2-brassinosteroid insensitive kinase 1 complex, which further leads to FLS2 internalization (Robatzek et al., 2006; Mbengue et al., 2016; Iwatate et al., 2020; Ma et al., 2020).
Other examples of transmembrane proteins where phosphorylation plays a prominent role in their internalization are NIP5;1, a PM localized boric acid channel, and the chitin receptor LYK5. Phosphorylation of NIP5;1 controls its endocytosis and is required to maintain its outer lateral polar localization. Phosphorylation of LYK5 is mediated by the chitin elicitor receptor kinase1 upon chitin binding, and causes transient relocalization of LYK5 to late endocytic compartments (Erwig et al., 2017; Wang et al., 2017). Phosphorylation of transmembrane cargoes can therefore act as a potent regulatory mechanism, and can either be independent of or work together with ubiquitination to drive sorting.
Outlook
Plants continuously need to adapt their cellular homeostasis to the changes in their environment. For this reason, the perception of stress, biotic or abiotic, and an adequate response to stimuli is critical. Perception of stress involves transmembrane receptors undergoing highly coordinated synthesis, endocytosis, recycling, and degradation (Rodriguez et al., 2020). Degradation and recycling should be perfectly balanced to maintain an equilibrium. With the fluorescent-based tools we have at hand in plant cell biology, it remains challenging to distinguish whether an observation of enhanced PM levels of a certain transmembrane protein is caused by defective endocytosis, or by defective degradation that leads to enhanced recycling. The recent results with BOR1 represent an excellent example of this (Yoshinari et al., 2019). We need tools that allow us to specifically label proteins at the PM without perturbing their functionality and to effectively differentiate between recycling and degradation. In light of this, the recent report of possibly using SNAP-tag compatible dyes, which do not penetrate plant cells on their own, might be a major step in the right direction, as these dyes allow for the first time discrimination between the pool of endocytosed and de novo produced proteins at the TGN (Iwatate et al., 2020). For a better understanding of the interplay of these trafficking processes in maintaining the equilibrium in plant cells, we need to better understand how cargoes undergo internalization and how this internalization relates to their activities (e.g. in the case of receptor-mediated signaling). We, therefore, have to link cargoes and trafficking complexes more extensively, for example by performing differential proteomics experiments such as those done for AP3 and AP4 (Pertl-Obermeyer et al., 2016). Knowledge about the interaction between cargo proteins and their adaptor complexes that would allow directed mutagenesis to interfere with their trafficking is still largely missing. For example, AP2 is known to interact with tyrosine- and dileucine-based motifs in different organisms, but in plants, we only have a few concrete examples for this, and there is even evidence that AP2 dependent trafficking in plants works independently of these motifs.
Next to the multi-subunit adaptor complexes, monomeric adaptor proteins also play a role. Examples are ENTH and ANTH proteins (Holstein and Oliviusson, 2005; Song et al., 2012). Very recently, a study elegantly showed that retrieval of the Arabidopsis vesicle-associated R-SNARE VAMP72 from the PM is clathrin-dependent and specifically relies on the interaction between VAMP72 and the ANTH-domain monomeric adaptor proteins PICALM1a/ECA1 and PICALM1b. Defective retrieval of these SNARES did not cause cytokinesis failure as seen in the respective mutant background (Zhang et al., 2011), indicating that cell plate formation can run solely on de novo synthesis of these SNAREs (Fujimoto et al., 2020). Yeast and mammalian genomes furthermore encode for two additional families of monomeric adaptors: Golgi-localizing, Gamma-adaptin ear domain homology, ARF-binding proteins (GGAs), and stonins, which share partial homology with adaptins and can recognize similar motifs (Boehm and Bonifacino, 2001). GGA and stonins are, however, absent from the Arabidopsis genome. Whether their functions are substituted by other monomeric adaptors or by TPC remains to be determined. Indeed, TPC shares an evolutionary history with the AP complexes and it possesses the subunits that would allow for cargo recognition. Although it has been shown to interact with Cellulose Synthase Subunits (Sánchez-Rodríguez et al., 2018), we lack understanding of the specific residues and domains present in this complex that might interact with the motifs in the cargo proteins. Mutating these interacting residues in the respective adaptor complexes would provide us with tools to reveal the processes they regulate.
Supplemental data
The following supplemental materials are available in the online version of this article.
Supplemental Table S1 A comprehensive summary of the Arabidopsis multi-subunit HTAC-CC and their associated mutant phenotypes.
What is the role of the TPC/TSET complex in cargo recognition at the plasma membrane?
How can we discriminate plasma membrane retention of cargo proteins from enhanced recycling due to impaired degradation?
To which extent is tyrosine-based motif recognition by AP2 essential for endocytic internalization in plants?
How can we uncouple the role of TPC/TSET in endocytosis and in autophagy?
Does the endocytic-autophagosomal pathway starting at the ER-PM contact sites serve as an alternative pathway to degrade endocytosed cargo proteins?
Acknowledgments
The authors would like to apologize to those researchers whose work is not discussed in sufficient detail here due to space limitations and to maintain a certain focus. We would also like to thank Dr. Roman Pleskot (Institute of Experimental Botany of the Czech Academy of Sciences, Prague, Czech Republic) for critical reading of the manuscript, for helping out with the graphical representations in Figure 1A and for valuable input, and Dr. Eugenia Russinova (VIB/Ghent University, Ghent Belgium) for critical reading of the manuscript and valuable input. This work was funded by the European Research Council (T-Rex project number 682436 to D.V.D.) and by the National Science Foundation Flanders (FWO; G009415N to D.V.D.).
Conflict of ineterst statement. The authors declare to have no conflict of interest.
D.A. wrote the initial draft. D.A. and D.V.D. finalized the manuscript. The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in theInstructions for Authors (https://dbpia.nl.go.kr/plphys/pages/general-instructions) is: Daniёl Van Damme ([email protected]).
References
Author notes
Senior author.



