-
PDF
- Split View
-
Views
-
Cite
Cite
Ricky J. Milne, Katherine E. Dibley, Wendelin Schnippenkoetter, Martin Mascher, Andy C.W. Lui, Lanxiang Wang, Clive Lo, Anthony R. Ashton, Peter R. Ryan, Evans S. Lagudah, The Wheat Lr67 Gene from the Sugar Transport Protein 13 Family Confers Multipathogen Resistance in Barley , Plant Physiology, Volume 179, Issue 4, April 2019, Pages 1285–1297, https://doi.org/10.1104/pp.18.00945
Close - Share Icon Share
Abstract
Fungal pathogens are a major constraint to global crop production; hence, plant genes encoding pathogen resistance are important tools for combating disease. A few resistance genes identified to date provide partial, durable resistance to multiple pathogens and the wheat (Triticum aestivum) Lr67 hexose transporter variant (Lr67res) fits into this category. Two amino acids differ between the wild-type and resistant alleles – G144R and V387L. Exome sequence data from 267 barley (Hordeum vulgare) landraces and wild accessions was screened and neither of the Lr67res mutations was detected. The barley ortholog of Lr67, HvSTP13, was functionally characterized in yeast as a high affinity hexose transporter. The G144R mutation was introduced into HvSTP13 and abolished Glc uptake, whereas the V387L mutation reduced Glc uptake by ∼ 50%. Glc transport by HvSTP13 heterologously expressed in yeast was reduced when coexpressed with Lr67res. Stable transgenic Lr67res barley lines exhibited seedling resistance to the barley-specific pathogens Puccinia hordei and Blumeria graminis f. sp. hordei, which cause leaf rust and powdery mildew, respectively. Barley plants expressing Lr67res exhibited early senescence and higher pathogenesis-related (PR) gene expression. Unlike previous observations implicating flavonoids in the resistance of transgenic sorghum (Sorghum bicolor) expressing Lr34res, another wheat multipathogen resistance gene, barley flavonoids are unlikely to have a role in Lr67res-mediated resistance. Similar to observations made in yeast, Lr67res reduced Glc uptake in planta. These results confirm that the pathway by which Lr67res confers resistance to fungal pathogens is conserved between wheat and barley.
Fungal diseases such as rust pose a constant threat to global grain production. Disease resistance conferred by plant resistance genes is preferred over fungicides to reduce the impact of fungal diseases. However, instances exist where the breakdown of resistance has resulted in major crop losses (Singh et al., 2015), exemplified by the emergence of the Ug99 stem rust race, which was virulent against genetic resistance present in many commercial wheat (Triticum aestivum) cultivars (Pretorius et al., 2000). Due to the rapid evolution of these pathogens, plant resistance genes can be quickly overcome; hence, new sources of genetic resistance are required. Recently, a number of adult plant resistance (APR) genes have been cloned that differ from the archetypal nucleotide-binding leucine-rich repeat (NLR)-type resistance genes – Lr34, which encodes an ABC transporter (Krattinger et al., 2009), and Lr67, which encodes a hexose-proton symporter (Moore et al., 2015). These genes function mainly during the adult stage of plant development to confer partial resistance or slowed pathogen growth not associated with a hypersensitive response (Ellis et al., 2014). Both genes confer a similar resistance phenotype coupled to leaf tip necrosis and their combined resistances are nonadditive. Unlike NLRs, this class of APR genes does not rely on a gene-for-gene interaction with pathogen avirulence genes (Dodds et al., 2009), and hence are broadly effective against many strains and species of fungi. As such, the highly variable nature of pathogen genetics does not appear to help overcome partial resistance genes (Ellis et al., 2014). This could be due to physiological effects reducing pathogen growth. Generally, single partial resistance genes alone do not confer sufficient pathogen resistance, however it is important to note that partial resistance genes can be used in concert with NLRs to give a stronger and more durable resistance.
Sugar transport proteins (STPs) are a large subfamily of the monosaccharide transporter superfamily and there are 14 STPs in Arabidopsis (Arabidopsis thaliana) and 29 STPs in rice (Oryza sativa) (Büttner et al., 2000; Johnson and Thomas, 2007). Within the STP subfamily, Lr67 belongs to the widely conserved STP13 clade, so further understanding of its function has the potential for use in other crops where no information on resistance genes is available. STP13 proteins function as high affinity hexose-proton symporters that transport hexoses from the apoplasm to the cytosol (Slewinski, 2011). Interestingly, STP13 transporters in species other than wheat have also been implicated in the plant defense response, and as such respond to infection by various pathogens in Arabidopsis and grapevine (Vitis vinifera) (Hayes et al., 2010; Lemonnier et al., 2014; Yamada et al., 2016). Two amino acids differ between the wheat wild-type STP13, or “susceptible” (Lr67sus), and “resistant” (Lr67res) protein variants (G144R, V387L), and interestingly, these changes render Lr67res incapable of hexose transport (Moore et al., 2015). A number of potential scenarios may explain how Lr67res confers multipathogen resistance. The first being nutrition may be limited for fungal pathogens that feed via haustoria by the altered carbon partitioning caused by Lr67res. Alternatively, reduced apoplasmic hexose could lead to an altered hexose/Suc ratio and trigger sugar signaling, similar to that mediated by increased invertase activity observed during pathogen invasion, resulting in a defense response (Sonnewald et al., 2012; Proels and Hückelhoven, 2014). A third possibility is that Lr67res may acquire an altered function that leads to reduced pathogen growth by an unknown mechanism.
The focus of this study was to investigate whether the Lr67res gene can confer resistance to biotrophic pathogens in barley (Hordeum vulgare), and whether phenotypes and transcriptional profiles similar to those of Lr34res barley (Risk et al., 2013) are exhibited by Lr67res barley. Our results indicate that Lr67res-mediated resistance can be transferred to barley and is effective against multiple pathogen species. The barley ortholog of Lr67, HvSTP13, was functionally characterized in yeast and it was revealed that Lr67res is capable of reducing Glc uptake by HvSTP13. Lr67res also reduced Glc uptake into roots of Lr67res barley lines and the likelihood of this reduction in Glc transport underlying the resistance phenotype is considered.
RESULTS
Functional Properties of HvSTP13 Are Identical to Those of Lr67sus from Wheat
The barley HvSTP13 gene is orthologous to the wheat Lr67 gene and both fall within the STP13 clade of hexose transporters (Moore et al., 2015). Predicted protein sequences of HvSTP13 and Lr67sus are 98.8% identical (509/515 residues), hence functional characterization of HvSTP13 in the hexose transport-deficient yeast, EBY.VW4000, aimed to determine if HvSTP13 and Lr67sus share similar functional properties. HvSTP13 enabled hexose transport when the HvSTP13 gene was expressed in yeast cells (Fig. 1, A and B). A Glc affinity of 98 ± 5 µM and a lower Fru affinity of 410 ± 20 µM were exhibited by HvSTP13, consistent with other STP13s (Fig. 1, C and D). Glc accumulated in the yeast cell at concentrations about 30-fold higher than the external solution (and about 6-fold for Fru) indicating the uptake is energised, as expected of a hexose-proton symporter. Like most homologous hexose transporters, HvSTP13 is reliant on the proton gradient as [14C]Glc uptake was inhibited by the protonophore 2,4-dinitrophenol (DNP; Fig. 2A). The sulfhydryl modifiers, N-ethylmaleimide (NEM) and diethyl pyrocarbonate (DEPC) reduced Glc uptake by HvSTP13 (Fig. 2A). Reduction of Glc uptake by phlorizin, a glycoside of phloretin known to inhibit sugar transport, is consistent with HvSTP13 being a sugar transporter (Fig. 2A). Maximal uptake by HvSTP13 was observed at pH 5 and a high level of uptake was retained between pH 4 and pH 7 (Fig. 2B).
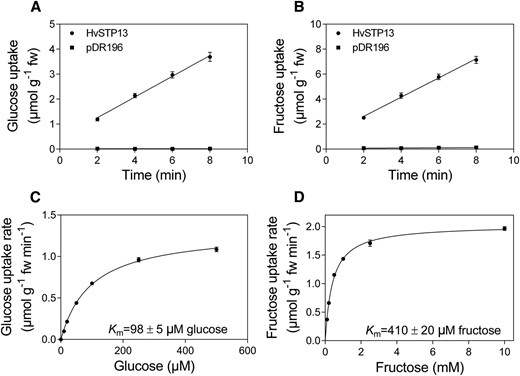
Hexose transport capability and affinity of HvSTP13 after heterologous cDNA expression in EBY.VW4000 yeast. Time course of Glc (A) and Fru (B) accumulation from 100 µM and 1 mm uptake solutions, respectively, by yeast transformed with HvSTP13 or pDR196 empty vector. Concentration-dependent Glc (C) and Fru (D) uptake by HvSTP13 after a 4-min incubation. Uptake experiments were carried out at pH 5. Mean ± se (vertical bars) of three biological replicates; fw, fresh weight.
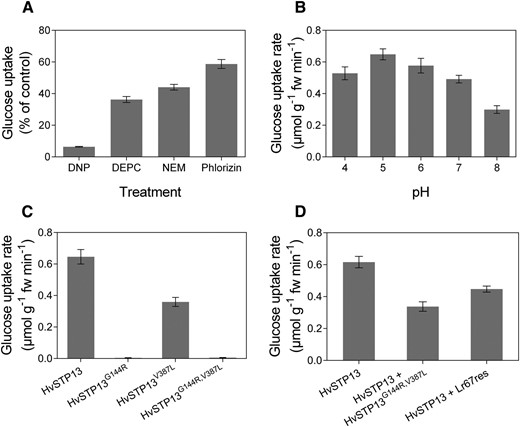
Effect of external pH, inhibitors and point mutations on Glc transport by HvSTP13. A, Glc uptake expressed as proportion of control by HvSTP13-transformed yeast after 30 s preincubation with the protonophore 2,4-DNP (100 µM), the sulfhydryl reagents DEPC and NEM (0.5 mm and 2 mm respectively), and the sugar transporter inhibitor phlorizin (2 mM). B, Glc uptake rate of yeast transformed with HvSTP13 in 25 mm MES-HEPES solution at indicated pH value. C and D, Glc uptake rates of yeast transformed with HvSTP13 (C) and site-directed mutants (D) and HvSTP13 cotransformed with HvSTP13 G144R,V387L or Lr67res. Uptakes in A, C, and D were carried out at pH 5. Mean ± se (vertical bars) of three biological replicates; fw, fresh weight.
Glc uptake was also tested for site-directed mutants of HvSTP13 with the Lr67res-specific mutations, G144R and V387L. As observed for the wheat Lr67res (Moore et al., 2015), HvSTP13G144R lacked Glc transport capability, whereas Glc uptake by HvSTP13V387L was approximately half that of the wild-type HvSTP13 (Fig. 2C). Introduction of G144R and V387L together into HvSTP13 also resulted in no Glc uptake (Fig. 2C). To test the dominant-negative interaction hypothesis of Lr67res (Moore et al., 2015), Glc uptake was measured when HvSTP13 and HvSTP13 G144R,V387L were coexpressed in yeast. Glc uptake was reduced by half when both were coexpressed in yeast (Fig. 2D). Lr67res, when coexpressed with HvSTP13, also reduced Glc uptake in comparison to HvSTP13 alone (Fig. 2D). This observation indicates that the proposed dominant-negative interaction may occur between HvSTP13 and either HvSTP13G144R,V387L or Lr67res in yeast.
Lr67res Mutations Were Not Found in a Collection of Wild Barley Accessions
Exome sequence data of 267 barley accessions (Russell et al., 2016) were mined to identify whether G144R, V387L or other mutations were present in the HvSTP13 genes of these lines. The collection of accessions primarily consisted of barley wild relatives and landraces. Ten nonsynonymous variants were identified (Table 1), however the transmembrane region 4 (TMR 4)-located G144R and TMR 10-located V387L were not represented in any of the accessions. A mutation predicted to be within TMR 4 of the HvSTP13 protein was identified in this collection (G148V). Six of the remaining mutants were predicted to be located in TMRs 10-12, the cytoplasmic C terminus and TMR 7-8 loop domain (Table 1). To examine conservation and evolutionary constraints of amino acids in the HvSTP13 sequence, EVfold analysis was run using the online server (http://evfold.org/evfold-web/newmarkec.do), which compared HvSTP13 to 50,300 related sequences. This server predicts important amino acids related to protein function based on sequence conservation and coupling between amino acids across evolutionary time (Hopf et al., 2012; Marks et al., 2012). The G148 amino acid was predicted to be the most highly conserved of the identified variants from wild barley accessions at 82% (Table 1). For comparison, the predicted conservation values of G144 and V387 are 94% and 17% respectively. G144 ranked as the third most highly conserved amino acid in the protein sequence, behind E160 (97%) and G108 (96%).
Naturally occurring nonsynonymous mutants in the barley STP13 transporter. AA change data generated from (Russell et al., 2016), EVfold data from the EVfold server (www.evfold.org).
| Amino acid change . | EVfold value for conservation . | Predicted location (transmembrane/cytoplasmic) . |
|---|---|---|
| G148V | 82% | TMR 4 |
| S319A | 25% | TMR 7-8 extracellular loop |
| G403V | 42% | TMR 10 |
| L436V | 9% | TMR 11 |
| F450Y | 12% | TMR 12 |
| I454V | 19% | TMR 12 |
| V465L | 24% | TMR 12 |
| D484E | — | C terminus (intracellular) |
| K485R | — | C terminus (intracellular) |
| D501G | — | C terminus (intracellular) |
| Amino acid change . | EVfold value for conservation . | Predicted location (transmembrane/cytoplasmic) . |
|---|---|---|
| G148V | 82% | TMR 4 |
| S319A | 25% | TMR 7-8 extracellular loop |
| G403V | 42% | TMR 10 |
| L436V | 9% | TMR 11 |
| F450Y | 12% | TMR 12 |
| I454V | 19% | TMR 12 |
| V465L | 24% | TMR 12 |
| D484E | — | C terminus (intracellular) |
| K485R | — | C terminus (intracellular) |
| D501G | — | C terminus (intracellular) |
| Amino acid change . | EVfold value for conservation . | Predicted location (transmembrane/cytoplasmic) . |
|---|---|---|
| G148V | 82% | TMR 4 |
| S319A | 25% | TMR 7-8 extracellular loop |
| G403V | 42% | TMR 10 |
| L436V | 9% | TMR 11 |
| F450Y | 12% | TMR 12 |
| I454V | 19% | TMR 12 |
| V465L | 24% | TMR 12 |
| D484E | — | C terminus (intracellular) |
| K485R | — | C terminus (intracellular) |
| D501G | — | C terminus (intracellular) |
| Amino acid change . | EVfold value for conservation . | Predicted location (transmembrane/cytoplasmic) . |
|---|---|---|
| G148V | 82% | TMR 4 |
| S319A | 25% | TMR 7-8 extracellular loop |
| G403V | 42% | TMR 10 |
| L436V | 9% | TMR 11 |
| F450Y | 12% | TMR 12 |
| I454V | 19% | TMR 12 |
| V465L | 24% | TMR 12 |
| D484E | — | C terminus (intracellular) |
| K485R | — | C terminus (intracellular) |
| D501G | — | C terminus (intracellular) |
Lr67res Responds to Pathogen Infection in Transgenic Barley
The complete genomic fragment (7133 bp) of Lr67res including 1318 bp of native promoter and 1512 bp of 3′ UTR sequence (Moore et al., 2015) was introduced into barley cv Golden Promise by Agrobacterium-mediated transformation. Three independent transgenic lines containing the full-length genomic fragment were utilized for further analysis. Subsequent analyses were carried out on the T1-T3 generations. Southern blot analysis showed that lines B15-11 and B15-6b contained a single copy of the Lr67res transgene whereas line B16-2b contained three copies (Supplemental Fig. S1).
Expression of the wheat Lr67res transgene was quantified in the three barley Lr67res transgenic lines and a control negative sibling (sib) line without the Lr67res transgene. The third leaf was sampled from plants at the five leaf stage. Lr67 expression was 1.5 to 4.5-fold higher in all Lr67res barley lines compared to Thatcher and Thatcher + Lr67 near-isogenic wheat lines at the same developmental stage (Fig. 3A). Lr67 expression in adult wheat flag leaves after head emergence was approximately 2-fold higher than in leaf three of wheat at the seedling stage. The Lr67res single copy line B15-11 exhibited the lowest Lr67res expression of the three lines, whereas the three copy line B16-2b was ∼ 2-fold higher and the second single copy line, B15-6b, was ∼ 3-fold higher than the control line (Fig. 3A). Transcript level differences may be due to the genomic locations of the transgenes. Lr67res transcripts were not detected in negative sib lines without the Lr67res transgene (Fig. 3A).
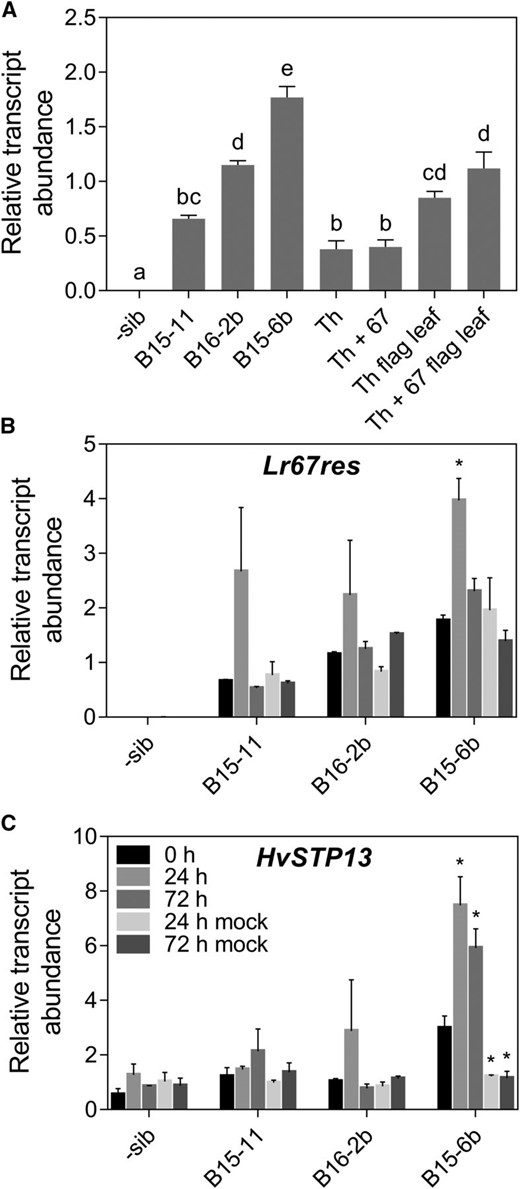
Expression of Lr67 and HvSTP13 in wheat and barley leaves. A, Expression of the wheat Lr67res gene in the third leaf of transgenic barley lines and negative sibling, and the wheat near isogenic lines Thatcher (Th), Thatcher + Lr67 (Th + 67) in the absence of pathogen infection. Thatcher and Thatcher + Lr67 flag leaves were also sampled at head emergence (Th flag leaf, Th + 67 flag leaf). B and C, Expression of (B) Lr67res and (C) HvSTP13 in the third leaf of Lr67res transgenic barley lines and negative sibling inoculated with barley leaf rust or mock inoculated. Legend applies to B and C. Mean ± se (vertical bars) of three biological replicates. Levels of statistical significance shown in A (a-e; ANOVA); *P < 0.05 in B and C (t test comparing 0 h time point to other time points).
After inoculation of barley plants with barley leaf rust (Puccina hordei), Lr67res was induced two to 3-fold at 24 h postinoculation (hpi) in the Lr67res lines (Fig. 3B, significant in B15-6b). Lr67res expression returned to basal levels at 72 hpi and induction was not observed in mock-infected controls. Transcripts of the most closely related ortholog of Lr67 in barley, HvSTP13, were also quantified postinoculation. An approximately two to 3-fold induction was observed 24 hpi in the two highest expressing Lr67res lines (B16-2b and B15-6b; significant in B15-6b). Little to no change was observed, however, in the third Lr67res transgenic line B15-11 and the negative sib line (Fig. 3C).
The Wheat Lr67res Gene Confers Resistance to Barley Leaf Rust and Powdery Mildew
Seedling resistance was exhibited by Lr67res barley plants inoculated with P. hordei at the five leaf stage (Fig. 4, third leaf is shown; see Supplemental Fig. S2 for whole leaves). Pustules were apparent in negative sib lines at 14 d postinoculation, however pustules were absent on the two highest expressing Lr67res lines (B15-6b, B16-2b). A very small amount of sporulation occurred on the lowest expressing Lr67res barley line, B15-11. No hypersensitive response was associated with Lr67res-mediated resistance in barley. Lr67res lines displayed severe leaf tip necrosis (LTN) and early senescence in the absence of pathogen infection (Supplemental Fig. S3), identical to observations made when Lr34res was expressed in barley (Risk et al., 2013). Some brown pigmentation was observed on leaves of transgenic barley, however this was also observed in the absence of pathogen infection. Resistance to barley powdery mildew (Blumeria graminis f. sp. hordei) was also exhibited by Lr67res barley in comparison to negative sib lines (Supplemental Fig. S4). Little to no B. graminis sporulation was present on Lr67res lines.
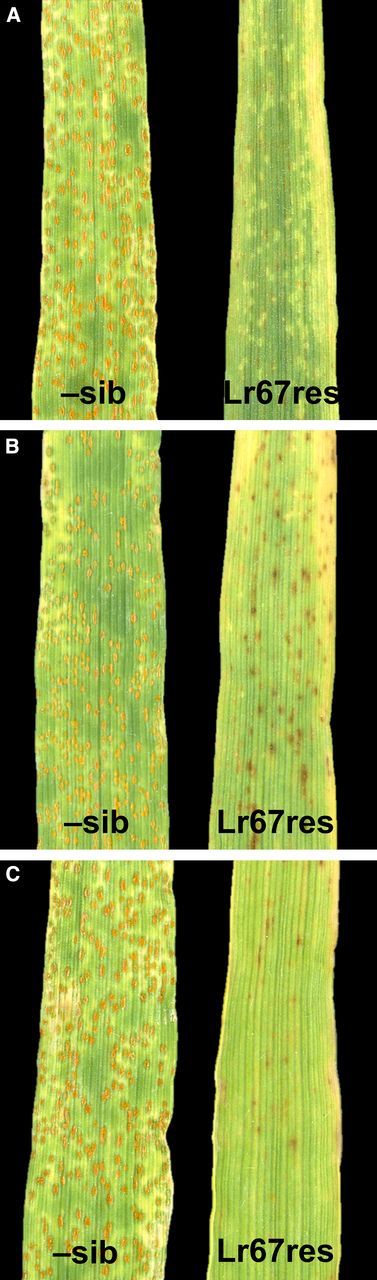
Barley leaves infected with P. hordei. The third leaf from negative sibling lines (–sib) and Lr67res lines 14 d postinoculation. (A) Single copy line B15-11, (B) three-copy line B16-2b, (C) single copy line B15-6b. Images are representative of three biological replicates.
Pathogenesis-Related Gene Expression Is Influenced by the Presence of Lr67res in Barley
Introduction of the wheat Lr34res transgene in barley caused higher expression of pathogenesis-related (PR) genes in comparison to negative sib lines without the Lr34res gene, in the absence of pathogen infection (Risk et al., 2013); hence, we investigated expression of the PR genes, PR1, PR2 and PR3 in Lr67res transgenic barley. Higher PR gene expression was also observed in some Lr67res barley lines in the absence of infection compared to the negative sib lines (Fig. 5). Negative sib lines exhibited a significant 10 to 20-fold increase of PR gene expression at 72 hpi, indicating responsiveness of these three genes to P. hordei. At 72 hpi, PR1 expression (Fig. 5A) was approximately 3-fold greater than PR2 (Fig. 5B) and PR3 expression (Fig. 5C). Lr67res lines however exhibited different PR gene expression patterns compared to the negative sib line and were either induced earlier at 24 hpi, or had higher basal levels of expression than the negative sib line. PR1, PR2 and PR3 were induced significantly (10-fold, 3-fold and 4-fold respectively) at 24 hpi in line B15-11, which is earlier than the negative sib line. PR1 expression was similar at 24 and 72 hpi in B15-11, whereas PR2 and PR3 expression at 72 hpi was reduced to the preinoculation level. PR1 and PR2 basal expression in the B15-11 line at 0 hpi was similar to the negative sib line, but PR3 expression levels were 3-fold higher in the B15-11 line than the negative sib line. The B16-2b and B15-6b lines both exhibited comparable expression profiles for the three PR genes. PR1 transcript levels were 7 to 10-fold higher in the two Lr67res lines at 0 hpi than the negative sib line and remained at a similar level across the remaining time points including mock-inoculated controls (Fig. 5A).
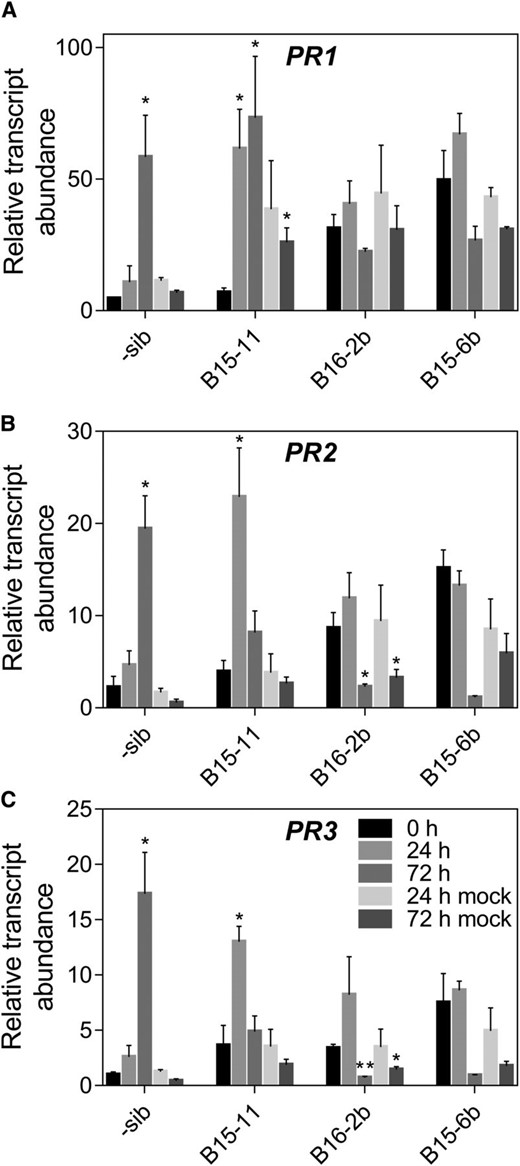
Expression of pathogenesis-related (PR) genes in transgenic barley in response to pathogen inoculation. Expression of (A) PR1, (B) PR2, (C) PR3 in the third leaf of Lr67 barley transgenic lines and a negative sibling line at 0, 24 and 72 h postinoculation with P. hordei or mock inoculated. Legend in C applies to all panels. Mean ± se (vertical bars) of three biological replicates. *P < 0.05, **P < 0.01 (t test comparing 0 h time point to other time points).
Glucose Uptake and Gene Expression of Barley Seedlings
Since AtSTP13 was upregulated in Arabidopsis seedling roots by NaCl and ABA treatments, and was found to be the primary contributor to root Glc uptake under salt stress (Yamada et al., 2011), a similar approach was used to test whether Lr67res influenced Glc uptake in barley seedling roots. Barley root Glc uptake in the presence of the protonophore CCCP was diminished to ∼ 10% of uptake without CCCP in negative sib seedlings lacking Lr67res, signifying that at least ∼ 90% of Glc uptake observed could be accounted for by proton-coupled transport, likely by STPs (Supplemental Fig. S5A). However, unlike Arabidopsis, Glc uptake was the same in control-treated and NaCl-treated seedlings (Supplemental Fig. S5A) despite HvSTP13 transcripts being induced significantly during the salt treatment in root tissue (Supplemental Fig. S5B). HvSTP13 transcripts of roots were also upregulated during treatment with ABA, however Glc uptake was reduced significantly in ABA treated seedlings compared to controls (Supplemental Fig. S5A).
Glc uptake by Lr67res seedlings was tested in the presence of 100 µM ABA and it also reduced Glc uptake into these lines (Fig. 6A; significant in Lr67res lines). Interestingly, in both control and ABA-treated seedlings, Glc uptake into the Lr67res lines was lower than that of the negative sib line, suggesting Lr67res may be capable of altering Glc transport in planta, as observed in yeast (Fig. 2D; Moore et al., 2015). Lr67res transcripts quantified in seedling shoots and roots revealed the Lr67res transgene was not induced in shoots or roots as a result of treatment (Fig. 6B). The native HvSTP13 gene was however induced significantly in roots after treatment (Fig. 6C). The absence of HvSTP13 induction in the shoot indicates the ABA signal may not be transmissible from the root to the shoot.
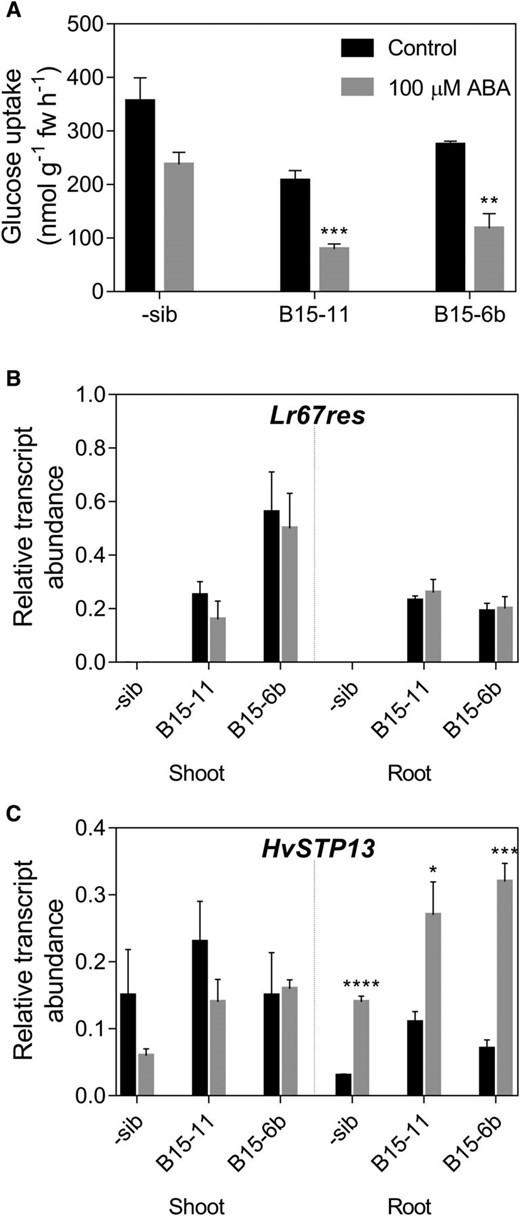
Glc uptake in roots and gene expression in barley seedlings. A, Root Glc uptake from a 1 mm Glc solution by the negative sibling line, B15-11 and B15-6b Lr67res lines. B and C, Expression of Lr67res (B) and HvSTP13 (C) in seedling shoot and root tissues at the same developmental stage and treatments as in (A). Legend in A applies to all panels. Mean ± se (vertical bars) of four biological replicates. *P < 0.05, **P < 0.01; ***P < 0.001; ****P < 0.0001 (t test comparing control and ABA-treated samples).
Flavonoid Production as a Result of Lr67res
Observations in Lr34res sorghum (Sorghum bicolor) of elevated flavonoid production in response to inoculation with Puccinia purpurea (Schnippenkoetter et al., 2017) led us to investigate the possibility of flavonoid production induced by Lr67res in barley. A specific class of flavonoids, primarily flavones and their C-glycosides were detected in barley samples. Isovitexin and vitexin were the two most abundant compounds detected (Supplemental Fig. S6). There was a trend that the single copy B15-11 line accumulated some of the flavone metabolites, luteolin, orientin and isoorientin, at higher levels than the negative sib line (Supplemental Fig. S7). However it does not appear that the levels were enhanced or induced by inoculation with P. hordei.
DISCUSSION
In this study we demonstrate that transformation of barley with the wheat Lr67res gene confers resistance to the barley-adapted pathogens, P. hordei and B. graminis f. sp. hordei, the causal agents of barley leaf rust and powdery mildew, respectively. This finding suggests that, similar to the wheat Lr34res gene that confers multipathogen resistance in barley (Risk et al., 2013; Chauhan et al., 2015), the underlying pathway and mechanisms required for Lr67res to confer pathogen resistance in wheat are also conserved in barley. This is not surprising given that in addition to hexaploid wheat, Lr34res confers resistance to pathogens that are adapted to other monocot crops – barley, rice, durum wheat (Triticum turgidum), sorghum and maize (Zea mays) (Risk et al., 2013; Krattinger et al., 2016; Rinaldo et al., 2017; Schnippenkoetter et al., 2017; Sucher et al., 2017). We anticipate that Lr67res is also likely to confer multipathogen resistance when transformed into these species. It remains unknown however whether Lr67res and/or Lr34res resistance is transferrable to eudicots.
HvSTP13 Functional Properties and Reduction of Hexose Transport by Lr67res
Both HvSTP13 and the wheat Lr67sus proteins (Moore et al., 2015) share similar functional properties with other characterized STP13 hexose transporters. Treatment of yeast expressing HvSTP13 with the protonophore DNP, known to disrupt the proton gradient (de la Peña et al., 1982), reduced Glc uptake by more than 90% (Fig. 2A), indicating reliance of HvSTP13-mediated Glc transport on the proton gradient. Similar effects were also observed for the tomato (Solanum lycopersicum), Arabidopsis and grapevine STP13s (Gear et al., 2000; Nørholm et al., 2006; Hayes et al., 2007). Phlorizin (a phoretin glycoside) is known to act as a competitive inhibitor of uptake by hexose transporters (Felle et al., 1983; Gear et al., 2000; Moore et al., 2015) and this may be the case for HvSTP13 (Fig. 2A). Reduction in HvSTP13-mediated hexose transport by the His modifier DEPC signifies the importance of conserved His residues in hexose transport (Lu and Bush, 1998). The membrane permeable sulfhydryl group modifier, NEM, caused a reduction in hexose transport suggesting sulfhydryl groups may influence hexose transport ability. The Glc affinity of HvSTP13 (∼ 100µM; Figure 1C) and its broad pH optimum (Fig. 2B) are consistent with other transporters whose primary role is retrieval of hexoses from a somewhat acidic apoplasm. Functional similarities conferred by high sequence identity between monocot and dicot STP13s may suggest a conserved function in planta.
As expected, due to the high conservation of the G144 amino acid across many hexose transporters including those outside the STP family, HvSTP13G144R lacked Glc transport capability in yeast (Fig. 2C). The change of Gly to Arg is major in terms of amino acid properties – Arg has a larger and positively charged side chain compared to Gly, hence the disruption of normal hexose transport function. Combined with observations of the wheat Lr67res ortholog (Moore et al., 2015), these data support the hypothesis that G144 is critical for normal hexose-proton symport. Since Glc uptake by HvSTP13 in yeast was higher than that observed in yeast transformed with both HvSTP13 and Lr67res (Fig. 2D), a dominant-negative interference by Lr67res, leading to a reduction of hexose transport, is also plausible in Lr67res transgenic barley plants. The lower rates of Glc uptake into Lr67res barley seedlings compared with the negative sib (Fig. 6) support this hypothesis. Based on these observations, we also predict that transformation of HvSTP13G144R into barley would confer a similar resistance phenotype to Lr67res.
Neither of the Lr67-specific mutations, G144R or V387L, was identified in wild barley accessions. The G144 residue is highly conserved (94%; Table 1) and is required for normal hexose transport capability, hence deleterious mutants would likely be selected against. This may explain why the Gly to Arg mutant is rare and to our knowledge has only been identified in muskmelon (Cucumis melo), as revealed by EVfold analysis of HvSTP13. The C. melo sequence (NCBI accession XP_008439909) most closely aligns with AtSTP1 (NCBI accession AEE28705) and contains an Arg at position 145 in place of the conserved Gly. It is unknown whether this mutation would render the transporter incapable of Glc transport, or enhance disease resistance. Future experimental work should target the highly conserved barley G148 amino acid, which is predicted to be located in TMR 4 close to the highly conserved G144 residue, to determine if the G148V variant has reduced hexose transport. If this proves to be the case, the effect of a G148V mutation could be assessed in terms of disease resistance. Other variants of STP13 are yet to be tested so it remains unknown if only mutation of G144 and V387 lead to resistance. Based on the loss of hexose transport capability by the G144R mutant, we hypothesize that this amino acid alone may be capable of conferring partial resistance like the reference Lr67res allele. This is currently being investigated in wheat lines that only carry the G144R mutation in the Lr67 gene.
In planta Function of STP13
STP13 gene expression in various species responds to a range of biotic and abiotic stimuli such as wounding, cold, salt, osmotic stress, ABA and microbial invasion (see Winter et al., 2007, further discussed below). Despite a number of studies on STP13, its physiological role is not overtly clear since stp13 knockouts or overexpression lines do not exhibit obvious phenotypes under normal growth conditions (Nørholm et al., 2006; Schofield et al., 2009). One possible reason may be that redundancy exists across sugar transporter families, for example STP1 may compensate for the absence of STP13 in stp13 knockout lines of Arabidopsis (Yamada et al., 2016). A general function proposed for STP13, among other hexose transporters, is apoplasmic hexose recovery as a result of Suc hydrolysis by invertase and passive leakage (Slewinski, 2011). This is logical in terms of response to bacterial pathogen invasion, to reabsorb apoplasmic hexoses thereby limiting pathogen nutrition. Work has been conducted on the STP13 of few species in response to abiotic and biotic stresses, such as grapevine and Arabidopsis. VvHT5, the STP13 ortholog from grapevine, was induced in mature leaves by wounding, exogenous abscisic acid (ABA) application and infection by powdery and downy mildews (Erysiphe necator and Plasmopora viticola respectively; Hayes et al., 2010). Experiments in Arabidopsis transformed with native VvHT5 promoter-GUS fusion constructs revealed that VvHT5 is induced in the vascular tissues of leaves following either powdery mildew infection or application of ABA, leading the authors to speculate that the function of VvHT5 was to enhance sink strength and the supply of sugars to cells under stress (Hayes et al., 2010). In Arabidopsis seedlings, AtSTP13 expression was induced in roots and leaves (approximately 6- to 10-fold) by ABA and salt treatments (Yamada et al., 2011). Additionally, AtSTP13 was induced by Botrytis cinerea (Lemonnier et al., 2014). Under such conditions, this transporter is predicted to mediate the uptake of hexose released from damaged cells. This function simultaneously energises the defense responses of neighboring cells and limits nutrient availability to the pathogen. Similarly, barley HvSTP13 was induced by salt and ABA (Fig. 6; Supplemental Fig. S5), although the fold change was far less than that observed in Arabidopsis (Yamada et al., 2011). These changes in expression however did not correlate with changes in Glc uptake in seedling roots and it is possible that other STPs may function in roots of barley and other monocots. Interestingly, ABA treatment reduced barley seedling Glc uptake, whereas salt treatment did not influence uptake. ABA may be affecting other processes leading to a reduction in Glc uptake. Unlike HvSTP13, Lr67res gene expression was not induced in transgenic barley by ABA. This may be due to the location of the inserted transgene in the genome, or the native promoter from wheat may not respond accordingly in barley.
Convincing evidence of a role for AtSTP13 in bacterial pathogen defense was also demonstrated in Arabidopsis leaves. Specifically, AtSTP13 physically interacts with the FLS2 flagellin receptor and the BAK1 associated receptor kinase phosphorylates AtSTP13, increasing the hexose transport rate (Yamada et al., 2016). FLS2 and BAK1 are components of basal or PAMP-triggered immunity (see Dodds and Rathjen, 2010 for review). Furthermore, AtSTP13 localization shifts from guard cell plasma membranes alone to mesophyll cell plasma membranes additionally to increase the area from which hexoses are retrieved. The key component of this response to bacterial pathogen infection is the increased hexose uptake by AtSTP13 driven by increased protein abundance and transport rate, thereby reducing apoplasmic hexoses available for the bacterial pathogen. Overexpression of AtSTP13 results in lower necrotrophic fungal pathogen development (B. cinerea; Lemonnier et al., 2014), but it remains unknown whether similar responses also occur during infection by haustorial-feeding pathogens. It is also unknown whether these observations in Arabidopsis also hold for other species, especially the monocots wheat and barley.
Seedling Resistance of Lr67res Barley
In bread wheat, Lr67res confers partial resistance at the adult plant stage only, and this is generally apparent only under field conditions and not glasshouse or cabinet growth conditions. It is unknown why resistance is more pronounced in the field. An explanation for why resistance occurs only at the adult plant stage in wheat may be related to the greater level of Lr67 expression in mature plants compared to seedlings (Fig. 3A). A similar pattern is exhibited by Lr34 in wheat (Risk et al., 2013). Transgenic Lr67res barley however exhibited strong resistance at the seedling stage of development, presumably due to higher expression of Lr67res in comparison to wheat at the seedling stage (Fig. 3A). Partial resistance to P. hordei is exhibited by barley cultivars carrying the APR gene Rph20 (Hickey et al., 2011) and is enhanced at lower temperatures (Singh et al., 2013). However, the barley cultivar used in this study, Golden Promise, does not carry Rph20, therefore this gene would not contribute to the observed phenotype. Durum wheat plants expressing Lr34res also show seedling resistance, which may indicate that a threshold of Lr67res transcript or protein abundance could be required to elicit the resistant phenotype (Rinaldo et al., 2017). Lr34res expression (and in some cases, Lr34res-mediated resistance) was influenced by temperature in both wheat and barley (Risk et al., 2012, 2013; Rinaldo et al., 2017); however, Lr67res barley lines were not tested under different growth conditions. Transgenic barley plants expressing Lr67res or Lr34res exhibited comparable levels of transgene expression at the seedling stage as the flag leaves of adult Lr67/Lr34 wheat plants (Fig. 3A; Risk et al., 2013). Alternatively, given that diploid barley has one orthologous gene (HvSTP13), as compared with hexaploid wheat which has two functional hexose transporter homeologues on the A and B genomes, the proposed dominant-negative interference on hexose transport (Moore et al., 2015) may have a stronger effect in diploids. The accelerated leaf tip necrosis phenotype was observed in both Lr67res and Lr34res barley in the absence of pathogen infection (Risk et al., 2013). Again, this may also be attributed to higher expression of each transgene in barely compared to wheat. Lr34res reduced grain yield when transgenically expressed in barley (Risk et al., 2013) and negative impacts upon plant vigor were also observed in high-expressing Lr34res transgenic sorghum lines, further supporting this notion (Schnippenkoetter et al., 2017). The dominant-negative explanation for Lr67 action, namely that the inactive Lr67res protein can bind to and inhibit the function of the hexose-transporting Lr67sus protein, will depend on the stoichiometry of Lr67res to Lr67sus proteins in plant cells. If there are excess active transporters there will not be enough dominant-negative binding partners to meaningfully inhibit hexose transport. Putative dimerization has been observed for other proton-coupled sugar transporters (SUTs; Liesche et al., 2011) as well as between Lr67sus and Lr67res using a split YFP assay (Moore et al., 2015). We anticipate an interaction may also occur between Lr67res and HvSTP13 in barley based on yeast and seedling root uptake data.
Interestingly, a number of pathways are constitutively active in Lr34res barley, including basal and inducible defense as indicated by high expression of genes involved in lignin biosynthesis and PR genes (Chauhan et al., 2015). Additionally elevated levels of salicylic acid (SA) and jasmonic acid (JA), hormones known to be involved in plant defense, were present in the absence of pathogen infection. Similar to Lr34res, expression of Lr67res in barley modifies constitutive and inducible PR gene expression. More rapid induction of PR genes postinoculation in Lr67res barley (or constitutively expressed) compared to the negative sib (Fig. 5) suggests defense pathways are primed and either already active or activated sooner after pathogen infection due to the presence of the Lr67res gene. It would be worthwhile comparing a barley hvstp13 knockout line with an Lr67res line to determine whether different classes of genes are being induced and whether hvstp13 knockout lines exhibit multipathogen resistance.
Does Reduced Glucose Transport Account for the Resistance Phenotype in Lr67res Transgenics?
To date, evidence suggests that increased hexose transport by AtSTP13 correlates with reduced necrotrophic and bacterial pathogen development (Lemonnier et al., 2014; Yamada et al., 2016). This differs from observations made of Lr67res, where reduced hexose transport correlates with reduced biotrophic pathogen development (Moore et al., 2015); hence, a number of aspects of the dominant-negative interference hypothesis by Lr67res remain unexplained and require further investigation. Lr67res indeed reduces Glc transport in planta in Lr67res barley roots (Fig. 6) and we assume this may also be the case in leaves. However, these observations are at odds with those made in Arabidopsis. Increased STP13-mediated Glc uptake led to greater resistance whereas lower Glc uptake in stp1 stp13 knockouts caused susceptibility (Yamada et al., 2016). An argument against the dominant-negative interference hypothesis is that other transporters such as STP1 could compensate for this loss of function to maintain hexose fluxes, since a double knockout (stp1 stp13) was required to observe a phenotype. It was previously proposed that Lr67res reduces apoplasmic Glc retrieval (Moore et al., 2015), increasing the apoplasmic hexose/Suc ratio hence, inducing sugar signaling and resulting in reduced pathogen growth (Sonnewald et al., 2012; Proels and Huckelhoven, 2014). This prompts the question, why wouldn’t similar sugar signaling be activated in stp1 stp13 knockout lines, leading to resistance? It is worth noting that comparison between different pathosystems is being made, bacterial and necrotrophic in Arabidopsis, and biotrophic in wheat, which may explain differences between STP13 and Lr67res-mediated resistance. Interestingly the introduction of additional mutations in the wheat Lr67res gene resulted in a loss of resistance (e.g. C95Y, G208D; Moore et al., 2015), giving weight to the hypothesis that Lr67res may possess an altered or gain of function. Future experimental work will investigate this possibility and whether these mutations can restore Glc transport.
Demonstration of Lr67res functionality in conferring multipathogen resistance in barley signifies the potential of this strategy for the development of multipathogen resistance in other crops. STP13 alleles from wild barley relatives may provide additional sources of resistance, and may provide better insights into the functional mechanism of Lr67res-mediated resistance.
CONCLUSIONS
The barley ortholog of Lr67, HvSTP13, has similar functional properties to the wheat wild-type Lr67sus. Glc uptake by HvSTP13 was reduced in yeast by Lr67res and in planta Glc uptake was lower in Lr67res barley lines. The wheat Lr67res gene functions to confer resistance to multiple pathogens in barley, however it remains to be determined whether this is due to lower Glc transport or another unknown mechanism.
MATERIALS AND METHODS
Production of Stable Transgenic Lr67res Barley Lines
The Lr67res genomic fragment of 7133 bp containing introns, 1318 bp of native promoter and 1512 bp of 3′ untranslated region (UTR), was inserted into pVec8 at the cohesive ended NotI site and transformed into Agrobacterium tumifaciens strain AGL0, which was used to transform barley cultivar Golden Promise as previously described (Harwood, 2014). DNA isolation and genomic blot procedures were performed as described (Lagudah et al., 1991) using plants from the T1 generation and primers in Supplemental Table S1 to generate a probe. Further experiments were conducted on T1 – T3 generation plants that were a mixture of homozygotes and heterozygotes. Homozygous lines were not selected since the resistance phenotype was observed in all Lr67res positive genotyped lines. Transformants were genotyped using a KASPar assay with primers in Supplemental Table S1 as described (Moore et al., 2015).
Plant Growth and Pathogen Infection
Plants were maintained under growth cabinet conditions with 16/8-h light/dark and constant temperature of 13°C, as per Risk et al. (2013). Plants were infected at the five-leaf stage. P. hordei pathotype 5457P+ (kindly provided by Plant Breeding Institute, NSW, Australia) was used to infect barley cv Golden Promise. This pathotype is avirulent on plants with resistance genes Rph5, 7, 11, 13, 14, 15 and 21; and virulent on lines with resistance genes Rph1, 2, 3, 4, 6, 8, 9, 10, 12 and 19 (Singh et al., 2018). Spores were heat shocked at 42°C for two minutes, mixed with talc powder and sprayed over plants. Mock controls were performed using talc without spores. Humidity (∼ 100%) was maintained in a sealed container at 20°C for 72 h postinoculation (hpi). Plants were acclimatised to the humidity and temperature change 24 h prior to inoculation. Thatcher and Thatcher + Lr67 near-isogenic wheat lines (Dyck and Samborski, 1979; Dyck et al., 1994) were also grown under identical conditions to compare transcript levels of Lr67 to transgenic barley lines. Flag leaves of these wheat lines were also sampled at the adult plant stage once the head had fully emerged. Natural infection of barley plants by Blumeria graminis f. sp. hordei resulting in severe mildew epidemics in the greenhouse were used as an inoculum source. Infected potted plants were interspersed among Lr67res barley and negative sib lines without the Lr67res transgene, and mildew symptom development was accordingly monitored.
RNA Isolation, cDNA Synthesis, and Quantitative PCR Analysis
Plants were sampled at 0, 24 and 72 hpi. Flag leaves of wheat lines were also sampled at the adult plant stage once the head had fully emerged. RNA was isolated from the third leaf of barley and wheat plants at the five-leaf stage using the QIAGEN RNeasy plant mini kit (QIAGEN, Chadstone Centre, VIC, Australia). On-column DNase digestion was performed and an additional DNase treatment was performed postextraction using Invitrogen Turbo DNase (Thermo Fisher, Scoresby, VIC, Australia). Minus-RT controls were tested to confirm elimination of genomic DNA contamination. cDNA was synthesized from 1 µg total RNA using Superscript III (Thermo Fisher) and an oligo d(T)20 primer.
Lr67 and HvSTP13 reverse transcription quantitative PCR (RT-qPCR) primers were designed in the 3′ UTR of each gene and were confirmed to be specific using standard PCR amplification and Sanger sequencing of amplified products. RT-qPCR was performed on a Bio-Rad CFX96 Real-Time PCR Detection System (Bio-Rad, Gladesville, NSW, Australia) using the iTaq Universal SYBR Green Supermix (Bio-Rad) according to the manufacturer’s instructions and an annealing temperature of 62°C. Primers used are listed in Supplemental Table S1. Efficiency and cycle threshold values were calculated using the LinRegPCR quantitative PCR data analysis program (Ruijter et al., 2009), and relative expression levels were calculated using the REST method (Pfaffl et al., 2002). Gene expression was measured relative to a combination of three genes as used by Risk et al. (2013), RLILP, Actin and β-tubulin 4. Sequence identity of each housekeeping gene between wheat and barley allowed identical primer sets to be used for both species (Supplemental Table S1).
Statistical analysis of data were carried out using Prism software (GraphPad, La Jolla, CA, USA), where one-way ANOVA followed by Tukey’s multiple comparison test were performed for data shown in Figure 3A. For all other RT-qPCR data, Prism software (GraphPad) was used to perform two-tailed Student’s t tests to compare between uninoculated and inoculated/mock inoculated samples.
Cloning HvSTP13, Generation of Yeast Strains and [14C]hexose Uptake Procedures
The full-length coding sequence of HvSTP13 was amplified from barley cv Golden Promise leaf cDNA by PCR using primers in Supplemental Table S1 and cloned into the pGEM-T Easy vector by TA cloning (Promega, Alexandria, NSW, Australia). Site-directed mutagenesis of HvSTP13 in pGEM-T Easy was used to introduce G144R and V387L mutations using a modified version of the QuikChange protocol (Agilent, Mulgrave, VIC, Australia). In short, primers were designed using the QuikChange Primer Design webpage (https://www.genomics.agilent.com/primerDesignProgram.jsp; see Supplemental Table S1) and Phusion polymerase (NEB, Ipswich, MA, USA) was used to amplify plasmids for 20 cycles followed by DpnI digestion and transformation into E. coli. Mutations were generated individually and a second round was used to introduce both G144R and V387L in a single construct. HvSTP13 and site-directed mutants were amplified from the pGEMt easy vector and cloned into the pDR196 vector (Rentsch et al., 1995) at the EcoRI and XhoI sites using specified primers (Table 1). Sequences were confirmed by Sanger sequencing and plasmids were transformed into EBY.VW4000 yeast, which is incapable of hexose uptake (Wieczorke et al., 1999), using the PEG1000 transformation procedure (Dohmen et al., 1991). To perform coexpression of HvSTP13 variants in yeast, the TRP1 gene was amplified from pGBKT7 BD (Takara Bio, Mountain View, CA, USA) using primers in Supplemental Table S1 before digestion with AatII and ClaI. The URA3 gene was excised from pDR196 using AatII and ClaI, and the TRP1 gene was ligated in its place to form the pDR196T vector backbone. Plasmids were then cotransformed in equal quantities using the PEG1000 protocol and selected on SDura–trp– media lacking uracil and Trp. Yeast uptakes were tested in both pDR196 and pDR196T vector backbones and no significant difference in Glc uptake was observed (Supplemental Figure S8). Yeast growth media and [14C]hexose uptake procedures used were as described (Milne et al., 2017).
Flavonoid Analysis
Barley plants were infected at the five-leaf stage with P. hordei as described previously. The third leaf was harvested at 0, 24 and 72 hpi. Mock-infected controls were also sampled at 24 and 72 hpi. Leaves were weighed, snap frozen in liquid nitrogen, freeze dried and reweighed. Leaves were then ground in liquid nitrogen and approximately 20 mg leaf powder was suspended in 8 volumes of HPLC-grade methanol (1:8, w/v) at 4°C overnight after vortexing. An equal volume of 2N HCl was added to all leaf extracts which were then incubated at 90°C for 1 h. Acid-hydrolyzed samples were subjected to reverse phase HPLC-qTOF-MS analysis. Briefly, acid-treated leaf extracts were separated on a C18 column (Phenomenex Synergi 4µ Fusion RP 80 A, 50 × 2 mm, Phenomenex, Torrance CA, USA). The solvent system used for compound separation was as follows: 0.5% (v/v) formic acid/water (A) and 0.5% (v/v) formic acid/methanol (B). Compound separation was achieved with a linear gradient of 10% to 65% B over 12 min at a flow rate maintained at 0.5 ml min−1 and the eluents were analyzed by a QTOF-mass spectrometer X500R system (AB SCIEX, Framingham, MA, USA) operated under positive ionization mode. Compound detection was performed by information-dependent acquisition. Identity of individual compounds was confirmed by comparing their retention time and MS/MS spectrum with authentic standards.
Seedling Glucose Uptake and Transcript Quantification
Seeds of barley Lr67res and the negative sib line lacking the Lr67res transgene were surface sterilized as per Kawasaki et al. (2018) and germinated on half-strength solid Murashige and Skoog (0.5x MS) media under a 16/8-h light/dark cycle and 23°C/18°C day/night temperature in a growth room. After seven days, four similar size seedlings per incubation were selected and transferred to 0.5x MS liquid media ± 125 mm NaCl or 100 µM abscisic acid (ABA) for 24 h. Seedlings were sampled at this point for gene expression by RT-qPCR as described previously. The remaining seedlings were transferred to 25 mL fresh 0.5x MS liquid media for two hours (± NaCl or ABA), supplemented with 1 mm Glc and 37 kBq [14C] Glc (Perkin Elmer, Glen Waverley, VIC, Australia) ± 50 µM Carbonyl cyanide m-chlorophenyl hydrazone (CCCP) for 2 h followed by four five-minute washes in 0.5x MS liquid media with 1 mm unlabeled Glc. Seedlings were blotted dry, weighed and incubated in 1 mL ethanol overnight at 80°C to extract soluble sugars. Whole seedlings and ethanol solution were added to 10 mL scintillant (Optima Gold, Perkin Elmer) in a scintillation vial, incubated overnight in darkness and dpm recorded on a Tri-Carb 2810 TR (Perkin Elmer). Statistical analysis of data were carried out using Prism software (GraphPad), where two-tailed Student’s t tests were used to compare between control and treated samples.
Accession Numbers
All are NCBI accessions. Lr67res genomic fragment: MK425206, Lr67res cDNA: KR604817.2, TaActin: AY663392.1, TaRLILP: AY059462.1, Taβ-tubulin 4: U76897, HvActin: AK356840.1, HvRLILP: AK359255.1, Hvβ-tubulin 4: AM502855.1, HvPR1: Z26321.1, HvPR2: AF515785.1, HvPR3: AK367847.1, HvSTP13: MK409638.
Supplemental Data
The following supplemental materials are available.
Supplemental Table S1. Primer sets used in this study.
Supplemental Figure S1. Southern blot of transgenic barley lines.
Supplemental Figure S2. Rust infected barley Lr67res transgenic and negative sib lines 14 d postinoculation with P. hordei.
Supplemental Figure S3. Whole plant phenotype before heading.
Supplemental Figure S4. Powdery mildew infected barley Lr67res transgenic and negative sib lines after natural inoculation in a growth cabinet by B. graminis f. sp. hordei.
Supplemental Figure S5. Glc uptake and gene expression in negative sib barley seedlings.
Supplemental Figure S6. Flavonoid analysis of the single copy B15-11 Lr67res barley line and negative sib line in the absence of pathogen infection.
Supplemental Figure S7. Flavonoid analysis of the single copy B15-11 Lr67res barley line and negative sib line infected with P. hordei.
Supplemental Figure S8. Four-minute Glc uptake by EBY.VW4000 yeast harboring HvSTP13 and HvSTP13G144R,V387L in different vector backbones, pDR196 and pDR196T.
ACKNOWLEDGMENTS
The authors would like to acknowledge Terese Richardson for producing stable transformed barley transgenic lines, Dr. Aki Kawasaki for assistance with seed surface sterilisation and Dr. Davinder Singh (Plant Breeding Institute, Cobbity, The University of Sydney) for kindly providing the P. hordei isolate 5457P+.
LITERATURE CITED
Author notes
This work was supported by funding provided by CSIRO OCE and Research Plus Postdoctoral Fellowship, Bill and Melinda Gates Foundation grant no. 0PP1131636.
Author for contact: [email protected]
Senior author.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: [email protected]
R.J.M. contributed to all experiments and drafted the manuscript. R.J.M. conducted yeast functional characterisation. R.J.M. and W.S. conducted genomic blot, pathogen infections and transcript analysis. R.J.M. and K.E.D. conducted seedling glucose uptake and transcript analysis. M.M., K.E.D. and R.J.M. conducted bioinformatic analysis and mined wild barley accession data. A.C.W.L., L.W., C.L. and R.J.M. conducted flavonoid analysis. R.J.M., K.E.D, A.R.A, P.R.R., C.L., E.S.L. conceived experimental plans. All co-authors commented on drafts and approved the manuscript.
Articles can be viewed without a subscription.



