-
PDF
- Split View
-
Views
-
Cite
Cite
Wei Zeng, Xinhua Dai, Jing Sun, Yifeng Hou, Xuan Ma, Xiaofeng Cao, Yunde Zhao, Youfa Cheng, Modulation of Auxin Signaling and Development by Polyadenylation Machinery, Plant Physiology, Volume 179, Issue 2, February 2019, Pages 686–699, https://doi.org/10.1104/pp.18.00782
Close - Share Icon Share
Abstract
Polyadenylation influences gene expression by affecting mRNA stability, transport, and translatability. Here, we report that Cleavage stimulation Factor 77 (AtCstF77), a component of the pre-mRNA 3ʹ-end polyadenylation machinery, affects polyadenylation site (PAS) selection in transcripts of some auxin signaling genes in Arabidopsis (Arabidopsis thaliana). Disruption of AtCstF77 reduced auxin sensitivity and decreased the expression of the auxin reporter DR5-GFP. Null mutations of cstf77 caused severe developmental defects, but were not lethal as previously reported. cstf77-2 genetically interacted with transport inhibitor response 1 auxin signaling f-box 2 auxin receptor double mutants, further supporting that polyadenylation affects auxin signaling. AtCstF77 was ubiquitously expressed in embryos, seedlings, and adult plants. The AtCstF77 protein was localized in the nucleus, which is consistent with its function in pre-mRNA processing. We observed that PASs in transcripts from approximately 2,400 genes were shifted in the cstf77-2 mutant. Moreover, most of the PAS shifts were from proximal to distal sites. Auxin treatment also caused PAS shifts in transcripts from a small number of genes. Several auxin signaling or homeostasis genes had different PASs in their transcripts in the cstf77-2 mutant. The expression levels of AUXIN RESISTANT 2/INDOLE-3-ACETIC ACID 7 were significantly increased in the cstf77-2 mutant, which can partially account for the auxin resistance phenotype of this mutant. Our results demonstrate that AtCstF77 plays pleiotropic and critical roles in Arabidopsis development. Moreover, disruption of AtCstF64, another component of the polyadenylation machinery, led to developmental defects and reduced auxin response, similar to those of the cstf77-2 mutant. We conclude that AtCstF77 affects auxin responses, likely by controlling PAS selection of transcripts of some auxin signaling components.
Auxin controls almost every aspect of plant growth and development, mainly by regulating gene expression at the transcriptional level (Salehin et al., 2015). Auxin is perceived by its receptor TRANSPORT INHIBITOR RESPONSE 1/AUXIN SIGNALING F-BOX (TIR1/AFB) and coreceptor AUXIN RESISTANT/INDOLE-3-ACETIC ACID (AUX/IAA). Auxin-regulated degradation of AUX/IAA proteins is a central step in auxin signaling. When auxin concentration is low, AUX/IAA repressors physically interact with auxin response factors (ARFs), preventing ARFs from binding to cis-elements of auxin-responsive genes. When auxin concentration is elevated, auxin binds to TIR1/AFBs, and enhances their interactions with AUX/IAA proteins. Subsequently, AUX/IAA proteins are ubiquitinated by the SCFTIR1/AFBs (SKP1-Cullin-F-box TIR1/AFBs) E3 ubiquitin ligases and are degraded by the 26S proteasome, freeing ARFs for transcription activation or repression (Dharmasiri et al., 2005a, 2005b; Kepinski and Leyser, 2005). Mutations in AUX/IAAs that affect the interaction between TIR1/AFBs and AUX/IAAs prevent AUX/IAA from degradation, leading to auxin resistance and developmental defects (Zenser et al., 2001; Dreher et al., 2006).
In eukaryotes, almost all pre-mRNAs are subjected to 3ʹ-end polyadenylation. A 3ʹ-end poly(A) tail of a mature mRNA affects mRNA localization, termination of transcription, mRNA stabilization, and translation. Polyadenylation of mRNA is generated by the polyadenylation machinery, which is composed of Cleavage and Polyadenylation Specificity Factors (CPSFs), Cleavage stimulation Factors (CstF), Cleavage Factors I and II, poly(A) polymerase, the scaffolding protein symplekin, and the nuclear poly(A) binding protein. The polyadenylation machinery cleaves the pre-mRNA at the polyadenylation site (PAS) and adds the poly(A) tail (Tian and Manley, 2017). In Arabidopsis (Arabidopsis thaliana), more than 70% of genes possess two or more PASs (Wu et al., 2011). The polyadenylation machinery appears to be conserved among eukaryotes (Hunt et al., 2012). It has been shown that several Arabidopsis genes, which encode polyadenylation machinery components, are involved in regulating plant development and in responses to environmental cues (Deng and Cao, 2017). Two conserved mRNA 3ʹ-end-processing factors, the Arabidopsis CstF77 and CstF64, are required in silencing of the key flowering gene FLOWERING LOCUS C (FLC). Interestingly, both CstF64 and CstF77 are required for 3ʹ processing of FLC antisense transcripts, but not for its sense transcripts (Liu et al., 2010). FY, the Arabidopsis homolog of yeast Pfs2p, is an RNA 3ʹ end-processing factor that interacts with the RNA-binding protein FLOWERING CONTROL LOCUS A (FCA) in controlling floral transition. The FCA/FY interaction is also required for the down-regulation of the floral repressor FLC (Simpson et al., 2003). In addition, AtCPSF30 was shown to be important in fertility, root development, stress, and plant hormone responses (Hunt, 2014). Recently, it was reported that the Arabidopsis CPSF30-l gene plays an essential role in nitrate signaling and regulates the nitrate transceptor gene NRT1.1 (Li et al., 2017).
In this article, we isolated an Arabidopsis CstF77 mutant in a genetic screen for mutants resistant to sirtinol and auxin (Blackwell and Zhao, 2003; Zhao et al., 2003; Cheng et al., 2004; Li et al., 2006). We show that mutations in AtCstF77 caused weak auxin resistance phenotypes and reduced auxin responses. Previous studies demonstrated that AtCstF77 was required for Arabidopsis development and that the homozygous cstf77-2 mutation caused lethality (Liu et al., 2010). However, under our growth conditions, the cstf77-2 mutation was not lethal and the plants were able to set viable seeds. Moreover, new null alleles generated using the clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein9 (Cas9) gene editing technology were also not lethal. We found that disruption of AtCstF64 also led to reduced auxin response and developmental defects similar to those of the cstf77-2 mutant. Our genome-wide poly(A) site sequencing (PAS-seq) and RNA-seq analysis of the cstf77-2 mutant and wild-type plants revealed that the PAS shifted in transcripts from 2,400 genes in the cstf77-2 mutant. Transcripts from several auxin signaling genes including AXR2/IAA7 displayed a PAS shift and an increase in expression levels. Our findings revealed that AtCstF77, AtCstF64, and alternative polyadenylation (APA) affect auxin signaling, and that AtCstF77 is important for plant development but it is not essential for Arabidopsis survival.
RESULTS
Isolation of a Sirtinol/Auxin-Resistant Mutant and Molecular Cloning of AtCstF77
To identify new components in auxin signaling, we carried out a genetic screen for sirtinol-resistant mutants from an Ethyl-methanesulfonate–mutagenized Arabidopsis Ler population. Sirtinol has been shown to be a useful chemical genetic tool in isolating auxin-resistant mutants (Zhao et al., 2003; Cheng et al., 2004). One of the sirtinol-resistant mutants a780 showed a weak auxin-resistant phenotype. In the dark, the a780 mutant was similar to wild-type plants when growing on Murashige & Skoog (MS) plates, but displayed longer hypocotyls and primary roots on MS plates containing 100 nm of the synthetic auxin 2,4-d (Fig. 1A). The mutant also showed an auxin-resistant root elongation phenotype in the light (Fig. 1B). The adult plants of a780 had shorter stature and shorter siliques than wild-type plants (Fig. 1C).
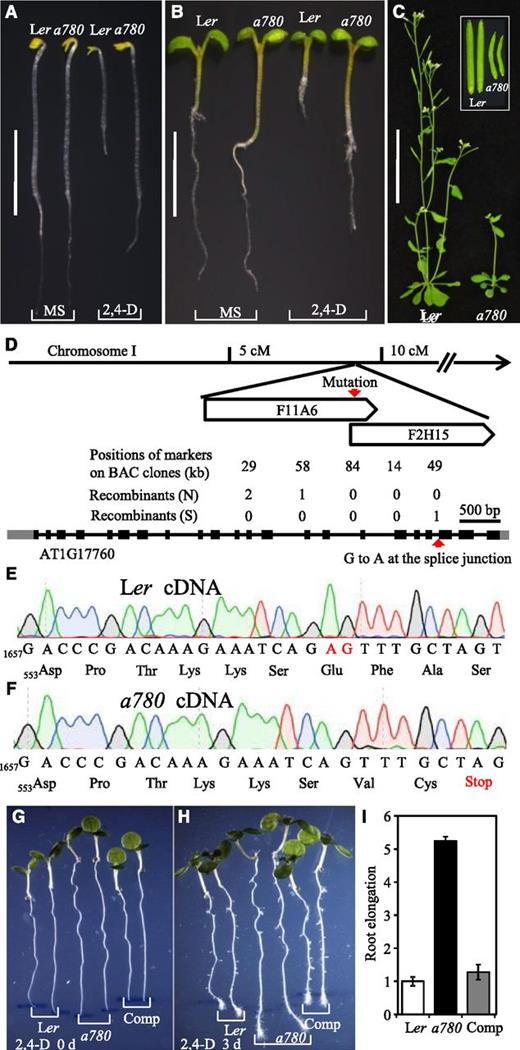
Isolation and molecular cloning of cstf77-a780. A and B, Seedlings grown in dark (A) and in light (B) on MS (left) and MS containing 100 nm 2,4-d (right). C, Adult plants of wild-type Ler and the a780 mutant. Inset: Siliques. D, Map-based cloning of CstF77. The mutation was mapped to an interval of 90 kb on Chromosome I. A nt conversion of G to A at the splicing junction between exon 20 and intron 19 was identified in At1g17760. E and F, The cDNA and deduced protein sequences of a part of At1g17760 in Ler (E) and the a780 mutant (F). Numbers refer to the corresponding nt and deduced amino acid. The nts 1676A and 1677G of At1g17760 cDNA in Ler labeled in red were missing in the a780 mutant, and this caused open-reading-frame shift and a premature stop codon. G to I, Complementation of the cstf77-a780 mutant by a genomic DNA fragment of At1g17760. Five-day-old seedlings were transplanted to an MS plate containing 100 nm 2,4-d and the positions of root tips were marked (G), then the seedlings were grown vertically for 3 d (H). Quantification of root elongation of the seedlings after auxin treatment, using that of Ler as an arbitrary unit. Data represent means ± sd (n = 15) (I). Scale bars = 5 mm (A), 5 mm (B), 5 cm (C).
We mapped the a780 mutation to an interval of approximately 90 kb on Chromosome I. Sequencing of all open reading frames in the region identified a G to A transition at the splice junction between intron 19 and exon 20 of At1g17760 in the a780 mutant (Fig. 1D). This mutation led to a 2-bp frame shift to the next “AG” in the mRNA of the gene because the original splicing acceptor “AG” changed to “AA.” This frame shift caused a premature stop codon in the mature mRNA (Fig. 1, E and F).
To confirm that the auxin-resistant phenotype of the a780 mutant was caused by the identified mutation in At1g17760, we performed a genetic complementation by transforming the a780 mutant with a genomic DNA fragment containing the coding region and regulatory sequences of At1g17760. The auxin-resistant phenotype was rescued by the At1g17760 genomic fragment (Fig. 1, G–I), demonstrating that the mutation in At1g17760 caused the observed auxin-resistant phenotype.
At1g17760 / AtCstF77 Plays Critical Roles in Growth and Development
At1g17760 encodes AtCstF77, which is a component in the pre-mRNA 3ʹ-end polyadenylation machinery. AtCstF77 has been reported to be required for 3ʹ-end processing of FLC antisense transcripts, and cstf77-1 was previously isolated as sof2, one of the suppressors of overexpressed FCA (Liu et al., 2010).
The a780 mutant (renamed as “cstf77-a780” hereafter) was in the Ler background, which made further characterization more difficult because many of our auxin mutants are in the Col-0 background. We obtained the cstf77-2 allele from the Nottingham Arabidopsis Stock Center, which was previously reported as a T-DNA insertion line in the Col-0 background (Liu et al., 2010). We found that the homozygotes of cstf77-2 were viable, although the segregation ratio from the heterozygotes was very low (Supplemental Fig. S1; Supplemental Table S1). At the seedling stage, the cstf77-2 mutant had short roots, a long hypocotyl, and few cells in the root meristem. The cotyledons of cstf77-2 seedlings were curled up. The cstf77-2 adult plants were dwarf and had reduced organ size, pale leaves, and late flowering time. We observed thinner stacks of thylakoids and lower chlorophyll contents in the cstf77-2 mutant than those in the wild type (Supplemental Fig. S2), which was consistent with the pale green leaves. Although the fertility of the cstf77-2 mutant was dramatically decreased, the cstf77-2 homozygous mutant was able to produce a small number of seeds. The progenies from cstf77-2 homozygotes were identical to their progenitors (Fig. 2, G–L).

Generation and characterization of additional alleles of cstf77. A, A schematic representation of CstF77 and positions of the T-DNA insertion in the cstf77-2 allele. Guide RNA target sequences of CstF77-C3 and CstF77-C4 are indicated. B, Expression analysis of CstF77 in Col-0 and the cstf77-2 mutant detected by RT-PCR. ACTIN2 served as a control. C to F, Partial genomic and cDNAs of cstf77-c3 (77-c3) and cstf77-c4 (77-c4) mutants. A 1-bp insertion and a 2-bp deletion were detected in the genomic DNA and cDNA of cstf77-c3 (C and D) and cstf77-c4 (E and F) mutants, respectively. G, The 7-d-old seedlings of Col-0, cstf77-2, cstf77-c3, cstf77-c4, and an F1 progeny of cstf77-2 × cstf77-c3. H, The adult plants of Col-0, cstf77-2, cstf77-c3, cstf77-c4, and an F1 progeny of cstf77-2 × cstf77-c3. I to K, Root length (I), hypocotyl length (J), and number of cells in root meristem (K) of the 7-d-old seedlings of Col-0, cstf77-2, cstf77-c3, and cstf77-c4. Data represent means ± sd (n = 15) with Student’s t test. **P < 0.001 compared to Col-0. L, Flowering time represented with Total Leaf Number of Col-0, cstf77-2, cstf77-c3, and cstf77-c4 plants. Data represent means ± sd (n = 15) with Student’s t test. **P < 0.001 compared to Col-0. Scale bars = 5 mm (G), 5 cm (H). TLN, total leaf number.
Because our results regarding the cstf77-2 mutant contrasted with those reported in Liu et al. (2010), we sought to confirm that the phenotype was indeed caused by the T-DNA insertion in this mutant. We generated two new alleles by using the CRISPR/Cas9 genome editing technology (Gao et al., 2016), and named them as cstf77-c3 and cstf77-c4. Sequencing of the genomic DNA of AtCstF77 in the mutants revealed a 1-bp insertion into Exon 4 in cstf77-c3 and a two-bp deletion at Exon 11 in cstf77-c4. Sequencing of the cDNAs confirmed that both CRISPR mutations led to premature stop codons in their mRNAs, suggesting that they were null alleles (Fig. 2, A–F). Plants of the cstf77-c3 and cstf77-c4 mutants displayed similar phenotypes to those in the cstf77-2 mutant at the seedling and adult stages. Genetic analysis of progenies of the cstf77-2 +/ −, cstf77-c3 +/ −, and cstf77-c4 +/ − mutants showed similar segregation ratios (Supplemental Table S2). To further confirm the phenotypes, we also crossed cstf77-2 with cstf77-c3. The F1 plants also showed the same phenotypes as those in the parent plants (Fig. 2, G and H). These results clearly demonstrate that AtCstF77 plays critical, but not essential, roles in Arabidopsis growth and development.
The cstf77 Mutants had Reduced Auxin Responses
The cstf77-a780 mutant was isolated from a genetic screen for sirtinol-resistant mutants, and was shown to be weakly auxin-resistant. We next tested the auxin responses of the cstf77 mutants using the standard root elongation assay. AXR1 encodes a protein related to ubiquitin-activating enzyme E1, and is a very important gene in auxin signaling (Leyser et al., 1993). The axr1-3 mutant is a strong auxin-resistant mutant (Lincoln et al., 1990). We found that cstf77 mutants displayed reduced auxin response, but were less resistant to auxin than axr1-3 (Fig. 3A). Because cstf77-2, cstf77-c3, and cstf77-c4 are likely null alleles and their mutants showed similar phenotypes, we used the cstf77-2 mutant in the next experiments. The DR5-GFP auxin response reporter has been widely used in analyzing auxin responses (Cui et al., 2016). We introduced DR5-GFP into the cstf77-2 mutant by crossing. The DR5-GFP signals were dramatically decreased in the cstf77-2 mutant in comparison to wild type (Fig. 3B). These results suggest that AtCstF77 is involved in auxin signaling.

Auxin responses of cstf77-2 mutants. A, Relative root elongation of cstf77-2 mutants to auxin treatment. Data represent means ± sd (n > 15). B, The DR5-GFP auxin response reporter (green fluorescence) was decreased in the cstf77-2 mutant. The red fluorescence was FM4-64 staining. Scale bars = 60 µm.
AtCstF64, an AtCstF77 Interacting Protein, is Also Involved in Auxin Signaling
AtCstF64 is another component of the polyadenylation machinery, and was reported to interact with AtCstF77 (Liu et al., 2010). To investigate whether AtCstF64 is also involved in auxin signaling, we obtained the cstf64-2 and cstf64-3 T-DNA insertion mutants from the Nottingham Arabidopsis Stock Center. Genotyping and sequencing identified the T-DNA insertion sites in the first and seventh introns of AtCstF64 (Fig. 4A). Reverse transcription PCR (RT-PCR) analysis revealed that the full-length cDNA was not detected in either allele (Fig. 4B), indicating that they were real knock-out mutants. Both cstf64 mutants displayed severe developmental defects and reduced auxin responses. These phenotypes were similar to those of the cstf77-2 mutant, although cstf64 mutants were less resistant to auxin than cstf77 (Fig. 4, C–E). These results indicate that AtCstF64 is also involved in auxin signaling. We noticed that the cstf64-3 mutant showed stronger auxin resistance and smaller rosette leaves than the cstf64-2 mutant (Fig. 4, D–E). It is noteworthy that the cstf64-2 allele produced transcripts that did not contain the first exon (Fig. 4B). Such transcripts may encode partially functional proteins. In contrast, the cstf64-3 allele had much shorter transcripts. The differences in transcript lengths may account for the phenotypic differences.

Auxin responses of the cstf64 mutants. A, Schematic representation of the gene structure, T-DNA insertion sites of cstf64-2 and cstf64-3 mutants, and locations of primers. B, RT-PCR analysis of CstF64 cDNA in the cstf64-2 and cstf64-3 mutants. ACT2 was used as a control. C and D, Wild-type, cstf64-2, cstf64-3, and cstf77-2 mutants at 7-d-old (C) and 35-d-old stages (D). E, Relative root elongation of cstf64 mutants in response to auxin treatment. Data represent means ± sd (n > 15).
The cstf77 Mutants Genetically Interact with Auxin Receptor Mutants
To further analyze the role of AtCstF77 in auxin signal transduction, we tested whether cstf77-2 genetically interacts with known auxin mutants. TIR1/AFBs are auxin receptors and their mutants show strong developmental defects (Dharmasiri et al., 2005a, 2005b). We crossed the cstf77-2 mutant with tir1-1 afb2-3 double mutants and analyzed the progenies of cstf77-2 +/ − tir1− / − afb2-3− / − in the F4 generation. All of the 16 cstf77-2 tir1 afb2 triple mutants that segregated from the 119 progenies displayed short root or rootless phenotypes, which is similar to tir1 afb2 afb3 and monopteros (mp) mutants (Supplemental Fig. S3; Supplemental Table S3). The mp-like short root or rootless phenotype has been observed in many auxin mutants, including bodenlos/iaa12, tir1 afb2 afb3, yuc1 yuc4 yuc10 yuc11, and ncp1 tir1 afb2 (Hardtke and Berleth, 1998; Hamann et al., 2002; Dharmasiri et al., 2005a, 2005b; Cui et al., 2016). The cstf77-2 tir1 afb2 mutant, but not the tir1 afb2 mutant, showed the mp-like phenotype. This result indicates that cstf77-2 enhanced the tir1 afb2 phenotype, which further supports that AtCstF77 plays a role in auxin signaling.
AtCstF77 is Ubiquitously Expressed and Localized in the Nucleus
To investigate the expression pattern of AtCstF77 and the subcellular localization of the protein, we generated transgenic Arabidopsis plants harboring the AtCstF77 genomic DNA fragment fused with the GFP or GUS gene. The transgenes were able to complement the developmental defects of the cstf77-2 mutant (Fig. 5A), indicating that the CstF77-GFP and CstF77-GUS fusion proteins were functional.
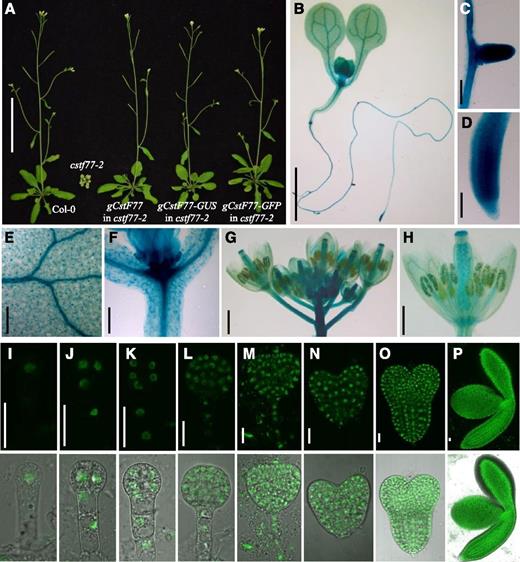
Expression patterns and subcellular localization of CstF77. A, Complementation of cstf77-2 by gCstF77-GUS and gCstF77-GFP. B to H, Expression of gCstF77-GUS in a 5-d-old cstf77-2 mutant seedling complemented with the transgene (B), lateral root (C), primary root (D), leaf (E), junction of leaf and hypocotyl (F), inflorescence (G), and flower (H). I to P, Expression of gCstF77-GFP in a cstf77-2 mutant plant complemented with the transgene at different stages of embryo development: 1-cell (I), 2-cell (J), 4-cell (K), globular (L), transition (M), heart (N), torpedo (O), and cotyledon (P) stages. The CstF77-GFP fusion protein was localized in the nucleus. Upper panel: GFP signals in dark field under a confocal microscope; lower panel: merged images of GFP and bright field. Scale bars = 5 cm (A), 2 mm (B), 100 µm (C and D), 200 µm (E and F), 1 mm (G), 500 µm (H), 20 µm (I–P).
At the seedling stage, CstF77-GUS was expressed in primary and lateral roots, hypocotyls, and new leaves, with high expression levels in vascular tissue and root tips (Fig. 5, B–F). In adult plants, the CstF77-GUS signals were detected in stems and flowers. The young flowers had strong expression. It was also highly expressed in pollen grains and in ovules (Fig. 5, G and H).
We examined the expression of CstF77-GFP during embryo development. From the single cell embryo to the mature embryo, the CstF77-GFP fusion protein was expressed at high levels. The CstF77-GFP fusion protein was highly expressed in embryos and localized in the nucleus (Fig. 5, I–P), consistent with its function in embryogenesis and pre-mRNA 3ʹ-end processing.
Genome-wide Analysis of the Effects of cstf77 and Auxin on Poly(A) Site Choice
To uncover the molecular mechanism of AtCstF77 in auxin signaling, we sequenced the transcriptome and PASs in wild-type and cstf77-2 mutant plants, with or without auxin treatments, by transcriptome sequencing and PAS-seq analysis (Shepard et al., 2011). Five-day-old seedlings were used in these experiments. In the auxin treatment experiments, seedlings were treated with 100 nm 2,4-d for 6 h; and in the control experiments, seedlings were treated with MS medium without 2,4-d. All samples were in triplicates.
Over 10 million 50-bp single-end clean reads were generated from each PAS-seq library. After removing the 3ʹ-end adaptors and 5ʹ-end 4-nucleotide (nt) barcodes, tags less than 20 nt in length were discarded and trimmed tags were mapped to the Arabidopsis genome (TAIR10) using the software Bowtie 2 (2.2.1; Langmead et al., 2009; Langmead and Salzberg, 2012), allowing two mismatches. Only uniquely mapped tags were kept for downstream analyses. Uniquely located tags with six or more continuous adenines downstream of the poly(A) junction in a 10-nt window were considered as internal priming tags and discarded (Shepard et al., 2011). Due to intrinsic heterogeneity, PASs located within a 24-nt window with tag per million (TPM) > 3 in the same gene were pooled and defined as a PAS cluster (PAC) as described in Wu et al. (2011). Approximately 25,000 PACs were detected in seedlings with the four different treatments. The PACs were found in 5ʹ-UTRs, coding sequences (CDSs), introns, 3ʹ-UTRs, and intergenic regions. We analyzed the distribution of different PACs. In wild type without auxin treatment, 61.4% PACs were in 3ʹ-UTRs, 23.62% in CDSs, 7.92% in 5ʹUTRs, 6.25% in intergenic regions, and only 0.81% in introns. In the cstf77-2 mutant without auxin treatment, the percentages of PACs in intergenic regions were almost doubled (6.26% vs. 13.23%); however, those in CDSs and 5ʹUTRs were slightly decreased to 18.64% and 5.61%, respectively. Auxin treatment slightly decreased the percentage of PACs in CDSs and 5ʹUTRs, but increased the percentage of PACs in 3ʹUTRs and intergenic regions (Fig. 6, A–E, Supplemental Table S4).

Genome-wide analysis of genes with differential APA in cstf77-2 and wild-type seedlings. A to E, Schematic representations and percentages of various types of poly(A) clusters in cstf77-2 versus Col-0 plants, with or without auxin treatment. 5ʹ UTR (A); CDS (B); intron (C); (D) 3ʹ UTR (D); intergenic region (E). F, Numbers of genes with a PAS shift in cstf77-2 versus wild-type plants. There were 1,777 (1298 + 479) genes in the samples with auxin treatment (referred to as “2,4-D” and the pie in blue), and 1,921 (1298 + 623) genes in the samples without auxin treatment (referred to as “control” and the pie in orange). The overlapping area refers to the 1,298 genes found in both samples. G, Numbers of genes with a PAS shift from distal to proximal or conversely in cstf77-2 versus Col-0 plants, with or without auxin treatment.
A total of 2,400 genes with PAS shifts were detected in all three replicates of cstf77-2 samples compared to wild type, with or without auxin treatments. Among them, 1,921 genes were detected in the cstf77-2 mutant without auxin treatment, while 1,777 genes were found in auxin treated cstf77-2 samples. A quantity of 1,298 genes were detected in both samples with/without auxin treatments, indicating that they were not affected by auxin treatment (Fig. 6F). In the control experiment, among the 1,921 genes with a PAS shift in the cstf77-2 mutant, 140 genes had a PAS shift from distal to proximal sites, and 1,781 genes had a PAS shift from proximal to distal sites. In the auxin treatment experiment, among the 1,777 genes in the cstf77-2 mutant that showed a PAS shift, 123 genes had a PAS shift from distal to proximal sites, while 1,654 genes had a PAS shift from proximal to distal sites (Fig. 6G).
We calculated and compared the nt frequencies around the PASs in wild type and the cstf77-2 mutant (see “Materials and Methods” for details). No obvious differences were observed in samples with or without auxin treatment (Supplemental Fig. S4). The differences between wild-type and cstf77-2 plants in the nt frequencies around the PASs might be one of the reasons for the PAS shifts in the cstf77-2 mutant.
Effects of the cstf77 - 2 Mutation on the Expression Levels of Genes with a PAS Shift
It is known that APA can generate proteomic and functional diversity, and also plays important roles in regulating gene expression (Tian and Manley, 2017). We analyzed the expression levels of genes with a PAS shift in wild type and the cstf77-2 mutant. The expression of most genes (72.7%) remained unchanged, and 13.9% were up-regulated and 13.4% were down-regulated in the cstf77-2 mutant. Auxin treatment slightly changed the percentages of the APA gene expression levels, in which 14.8% were up-regulated, 15.3% down-regulated, and 69.8% unchanged (Fig. 7, A–C).
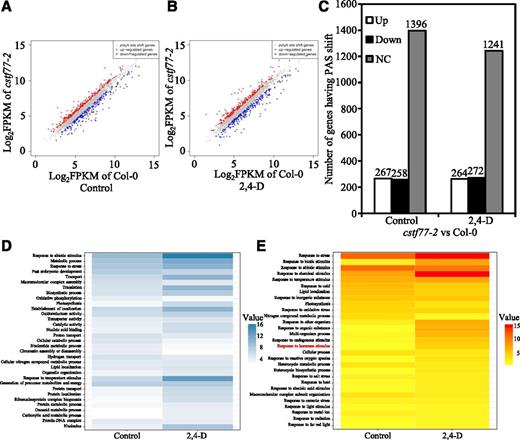
Expression and GO analysis of genes with a PAS shift. A and B, Comparison of gene expression levels of cstf77-2 versus Col-0 plants without 2,4-d treatment (A), and with 2,4-d treatment (B). Up-regulated genes are designated in red, down-regulated genes in blue, and not-changed genes in gray. C, Number of genes up-regulated (Up), down-regulated (Down), or unchanged (NC). D, GO analysis of genes with a PAS shift. E, GO analysis of genes with a PAS shift and expression level changes.
Gene ontology (GO) enrichment analysis of the genes with a PAS shift revealed that a number of cellular processes were affected in the cstf77-2 mutant compared to wild type. Genes in certain processes were highly enriched, such as response to abiotic stimulus, metabolic process, response to stress, transport, translation, and response to temperature stimulus (Fig. 7D). We also performed a GO analysis of the genes with a significant change of expression levels. A quantity of 536 genes in auxin-treated seedlings and 525 genes in seedlings without auxin treatments were identified, using the standards of gene expression level fold change > 1.3 and false discovery rate < 0.01. It was striking that many GO terms of response to stresses, hormones, or stimuli were enriched in the cstf77-2 mutant, suggesting that APA and APA-related gene expression are sensitive to endogenous stimuli and environmental stresses (Fig. 7E). It is consistent with the weak auxin insensitive phenotype displayed in the cstf77-2 mutant that many genes in hormone pathways were changed in the cstf77-2 mutant with auxin-treatment.
Auxin-Related Genes are Affected by the cstf77 Mutation
To further investigate how AtCstF77 modulates auxin signaling, we examined auxin-related genes in the lists of genes with a PAS shift. The PAS shift occurred at the 3ʹ-UTR, 5ʹ-UTR, and exons. CULLIN-ASSOCIATED AND NEDDYLATION DISSOCIATED 1 (CAND1), INDOLE-3-BUTYRIC ACID RESPONSE 1 (IBR1), IBR5, AXR2/IAA7, INDOLE-3-ACETIC ACID 9 (IAA9), IAA28, AUXIN RESPONSE FACTOR 2 (ARF2), ARF6, ARF9, ATP-BINDING CASSETTE B 19 (ABCB19), PIN-FORMED 7 (PIN7), SMALL AUXIN UPREGULATED RNA 16 (SAUR16), and D6 PROTEIN KINASE showed a PAS shift in the cstf77-2 mutant compared to wild type without auxin treatment. In the auxin-treated seedlings, CAND1, IBR1, IAA7, IAA9, IAA28, ABCB19, PIN7, LIKE AUX13 (LAX3), SAUR16, SAUR78, GH3.2, GH3.5, and D6 PROTEIN KINASE showed a PAS shift in the cstf77-2 mutant compared to wild type. CAND1, IBR1, IAA7, IAA9, IAA28, ABCB19, PIN7, and SAUR16 displayed a PAS shift, both in auxin-treated and untreated cstf77-2 seedlings compared to wild type. However, PAS shifts in LAX3, SAUR78, GH3.2, and GH3.5 were only found in the auxin-treated seedlings. These observations indicated that PAS shifts in some auxin-related genes were not affected by auxin treatment, but others were affected by both auxin and the cstf77-2 mutation (Fig. 8, A–F).

Poly(A) site shift of auxin-related genes in cstf77-2 versus Col-0 seedlings. A to F, schematic representation of positions and signal abundances of poly(A) in GH3.5 (A), LAX3 (B), IAA9 (C), IAA28 (D), IAA7 (E), ARF9 (F). Gene models are shown in the lower panels. Boxes and lines designate exons and introns, respectively.
We next analyzed the expression levels of the auxin-related genes that showed a PAS shift in the cstf77-2 mutant compared to wild type. In the auxin-treated seedlings, the expression levels of GH3.2, GH3.5, and LAX3 were decreased, while IAA7 was significantly increased in the cstf77-2 mutant. In the seedlings without auxin treatments, the expression of IAA7 in the cstf77-2 mutant was also significantly increased compared to wild type. Interestingly, the expression of IAA7 was up-regulated in the cstf77-2 mutant compared to wild type, with/without auxin treatments, suggesting that its expression is regulated by AtCstF77. It has been reported that the iaa7/axr2 gain-of-function mutant displays an auxin-resistant phenotype (Nagpal et al., 2000). We further validated the IAA7 expression in the cstf77-2 mutant by reverse transcription quantitative PCR (RT-qPCR). It was clear that the expression level of IAA7 in the cstf77-2 mutant was significantly increased (Supplemental Fig. S5), which would presumably lead to higher protein levels. The PAS shifts in IAA7, IAA9, GH3.5, ARF9, LAX3, and IAA28 were also confirmed by RT-qPCR (Supplemental Fig. S5).
DISCUSSION
In this article, we report the isolation and characterization of an auxin-resistant mutant cstf77-a780. We show that AtCstF77 plays critical roles in Arabidopsis growth and development. Disruption of AtCstF77 led to an auxin-resistant phenotype and caused severe developmental defects in embryogenesis, leaf development, and late flowering time. Genome-wide analysis of pre-mRNA 3ʹ-end polyadenylation in cstf77-2 and wild-type plants revealed a PAS shift in several auxin-related genes. We found that the expression of AXR2/IAA7 was increased in the cstf77-2 mutant, suggesting that AtCstF77 modulates auxin response by controlling mRNA 3ʹ-end polyadenylation of some auxin-related genes.
AtCstF77 is Critical for Arabidopsis Development
CstF77 is an important component of the mRNA 3ʹ-end polyadenylation machinery (Tian and Manley, 2017). Its role in mRNA polyadenylation is evolutionarily conserved from yeast, Drosophila, to human, and plants (Zhao et al., 1999; Liu et al., 2010). It has been shown that the counterparts of AtCstF77, in yeast RNA14 and Drosophila su(f), are essential for the viability of these organisms (Minvielle-Sebastia et al., 1991; Simonelig et al., 1996). It was previously reported that AtCstF77 was required for Arabidopsis development and that homozygous cstf77-2, which was reported as a null allele, was lethal (Liu et al., 2010). We show here that homozygous mutants containing null alleles cstf77-2, cstf77-c3, and cstf77-c4 displayed severe developmental defects, but they all survived under our growth conditions (Fig. 2, G and H). These findings indicate that although CstF77 is evolutionarily conserved from yeast to human and is critical for Arabidopsis development, it is dispensable. APA may offer sessile plants advantages in adapting to different environmental stresses during evolution. We observed that genes with altered expression were highly enriched in the cstf77-2 mutant in certain processes such as response to abiotic stimulus, metabolic process, response to stress, transport, translation, and response to temperature stimulus (Fig. 7D). We found that the cstf64-2 and cstf64-3 mutants were also resistant to auxin treatment, and their developmental phenotypes were similar to those observed in the cstf77-2 mutant. Our genetic results are consistent with the fact that CstF77 and CstF64 physically interact and that they function in the same protein complex in mRNA 3ʹ-end polyadenylation (Liu et al., 2010; Ruepp et al., 2011).
AtCstF77 Affects Poly(A) Site Choice
Our genome-wide analysis of cstf77-2 and wild-type plants identified PAS shifts in 2,400 genes, indicating that Arabidopsis CstF77 is involved in selecting PASs. Most of the PAS shifts were from proximal to distal sites (Fig. 6G). We found that the frequencies of A in the cstf77-2 mutant were slightly increased in Near Upstream Element and Cleavage Element regions, while the frequency of U was slightly decreased in Cleavage Element regions, compared to wild type (Supplemental Fig. S4, A and B). The differences between wild-type and cstf77-2 plants might be one of the reasons for the PAS shifts in the cstf77-2 mutant. We also analyzed the nt frequencies in samples with and without auxin treatments (Supplemental Fig. S4, A and C). The trends of profiles were similar to those observed in Hong et al. (2018). The slight difference in the frequencies of U in auxin-treated samples might be due to differences in durations of the auxin treatments in the two studies.
It was previously reported that CstF77 affects the relative utilization of alternative PASs in Drosophila and yeast (O’Hare, 1995). Our findings demonstrate that the role of CstF77 is conserved between plants and animals. It is known that 70% of Arabidopsis genes use more than one PAS (Wu et al., 2011). There are more than 20,000 genes having APA sites in Arabidopsis (Wu et al., 2011), indicating that many genes are not controlled by AtCstF77. The fact that cstf77 mutants displayed severe developmental defects indicates that the APA sites of these genes is important for plant development. This may also be true for AtCstF64, because of the similar developmental defects and auxin resistance in cstf77 and cstf64 mutants (Fig. 4), and the physical interaction between AtCstF77 and AtCstF64 proteins (Liu et al., 2010; Ruepp et al., 2011).
The ratio of PACs located in 5ʹUTRs, exons, introns, and intergenic regions was changed in the cstf77-2 mutant compared to Col-0. Particularly, the number of PACs located in intergenic regions increased two-fold in the cstf77-2 mutant (Fig. 6E). It is conceivable that the increases of PACs in intergenic regions were predominantly caused by read-through from the inefficient recognition of PASs in the cstf77-2 mutant. On the other hand, it was reported that intergenic PACs were equivalent to novel transcripts, 3ʹUTR, and antisense transcripts (Wu et al., 2015). Therefore, CstF77 might also regulate the transcriptome and proteome by affecting the distribution of PACs.
AtCstF77 Modulates Auxin Response at the Post-Transcriptional Level
APA in the 3ʹUTR produces transcripts with different lengths of 3ʹUTRs (Chakrabarti and Hunt, 2015). The longer 3ʹUTRs might have more microRNA target sites and AU-rich elements (AREs) than shorter ones, and the length of 3ʹUTRs may have a great influence on the stability and localization of mRNA (Tian and Manley, 2013). Interestingly, different ARE-associated RNA binding proteins have opposite effects on transcript stability. For example, an ARE binding protein-KSRP promotes mRNA decay by recruiting degradation machinery (Gherzi et al., 2004). In contrast, an embryonic lethal abnormal vision protein-HuR competes with the destabilizing protein-AUF1 for binding the same ARE, thus promoting mRNA stability (Lal et al., 2004). Compared to Col-0, the cstf77-2 mutation caused a shift in proximal polyadenylation sites to a distal polyadenylation site in the 3ʹUTR of IAA7 transcripts, leading to an increase of IAA7 transcripts with longer 3ʹUTRs. IAA7 transcripts with longer 3ʹUTRs have AREs, and some RNA binding proteins are likely to bind to the AREs to promote IAA7 mRNA stability. It is probably one of the reasons why IAA7 transcript levels increased in the cstf77-2 mutant compared to Col-0.
Recently, it was reported that auxin affects the choice of PASs in auxin-treated wild-type plants, and in the CPSF30 mutant oxt6, which showed altered sensitivity to auxin treatments (Hong et al., 2018). In their work, Hong et al. found that the usage frequency of APA sites was switched by auxin in 42 genes, including IAA14, ARF7, and ARF19. In addition, the usage change of PASs in ARF19 in the oxt6 mutant was smaller than those in wild type (Hong et al., 2018). In our work, we found 133 genes with a PAS shift in auxin-treated wild-type seedlings (Supplemental Table S5). There was no overlap between the 133 genes in our study and the 42 genes described in the study of Hong et al. (2018). The differences might be due to the different auxin treatment conditions in the two studies: a 6 h auxin treatment in our experiments, versus a 7 d auxin treatment in the study of Hong et al. (2018). On the other hand, the primary root elongation of cstf77 mutants was resistant to auxin, while the lateral root number in the oxt6 mutant was hypersensitive to auxin (Hong et al., 2018), suggesting AtCstF77 and CPSF30 may play different roles in modulating auxin signaling. Indeed, the APA of different genes in auxin signaling might be at least partially responsible for the altered auxin responses in their mutants.
In this article, we show that AtCstF77 and alternative poly(A) affect auxin signaling and modulates auxin responses. The cstf77 mutant displayed an auxin-resistant phenotype, and reduced DR5-GFP expression. The observed PAS shift in AXR2/IAA7 and the increased expression levels of IAA7 in the cstf77 mutants partially accounts for the auxin-resistant phenotypes of cstf77 mutants. Moreover, cstf64 mutants also displayed auxin resistance and developmental defects similar to the cstf77 mutants. These findings clearly demonstrate that AtCstF77 and APA play important roles in modulating auxin response and plant development.
MATERIALS AND METHODS
Plant Materials
Arabidopsis (Arabidopsis thaliana) seeds were sterilized with 75% and 100% (v/v) ethanol, and sown on 0.5× MS medium supplemented with 0.8% (w/v) agar. Plates were placed at 4°C for 2 d in the dark, then transferred to culture room at 22°C under the 16-h light/8-h dark condition.
All mutants were in the Col-0 background except cstf77-a780, which was in the Ler background. To identify the mutation causing the auxin-resistant phenotypes in the a780 mutant, a map-based cloning approach was used. The mutant was crossed to the Col-0 ecotype to generate F1 plants, and selfed to obtain the F2 mapping population. About 2,000 auxin-resistant F2 individuals were analyzed.
The cstf77-2 (GK_136D03), cstf64-2 (SAIL_794_G11), and cstf64-3 (SAIL_31_H03) T-DNA insertion lines were obtained from the Nottingham Arabidopsis Stock Center. Two tandem T-DNA insertion sites were identified in the second intron of the gene CstF77 in cstf77-2, 232 bp and 246 bp after the ATG start codon, respectively. The cstf77-2 mutant was genotyped by PCR using primers cstf77-2-LP, cstf77-2-RP with GK-LB1 (Supplemental Table S6). In the cstf64-2 mutant, a T-DNA insertion site was identified in the first intron of the gene CstF64, 99 bp after the ATG start codon. The cstf64-2 mutant was genotyped by PCR using primers cstf64-2-LP, cstf64-2-RP with SAIL_LB1. In the cstf64-3 mutant, a T-DNA insertion site was identified in the seventh intron of the gene CstF64, 1,338 bp after the ATG start codon. The cstf64-3 mutant was genotyped by PCR using primers cstf64-3-LP and cstf64-3-RP with SAIL_LB1. RT-PCR analysis of cstf77-2, cstf64-2, and cstf64-3 mutants was carried out by using a combination of primers: cstf77-2-LP with CstF77-RP, CstF64-Primer1-LP with cstf64-2-RP, and CstF64-Primer2-LP with cstf64-3-RP, respectively. The truncated transcripts of CstF64 were amplified using CstF64-Primer3-LP with CstF64-Primer3-RP, with CstF64-Primer4-LP, with CstF64-Primer3-RP, respectively (Supplemental Table S7).
The cstf77-c3 and cstf77-c4 mutants were generated by using CRISPR/Cas9 technology (Gao et al., 2016). The mutants were genotyped by PCR amplification using cstf77-c3-LP and cstf77-c3-RP (or cstf77-c4-LP and cstf77-c4-RP) primers, and the PCR product was digested with RsaI (for cstf77-c3) or XhoI (for cstf77-c4). The tir1-1 and afb2-3 mutants were genotyped as described in Ruegger et al. (1998) and . Sequences of the primers are listed in Supplemental Table S6.
Constructs and Plants Transformation
For complementation of the cstf77-2 mutant, a genomic DNA fragment containing the coding region, upstream and downstream regulatory regions of at1g17760 was amplified and cloned into the binary vector pPZP211 to generate the pPZP211-gCstF77 construct, which was introduced into the cstf77-2 +/− mutant plants via Agrobacterium strain GV3101 using the floral dipping method (Clough and Bent, 1998). The transgenic seedlings were selected on 0.5× MS plates containing 50 μg/mL kanamycin.
For generation of the cstf77-c3 and cstf77-c4 mutants by CRISPR/Cas9 technology, CRISPR/Cas9 targets CstF77-C3 or CstF77-C4 were fused to the AtU6 promotor through two rounds of PCR using primers CRISPR-CstF77-C3-LP with CRISPR-CstF77-C3-RP or CRISPR-CstF77-C4-LP with CRISPR-CstF77-C4-RP, which generated AtU6-CstF77-C3 or AtU6-CstF77-C4 sgRNA expression cassettes. The cassettes were cloned into the pHDE/35S-Cas9 vector digested with PmeI by Gibson assembly (Gibson et al., 2009). The construct was introduced into Col-0 plants via Agrobacterium strain GV3101 using the floral dipping method (Clough and Bent, 1998). The positive transgenic seedlings were selected on 0.5× MS plates containing 20 μg/mL hygromycin.
The pPZP211-gCstF77-GUS and pPZP211-gCstF77-GFP constructs were generated from the pPZP211-gCstF77 construct by inserting the GUS or GFP gene immediately before the stop codon of the CstF77gene, respectively. The constructs were introduced into the cstf77-2 +/− mutant plants via Agrobacterium strain GV3101 using the floral dipping method. The positive transgenic seedlings were selected on 0.5× MS plates containing 50 μg/mL kanamycin. Sequences of the primers are listed in Supplemental Table S8.
Histochemical GUS Staining
GUS staining was performed as described in Cheng et al. (2006). The gCstF77-GUS transgenic seedlings and flowers were staining in the buffer at 37°C for 12 h.
Auxin Response Analysis
Seedlings grown vertically for 5 d were transferred to 0.5× MS plates containing various concentrations of 2,4-d and the root tips of all transferred seedlings were marked. After being grown vertically for another 3 d, root elongation was quantified using the software ImageJ (NIH).
RNA Isolation and RT-qPCR Analysis
Total RNA was extracted from 7-d-old seedlings using TRNzol reagent (Tiangen). First-strand cDNA was reverse-transcribed using M-MLV Reverse Transcriptase (Promega). RT-qPCR was performed with a LightCycler96 system (Roche) using SYBR Premix Ex Taq (TaKaRa). Sequences of the primers are listed in Supplemental Table S9.
PAS-Seq
PAS-Seq was performed as previously described with minor modifications (Shepard et al., 2011; Zhang et al., 2015). poly(A)+RNA was purified using the mRNA purification kit (Invitrogen), and 0.4 μg to 0.5 μg poly(A)+RNA was fragmented by heating at 95°C for 30 min (Cloonan et al., 2008; Fu et al., 2011; Zhang et al., 2015). Reverse transcription (Superscript, Invitrogen) was carried out as follows: poly(A)+RNA was incubated with the HITS-3ʹ adaptor at 65°C for 5 min and placed on ice for 2 min, then 4 μL 5× buffer, 2 μL 0.1 m DTT, 1 μL RNaseOUT (Invitrogen), and 1 μL Superscript II (Invitrogen) were added. After the mixture was incubated at 42°C for 30 min, the HITS-5′ adaptor (a SMART oligo) was added and incubated for another 30 min. The cDNA was purified using the PCR Cleanup kit (Qiagen) and the second strand cDNA was synthesized by three cycles of PCR (95°C 10 s, 60°C 30 s, 72°C 20 s) using Phusion DNA polymerase (New England Biolabs) and the primer PE 1.0 paired with the primer PE 2.0. PCR products were run on a 2% agarose gel and 200-dp to 300-bp bands were excised and purified. Gel-extracted DNA was amplified by 15 cycles of PCR (95°C 10 s, 60°C 30 s, 72°C 20 s) using Phusion DNA polymerase (New England Biolabs) and the primer PE 1.0 paired with the primer PE 2.0. PCR products were purified using the PCR Cleanup kit (Qiagen). Sequences of the oligos are listed in Supplemental Table S10.
Analysis of RNA-Seq
A quantity of 14 million to 30 million 125-bp paired-end clean reads were generated from each strand-specific RNA-seq library. All clean reads were aligned to the Arabidopsis genome (TAIR10) using the software TopHat2 (2.0.11) with no more than five mismatches (Trapnell et al., 2009; Kim et al., 2013). Reads with unique genome location were used for subsequent analysis. The expression level of each gene was calculated by counting the number of sequenced tags mapped to the gene exon region and normalized by mapped fragments per kilobase of exon per million mapped fragments. To ensure RNA-Seq data reliability, three biological replicates were performed for Col-0/ cstf77-2 mutant, Col-0 treated with 2,4-d/cstf77-2 mutant treated with 2,4-d. Pearson’s correlation coefficient (r) was used to evaluate the consistency of replicate libraries. All replicate data sets were highly consistent (r > 0.967, P value < 2.2e-16). Differentially expressed genes were detected using the software edgeR (Robinson et al., 2010). Genes with a false-discovery–rate value less than 0.01 were regarded as differentially expressed genes.
Analysis of PAS-Seq
PAS-Seq analysis was performed as described in Zhang et al. (2015). Over 10 million 50-bp single-end clean reads were generated from each PAS-seq library. The 3ʹ-end adaptors and 5ʹ-end 4 nt barcodes were removed through a FASTX toolkit (http://hannonlab.cshl.edu/fastx_toolkit). Tags less than 20 nt in length were discarded and trimmed tags were mapped to the Arabidopsis genome (TAIR10) using Bowtie 2 (2.2.1; Langmead et al., 2009; Langmead and Salzberg, 2012), allowing two mismatches. Only uniquely mapped tags were kept for downstream analyses. Uniquely located tags with six or more continuous adenines downstream of the poly(A) junction in a 10-nt window were considered as internal priming tags and discarded (Shepard et al., 2011). Due to intrinsic heterogeneity, PASs located within a 24-nt window with TPM > 3 in the same gene were pooled and defined as a PAC as described (Wu et al., 2011). To ensure PAS-Seq data reliability, three biological replicates were performed for Col-0/ cstf77-2 mutant,Col-0 treated with 2,4-d/ cstf77-2 mutant treated with 2,4-d. All replicate data sets were highly consistent (r > 0.968, P value < 2.2e-16). PACs in at least two biological replicates were kept and considered as PACs of each sample. For statistical analysis of APA shifts, genes with at least two PACs (TPM > 3) were selected for analysis of differential usage. We used Fisher’s exact test to compare the APA shift events between the two most predominant PACs as described in Wu et al. (2011). A pair of PAC shifts with P < 0.05 were regarded as significant APA shift events.
Analysis of nt Frequency Surrounding the Poly(A) Sites
PACs that overlap with known 3ʹ UTRs were subjected to poly(A) usage analysis. The nucleotide frequency within the region of 200-nt upstream and 50-nt downstream of the terminus of the 3ʹ UTR was calculated using an in-house Perl script and was plotted using R (version 3.5.1).
Accession Numbers
Sequence data of the genes described in this article can be found in the GenBank/EMBL data libraries under the accession numbers AT1G17760 (AtCstF77), AT1G71800 (AtCstF64), and AT3G23050 (AXR2/IAA7). RNA data has been deposited in the Genome Sequence Archive database, under accession number CRA002846 (https://bigd.big.ac.cn/search?dbId=gsa&q=cra002846).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Phenotypic analysis of seed development of wild-type and cstf77 plants.
Supplemental Figure S2. Chloroplast and chlorophyll contents in wild-type and cstf77-2 plants.
Supplemental Figure S3. Genetic interaction between cstf77-2 and tir1-1 afb2-3 mutants.
Supplemental Figure S4. Single nt profile surrounding the PASs of all PACs.
Supplemental Figure S5. Confirmation of the six auxin-related genes with a PAS shift shown in Figure 8, by RT-qPCR analysis.
Supplemental Table S1. Analysis of test-crosses.
Supplemental Table S2. Genetic analyses of cstf77-2, cstf77-c3, and cstf77-c4.
Supplemental Table S3. Genetic analyses of tir1-1 afb2-3 cstf77-2.
Supplemental Table S4. Distribution of poly(A) clusters.
Supplemental Table S5. Genes having poly(A) shift in wild type.
Supplemental Table S6. Genotyping primers used for cstf77-2, cstf77-c3, cstf77-c4, cstf64-2, and cstf64-3 identification.
Supplemental Table S7. RT-PCR primers.
Supplemental Table S8. Primers used for construction.
Supplemental Table S9. Primers used for RT-qPCR.
Supplemental Table S10. Oligos used for PAS Seq.
ACKNOWLEDGMENTS
We thank Dr. Mark Estelle for providing the tir1 afb mutant seeds.
LITERATURE CITED
Author notes
This work was supported by the National Natural Science Fund of China (grant nos. 31270330, 91217310, 91017008, and 31171389), the National Basic Research Program of China (grant no. 2014CB943400), and the Hundred Talents Program of the Chinese Academy of Sciences (all to Y.C.). This work was also supported by the U.S. National Institutes of Health (grant no. R01GM114660 to Y.Z.), and by the National Natural Science Foundation of China (grant nos. 31788103 and 91540203 to X.C.) and the Strategic Priority Research Program of the Chinese Academy of Sciences (grant no. XDB27030201 to X.C.).
These authors contributed equally to the article.
Senior authors.
Author for contact: [email protected].
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Youfa Cheng ([email protected]).
Y.C. and Y.Z. conceived and designed research; W.Z., X.D., and Y.C. performed most of the experiments; W.Z., X.D., J.S., Y.H., X.M., X.C., Y.Z., and Y.C. analyzed data; and Y.Z. and Y.C. wrote the article.
Articles can be viewed without a subscription.



