-
PDF
- Split View
-
Views
-
Cite
Cite
Britta Schillheim, Irina Jansen, Stephani Baum, Alexander Beesley, Carsten Bolm, Uwe Conrath, Sulforaphane Modifies Histone H3, Unpacks Chromatin, and Primes Defense, Plant Physiology, Volume 176, Issue 3, March 2018, Pages 2395–2405, https://doi.org/10.1104/pp.17.00124
Close - Share Icon Share
Abstract
Modern crop production calls for agrochemicals that prime plants for enhanced defense. Reliable test systems for spotting priming-inducing chemistry, however, are rare. We developed an assay for the high-throughput search for compounds that prime microbial pattern-induced secretion of antimicrobial furanocoumarins (phytoalexins) in cultured parsley cells. The screen produced 1-isothiocyanato-4-methylsulfinylbutane (sulforaphane; SFN), a secondary metabolite in many crucifers, as a novel defense priming compound. While elucidating SFN’s mode of action in defense priming, we found that in Arabidopsis (Arabidopsis thaliana) the isothiocyanate provokes covalent modification (K4me3, K9ac) of histone H3 in the promoter and promoter-proximal region of defense genes WRKY6 and PDF1.2, but not PR1. SFN-triggered H3K4me3 and H3K9ac coincide with chromatin unpacking in the WRKY6 and PDF1.2 regulatory regions, primed WRKY6 expression, unprimed PDF1.2 activation, and reduced susceptibility to downy mildew disease (Hyaloperonospora arabidopsidis). Because SFN also directly inhibits H. arabidopsidis and other plant pathogens, the isothiocyanate is promising for the development of a plant protectant with a dual mode of action.
To supply the future world population with food, crop production needs to double by 2050 (UN Food and Agriculture Organization, 2009). The required boost in crop yield largely depends on effective plant protection, which is mostly achieved today with synthetic agrochemicals. Although safer than ever, chemical crop protection raises ecological and health concerns (Mascarelli, 2013; Lamberth et al., 2013). Therefore, safe and eco-friendly pest and disease control products are needed (Lamberth et al., 2013).
Phytochemicals that prime the plant immune system for enhanced defense are promising for sustainable crop protection (Beckers and Conrath, 2007; Conrath et al., 2015). When primed, plants respond to very low levels of a stimulus with earlier, sometimes faster and often more intense activation of defense than unprimed plants. This frequently reduces pest and disease susceptibility (Conrath et al., 2002; 2006; 2015; Beckers and Conrath, 2007; Frost et al., 2008). In Arabidopsis (Arabidopsis thaliana), priming is associated with an elevated level of microbial pattern receptors (Tateda et al., 2014), accumulation of dormant cellular signaling enzymes (Beckers et al., 2009), and covalent modification to chromatin (notably histone H3K4me3, H3K9ac, and DNA hypomethylation; Jaskiewicz et al., 2011a; Luna et al., 2012; López et al., 2011). Together, these events seem to provide the memory to the initial infection in that they poise defense genes for enhanced transcription upon reinfection (Conrath, 2011; Jaskiewicz et al., 2011a; Luna et al., 2012; Conrath et al., 2015). Other molecular mechanisms of priming remained unknown. Priming does not run up a major fitness bill and is hardly prone to pest or pathogen adaptation (van Hulten et al., 2006; Martinez-Medina et al., 2016). Thus, triggering priming by phytochemicals represents a promising means for sustainable pest and disease control.
Previous work with synthetic chemicals provided proof of this concept. Benzo(1,2,3)thiadiazole-7-carbothioic acid S-methyl ester (BTH; common name acibenzolar-S-methyl), a mimic of the phytochemical salicylate (SA), in addition to activating some defense genes directly (Ryals et al., 1996), primes plants for enhanced defense (Katz et al., 1998; Kohler et al., 2002) and protects multiple crops from disease (Ryals et al., 1996). In 1996, BTH was commercially launched as a plant immune activator (Ruess et al., 1996) with the trade name “Actigard.” However, BTH’s economic success was limited, mainly because of its strictly protective activity. To overcome the practical limitations of BTH, and take advantage of the low risk of pathogen adaptation, BTH is nowadays combined with conventional fungicides to achieve the best possible plant protection. For example, Bion M is a mixture of BTH with the fungicide mancozeb. The mixture performs particularly well on vegetables, even if mancozeb levels are much reduced (Leadbeater and Staub, 2014).
Another strategy for exploiting defense priming in practical pest and disease control is by identifying compounds combining insecticidal or antimicrobial activity with defense priming in a same molecule. The strobilurin fungicide pyraclostrobin (trade name “Headline”), in addition to its fungicide activity, primes crops and ornamental plants in the greenhouse and field for resistance to disease associated with enhanced yield (Herms et al., 2002; Koehle et al., 2003; 2006) even in abiotic stress conditions (Holmes and Rueber, 2007). Because of their broad spectrum of protection and the distinctive yield benefit, pyraclostrobin and other strobilurin fungicides became top-selling agrochemicals (Bartlett et al., 2002).
Today, the commercial success of crop protectants often relies on their ability to combine insecticidal or antimicrobial activity with defense priming in the treated crop (Beckers and Conrath, 2007; Conrath et al., 2015). However, identifying such chemistry is difficult because test systems for priming activity are rare. We developed a high-throughput screen for compounds that prime microbial pattern-induced furanocoumarin (phytoalexin) secretion in suspension-cultured parsley (Petroselinum crispum) cells. The screen produced 1-isothiocyanato-4-methylsulfinylbutane (sulforaphane [SFN]) as a novel defense-priming compound in plants. Because SFN is a natural compound with antimicrobial and insect deterrent activity (Tierens et al., 2001; Halkier and Gershenzon, 2006), the isothiocyanate may qualify for the development of a sustainable plant protectant.
RESULTS
Identification of SFN as a Novel Defense-Priming Compound
To spot novel defense-priming compounds, we optimized an assay that measures the enhancement by priming agents of furanocoumarin secretion provoked in cultured parsley cells by a moderate concentration of Pep13 (Kauss et al., 1992), a molecular pattern in the plant pathogen Phytophthora sojae (Brunner et al., 2002). Over the past approximately 25 years, the parsley-Pep13 interaction helped to disclose biochemical and molecular biological aspects of defense priming (Kauss et al., 1992; Katz et al., 1998), and identify novel priming-inducing chemistry (Katz et al., 1998; Siegrist et al., 1998). For enhanced throughput, we performed the test with 1-mL aliquots of cell culture in 24-well microtiter plates (Fig. 1). Cell culture aliquots were supplemented with the candidate compound (in DMSO), the natural priming compound salicylate (in DMSO; positive control), or DMSO (solvent control; Fig. 1). Upon shaking for 24 h in the dark, Pep13 (50 pm) was added to spur furanocoumarin synthesis and secretion. After another 24 h on the shaker, fluorescence of secreted furanocoumarins was quantified in a microtiter plate reader (Fig. 1). Compounds that significantly enhanced Pep13-induced furanocoumarin secretion were considered active at priming for enhanced defense.

Scheme of the high-throughput screen for identifying plant immune stimulants. A quantity of 1-mL aliquots of a 3-d-old parsley cell culture was transferred to individual wells of a 24-well microtiter plate containing a candidate compound for priming (A, B, or C) or the known priming activator SA (positive control). All compounds were dissolved in DMSO (<1%). Thus, DMSO (1%) treatment served as a negative control. Upon incubation for 24 h on a shaker, Pep13 (50 pm) was added to appropriate wells. After shaking for another 24 h, the fluorescence of secreted furanocoumarins was quantified in a microtiter plate reader.
For unbiased screening, we randomly selected candidate compounds from commercial compound libraries. In three replications of the screening procedure, we identified SFN, an aliphatic isothiocyanate in many crucifers, as a novel defense-priming compound (Fig. 2A). Priming by SFN of Pep13-incuded furanocoumarin secretion was dose-dependent. At 25 μm, SFN was as active at priming as SA at 200 µm, whereas 60 μm SFN primed parsley cells better than 200 µm SA. No defense priming was seen when SFN was used at 120 µm (Fig. 2A). At this concentration, SFN noticeably harmed the cells, as made obvious by their mucilaginous appearance.
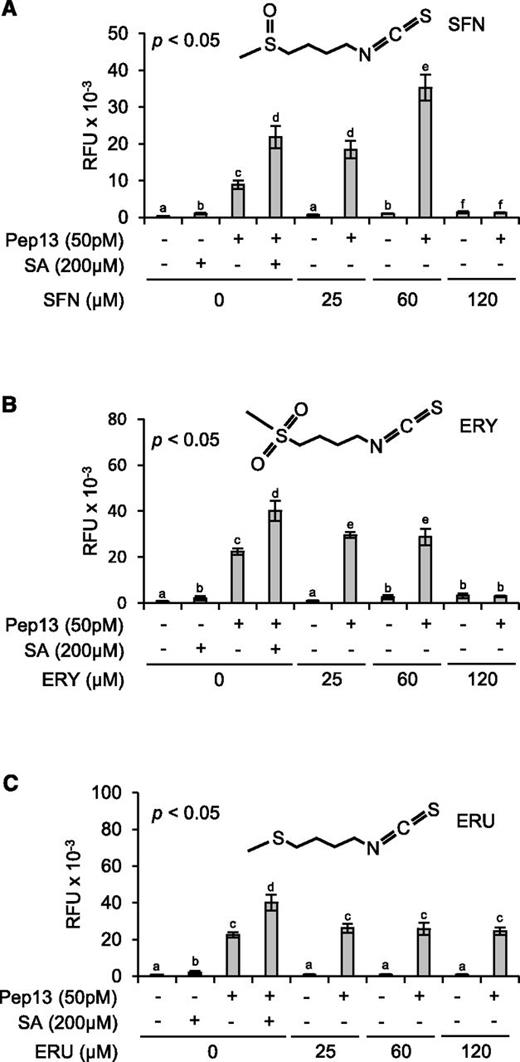
Role of the –N=C=S group and the oxidation state of sulfur in the side chain. A quantity of 1-mL aliquots of a 3-d-old parsley cell culture in microtiter plates was treated with SFN (A), ERY (B), ERU (C), or SA (A–C, positive control). All compounds were dissolved in DMSO (0.25%). Therefore, treatment with 0.25% DMSO served as an additional control. After 24 h on a shaker, 50 pm Pep13 was added. After another 24 h, furanocoumarin fluorescence in the wells was determined. Data were analyzed by one-way ANOVA followed by posthoc Student’s t test. Different letters denote statistically significant differences with 95% confidence. Data presented are means ± sd (n > 6). RFU, Relative fluorescence units.
Role of the –N=C=S Group and the Oxidation State of Sulfur in the Side Chain
The many biological activities of SFN have been assigned to its –N=C=S moiety that has various cellular nucleophilic targets (Zhang et al., 1992). To investigate whether the –N=C=S group in SFN is sufficient for priming and if oxidation of sulfur in the side chain would affect SFN’s priming capacity, 1-isothiocyanato-4-methylsulfonylbutane (erysolin [ERY]) and 1-isothiocyanato-4-methylsulfanylbutane (erucin [ERU]) were tested for priming activity (Fig. 2, B and C). In ERU, the sulfur atom is not oxidized at all. In SFN, it bears one, and in its sulfonyl analog ERY, two oxygen atoms. With reference to SA, ERY displayed weaker priming activity than SFN at 25 and 60 µm, and this activity was gone at 120 µm ERY (Fig. 2B). ERU at 25, 60, and 120 µm did not much, if at all, prime parsley cells for enhanced Pep13-provoked furanocoumarin secretion (Fig. 2C). Therefore, the oxidation state of sulfur in the side chain seems to be critical, and the –N=C=S moiety insufficient, for SFN’s priming activity.
SFN Induces Covalent Modification to Histone H3
In eukaryotes, trimethylation of Lys residue 4 in histone H3 (H3K4me3), acetylation of Lys-9 in histone H3 (H3K9ac), and some other histone modifications in the promoter or body of gene accompany gene activity (Li et al., 2007). After transcription, they provide a memory for enhanced future gene expression (Badeaux and Shi, 2013). In Arabidopsis, H3K4me3 and H3K9ac in the promoter and promoter-proximal region associate with the primed state of enhanced WRKY6/29/53 defense gene readiness, before reactivation of WRKY6/29/53 transcription (Jaskiewicz et al., 2011a). H3K9ac on the WRKY6/53 promoter region was also linked to transgenerational defense priming in this plant (Luna et al., 2012). Because of the assumed critical role of H3K4me3 and H3K9ac in defense gene priming and transcription, we wondered whether SFN would modify histone H3 in the promoter/promoter-proximal region of WRKY6, PLANT DEFENSIN1.2 (PDF1.2), and PATHOGENESIS-RELATED1 (PR1). These loci were selected because WRKY6 is a reliable reporter gene for defense priming in Arabidopsis (Jaskiewicz et al., 2011a), and PDF1.2 and PR1 serve as marker genes for the jasmonate (JA)/ethylene (ET) and SA signaling pathways, respectively (Uknes et al., 1992; Penninckx et al., 1996; Koornneef and Pieterse, 2008). The drought-responsive RAB18 (Lång and Palva, 1992) and Rubisco genes served as control loci.
Our chromatin immunoprecipitation (ChIP) experiments disclosed that a wettable powder (WP) formulation of SFN or BTH (which served as a positive control for defense priming) enhanced H3K4me3 and, with less confidence, H3K9ac in the promoter and promoter-proximal region of WRKY6 (Fig. 3, A and B). SFN induced both these histone modifications also in the promoter region of PDF1.2, whereas BTH seemed to reduce the two epi-marks on histone H3 in the PDF1.2 promoter (Fig. 3, C and D), although this was not significant. In contrast, BTH, but not SFN, significantly enhanced H3K4me3 and seemingly also H3K9ac in the promoter/promoter-proximal region of PR1 (Fig. 3, E and F). SFN and BTH either did not, or did only marginally, change H3K4me3 and H3K9ac in the promoter/promoter-proximal region of RAB18 and Rubisco (Fig. 3, G–J). These findings demonstrated that in the regulatory region of defense genes, SFN and BTH induce covalent modification of histone H3 that accompanies primed or unprimed defense gene transcription (Fig. 5, A–C).
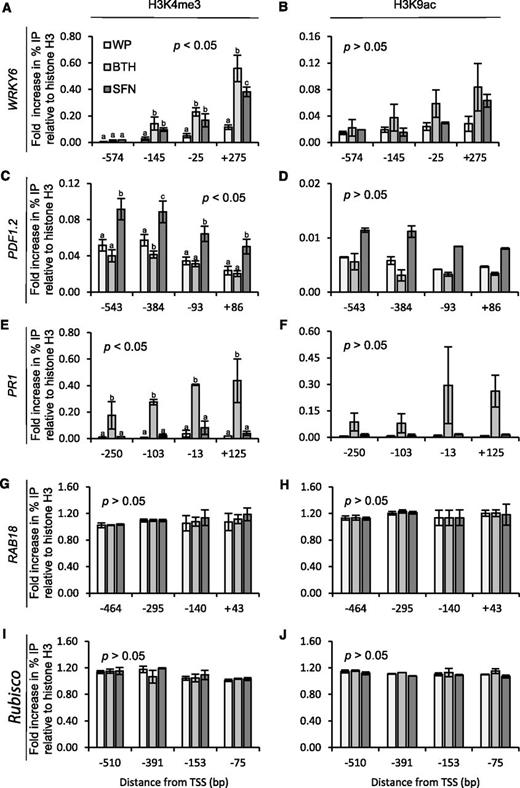
Histone H3 modification in the promoter of Arabidopsis defense genes upon plant treatment with SFN or BTH. Plants were sprayed with WP formulations of SFN (450 µm) or BTH (100 µm). Application of WP served as a control for these treatments. At 24 h after treatment, leaves were harvested and subjected to chromatin extraction, isolation, and immunoprecipitation with antibodies to the H3K4me3 (A, C, E, G, and I) or H3K9ac (B, D, F, H, and J) epitopes. DNA abundance in the precipitate was measured by qPCR with primers specific to WRKY6 (A and B), PDF1.2 (C and D), PR1 (E and F), RAB18 (G and H), or Rubisco (I and J). Data give the fold increase in amplicon abundance compared to a sample from untreated control plants. We analyzed the data for every given position of gene by one-way ANOVA followed by posthoc Student’s t test. Different letters denote statistically significant differences with 95% confidence. Data are means ± sd (n > 3).
SFN and BTH Unpack Chromatin at Defense Gene Promoters
Histone acetylation slacks the interaction of nucleosome neighbors, loosens the ionic DNA-histone interaction, and provides docking sites for regulatory proteins (Kanno et al., 2004). H3K4me3 is also known to recruit transcription coactivators to chromatin (Aasland et al., 1995). As the interaction of DNA with regulatory proteins in chromatin is less intense than the DNA-nucleosome interplay (Giresi and Lieb, 2009), nucleosome-depleted DNA elements interacting with regulatory proteins can experimentally be detected as open chromatin (Gaulton et al., 2010). To investigate whether modification of histone H3 by SFN and BTH (Fig. 3) would open chromatin in the promoter/promoter-proximal region of target genes, we performed formaldehyde-assisted isolation of regulatory DNA elements (FAIRE) associated with quantitative amplification of DNA using locus-selective PCR (FAIRE-qPCR; Giresi and Lieb, 2009). FAIRE is exceptionally powerful for identifying unpacked regulatory chromatin (Simon et al., 2012).
Figure 4A shows that treatment of Arabidopsis plants with SFN or BTH, both in WP, causes chromatin to open in the WRKY6 promoter/promoter-proximal region. Chromatin unpacking was detected within the −574- to +275-bp region relative to the transcription start site (TSS) and was most pronounced at −25 bp (SFN) and −145 bp (BTH) of all sites tested (Fig. 4A). In contrast to WRKY6, the PDF1.2 promoter opened in the −817- to +86-bp region after plant treatment with SFN, but less so upon treatment with BTH (Fig. 4B). Chromatin unpacking by SFN in the PDF1.2 promoter was maximal at −93 bp relative to the TSS (Fig. 4B). The PR1 promoter opened in the −650- to +125-bp region in response to BTH treatment with prominent peaks at −650-, −571-, and −103 bp, but did not open after SFN application (Fig. 4C). SFN and BTH did not open chromatin in the RAB18 promoter/promoter-proximal region (Fig. 4D). The overall chromatin unpacking recorded in the −295- to −464-bp region of RAB18 is likely due to the promoter start of the preceding, constitutively active, and antisense-oriented PLP3.b gene with a role in microtubule assembly. In the Rubisco promoter, chromatin seemed to be generally loosened after treatment with SFN and BTH, but typical peaks of chromatin opening were not detected (Fig. 4E).
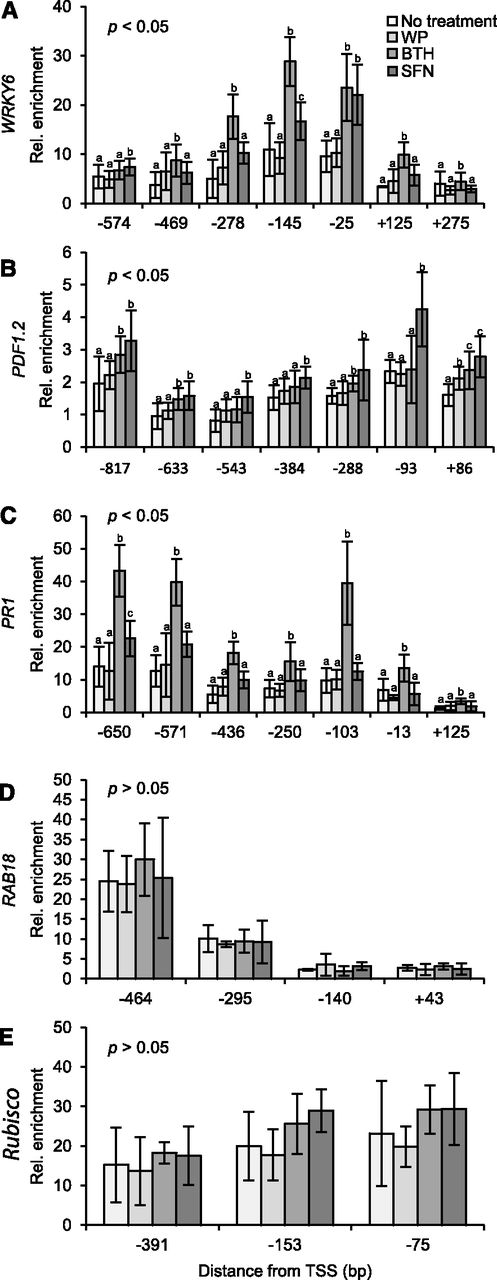
Detection of open chromatin in defense gene promoters/promoter-proximal regions. Arabidopsis plants were left untreated or sprayed with a WP formulation of SFN (450 µm) or BTH (100 µm). WP treatment served as a control for these treatments. After 24 h, we harvested leaves and subjected them to FAIRE. We performed qPCR with primers specific for different sites in the promoter/promoter-proximal region of WRKY6 (A), PDF1.2 (B), PR1 (C), RAB18 (D), and Rubisco (E). Data for every given position of gene were analyzed by one-way ANOVA followed by posthoc Student’s t test. Different letters denote statistically significant differences with 95% confidence. Data presented are means ± sd (n > 6).
A Dual Role for SFN in Defense Gene Activation
Because SFN caused covalent modification of histone H3 and chromatin opening in the WRKY6 and PDF1.2 but not PR1 regulatory regions (Figs. 3, A–F, and 4, A–C), we wondered whether the isothiocyanate affected the transcriptional response of WRKY6, PDF1.2, or PR1. To answer this question, we sprayed Arabidopsis plants with the WP formulation of SFN or BTH. Plants that were left untreated or sprayed with WP served as controls. At 24 h later, three leaves of an Arabidopsis plant were infiltrated with an aqueous solution of the microbial pattern flg22 to activate defense. After another 45 min, leaves were harvested and analyzed for accumulation of mRNA transcript of the WRKY6, PR1, and PDF1.2 defense genes (Fig. 5, A–C).
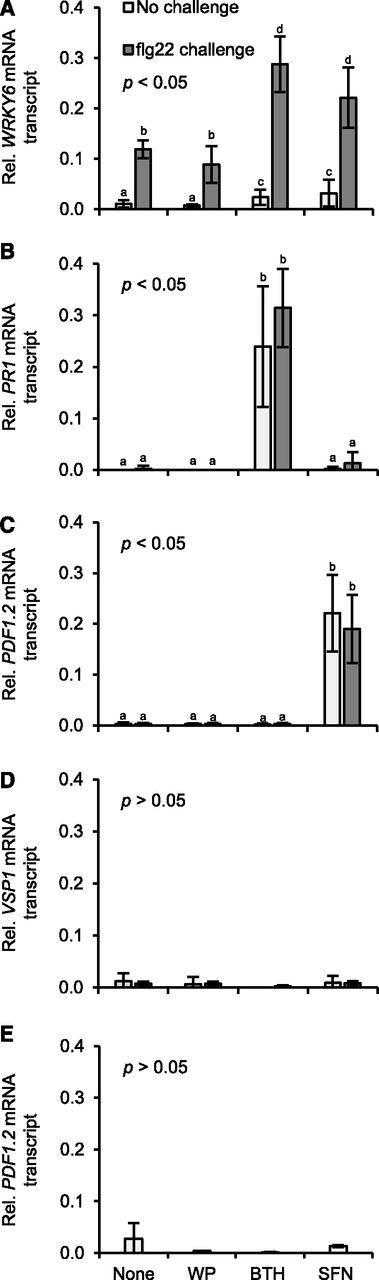
Unprimed or primed defense gene activation in Arabidopsis genotypes treated with SFN or BTH. Wild type (A–D) and ein2-1 (E) plants were left untreated, sprayed with a WP formulation of SFN (450 µm) or BTH (100 µm), or WP only. 24 h later, three leaves of half of the plants for each treatment were challenged by infiltrating flg22 (200 nm; A–D) or left unchallenged (A–E). After 45 min, we extracted RNA from leaves and subjected it to RT-qPCR analysis using gene-specific primers (Supplemental Table S1). Data were analyzed by one-way ANOVA followed by posthoc Student’s t test. Different letters denote statistically significant differences with 95% confidence. Data presented are means ± sd (n > 6).
Although flg22 at 200 nm triggered the accumulation of WRKY6 mRNA transcript, this accumulation was somewhat, but not significantly, reduced upon preexposure to WP (Fig. 5A). Treatment with SFN or BTH only faintly caused WRKY6 expression (Fig. 5A). However, treating Arabidopsis with either of the two compounds enhanced the later WRKY6 activation by flg22 (Fig. 5A). PR1 expression was not activated by SFN, but was induced upon treatment with BTH and this response was somewhat, but not significantly, augmented upon flg22 challenge (Fig. 5B). By contrast, SFN, but not BTH, activated PDF1.2 expression, with no further enhancement by subsequent flg22 challenge (Fig. 5C).
The activation of PDF1.2 by SFN (Fig. 5C) let us conclude that the isothiocyanate stimulates JA and/or ET signaling. In Arabidopsis, the two hormone signals are transduced via two distinct but interconnected pathways, which both lead to PDF1.2 expression (Zarate et al., 2007). However, only the JA signaling pathway also activates expression of the VEGETATIVE STORAGE PROTEIN1 (VSP1) gene (Zarate et al., 2007). To disclose the pathway by which SFN activates PDF1.2 in Arabidopsis (Fig. 5C), we thus examined the accumulation of VSP1 mRNA transcript in samples from appropriately treated plants. As shown in Figure 5D, neither WP, nor BTH or SFN, activated Arabidopsis VSP1 before or after flg22 treatment. This suggests involvement of ET rather than JA in SFN-induced PDF1.2 expression. Consistent with this assumption, SFN did not activate PDF1.2 expression in the Arabidopsis ETHYLENE INSENSITIVE2-1 (ein2-1) mutant (Fig. 5E), which is blocked in ET signaling (Alonso et al., 1999).
SFN and Plant Protection
Because SFN seems to stimulate ET signaling in Arabidopsis, the isothiocyanate is likely to reduce the susceptibility to nectrotroph pathogens in this plant. However, SFN directly inhibits the growth of many infectious fungi (e.g. Plectosphaerella cucumerina) and bacteria (e.g. Pseudomonas syringae) already in the absence of plant (Tierens et al., 2001). Therefore, we will not be able to distinguish whether a reduction of disease susceptibility to such pathogens in SFN-treated plants is due to defense priming, direct inhibition of the pathogen, or both these possibilities.
To investigate whether SFN affects the interaction of Arabidopsis with the infectious oomycete Hyaloperonospora arabidopsidis (Hpa), we sprayed Arabidopsis Col-0 plants with a WP formulation of SFN before we inoculated them with Hpa (race Noco; Uknes et al., 1992). The Noco race of Hpa causes downy mildew disease on Arabidopsis Col-0 (Coates and Beynon, 2010). Figure 6A discloses that pretreatment with SFN seems to reduce the susceptibility of Arabidopsis to downy mildew disease, as obvious by lower Hpa sporulation. This is suggesting that SFN activates defense priming, and by doing so, reduces the susceptibility to Hpa infection in Arabidopsis. However, because treatment of Arabidopsis leaves with Hpa conidiospores in a SFN solution causes an even stronger reduction of Hpa sporulation (Fig. 6B), the isothiocyanate seems to inhibit Hpa directly.
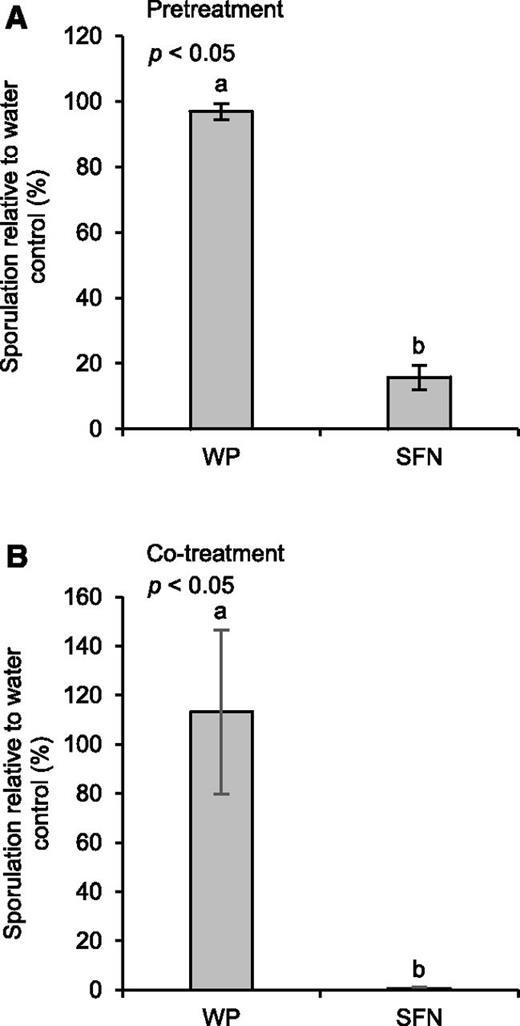
SFN reduces downy mildew disease. A, Plants were treated with water (control), WP, or a WP formulation of SFN (450 µm). Twenty-four hours later, we spray-inoculated plants with a suspension of Hpa conidiospores (5 × 104 spores per mL of water). B, Conidiospores (5 × 104 spores per mL) were suspended in water (control), WP, or a WP formulation of SFN (450 µm). Spore suspensions were mixed and used for spray inoculation of plants. In A and B, inoculated plants were kept at high humidity in short day. After 7 d, we determined the number of spores released by Hpa. Data were analyzed by one-way ANOVA followed by posthoc Student’s t test. Different letters denote statistically significant differences with 95% confidence. Data presented are means ± sd. A, n > 7; B, n > 14.
DISCUSSION
We developed an assay for the high-throughput identification of chemical compounds with potential for plant-immunity-based, sustainable agriculture. The test measures the extent of furanocoumarin secretion by parsley culture cells challenged with Pep13 (Kauss et al., 1992; Katz et al., 1998; Siegrist et al., 1998). The advantage of the test over competitive assays is the high sensitivity of fluorescence detection and the need of only two treatments (activation of priming and Pep13 challenge; Fig. 1) before final analysis. A recently introduced competitive high-throughput assay for identifying priming-inducing chemistry evaluates the enhancement of P. syringae pv tomato avrRpm1-induced cell death in an Arabidopsis cell culture by priming agents (Noutoshi et al., 2012). The identification of several immune-priming compounds verified the suitability of the assay for discovering priming-inducing chemistry. However, because of requirement of bacterial challenge, cytochemical staining, washing, dye extraction, and absorbance measurement, the screen is rather elaborate. The same holds true for a more recently introduced respiratory activity-monitoring system for discovering priming-inducing chemistry (Schilling et al., 2015), which is innovative but suffers from low throughput.
Our screen identified the glucosinolate metabolite SFN as a novel defense-priming compound in plants. In addition to Pep13-induced furanocoumarin secretion in parsley (Fig. 2A), SFN primed WRKY6 for enhanced expression upon flg22 treatment (Fig. 5A) and directly activated PDF1.2 (Fig. 5C) in Arabidopsis. Thus, SFN, just like SA and BTH (Katz et al., 1998; Thulke and Conrath, 1998), seems to have a dual role in the activation of defense genes in plants.
Glucosinolate metabolites were known to be important to the Arabidopsis defense response (Clay et al., 2009), but their mode of action remained incompletely understood. Unfortunately, Arabidopsis mutants with impaired SFN perception or transduction are not yet available. Because SFN, although at higher concentrations than those applied here, induced cell death when infiltrated into Arabidopsis leaves (Andersson et al., 2015), we cannot completely exclude that the SFN-induced priming (Figs. 2A and 5A) and direct activation of defense genes (Fig. 5C) is mediated by incidental cell death. In addition, SFN directly inhibits the growth of many infectious bacteria (e.g. P. syringae) and fungi (e.g. Pl. cucumerina) in the absence of plant (Tierens et al., 2001). Therefore, we will not be able to distinguish whether a reduction of disease susceptibility to such pathogens in SFN-treated plants is due to defense priming in the plant, direct inhibition of the pathogen, or both these possibilities. The weaker toxicity of SFN to some other plant pathogens (e.g. Alternaria brassicicola and Botrytis cinerea) could be countered by its stimulating effects on ET-dependent immunity in the plant (Fig. 5, C–E).
Because ERY was less active, and ERU not active, at priming (Fig. 2), the oxidation state of sulfur in the side chain seems to be critical, and the –N=C=S moiety insufficient, for SFN’s priming activity. SFN and ERY (but not ERU) also induce activity of the phase-II detoxification enzymes quinone reductase and glutathione S-transferase in murine hepatoma cells (Zhang et al., 1992), suggesting a same or similar mode of action of these compounds in plants and mammals. Whether SFN activates quinone reductase and glutathione S-transferase in plants, and whether this possible activation is relevant to defense priming, remains to be seen. However, in seeming analogy to Arabidopsis, SFN inhibits histone deacetylase and increases histone acetylation while preventing prostate cancer (Myzak et al., 2004; 2006; Gibbs et al., 2009; Ho et al., 2009), further supporting histone modification as a key mode of action of SFN.
SFN enhanced H3K4me3 and H3K9ac in the promoter/promoter-proximal region of WRKY6 and the JA-responsive PDF1.2 gene (Fig. 3, A–D), whereas the SA mimic BTH reduced the two epi-marks in the PDF1.2 promoter (Fig. 3, C and D). By contrast, BTH, yet not SFN, enhanced H3K4me3 and H3K9ac in the promoter/promoter-proximal region of the SA-responsive PR1 gene (Fig. 3, E and F). Hence, the negative cross talk of the JA/ET and SA signaling pathways, which enables plants to fine-tune their immune response to pathogens with different lifestyles (Koornneef and Pieterse, 2008), seems to be under epigenetic control (Alvarez et al., 2010; Caarls et al., 2015).
SFN treatment caused unprimed PDF1.2 transcription before, and primed WRKY6 transcription after, flg22 challenge (Fig. 5, A and C). WRKY6 priming and PDF1.2 transcription by SFN coincide with H3K4me3 and H3K9ac (Fig. 3, A–D), chromatin unpacking in the promoter/promoter-proximal region of gene (Fig. 4, A and B), and reduced downy mildew disease (Fig. 6). These findings point to histone modification, enhanced DNA accessibility, and an antimicrobial effect as modes of SFN action in plant disease alleviation. H3K4me3, H3K9ac, and chromatin unpacking were found in the promoter of unprimed as well as primed genes (Figs. 3 and 4; Jaskiewicz et al., 2011a). Therefore, histone modifications other than H3K4me3 and H3K9ac, co-occurrence with other histone marks, regulatory RNAs, and/or nonhistone proteins (e.g. transcriptional coactivators, chromatin remodeling factors, histone variants) seem to determine whether transcription of a given gene will be unprimed or primed (Conrath, 2011; Badeaux and Shi, 2013; Weiner et al., 2016).
In crucifers, including Arabidopsis, SFN and other isothiocyanates upon tissue disintegration are released from glucosinolates (1-thio-β-d-glucosides) by endogenous S-glycosyl hydrolases (myrosinases; Wittstock and Halkier, 2002). Whereas glucosinolates are considered biologically inactive isothiocyanate storage forms, SFN and other isothiocyanates serve as insect feeding and/or oviposition stimulants or deterrents (Halkier and Gershenzon, 2006). However, glucosinolates and their metabolites have also been shown to cause plant cell death (Andersson et al., 2015) and inhibit the growth of various infectious microbes (e.g. Pl. cucumerina, P. syringae, and Phakopsora pachyrhizi; Tierens et al., 2001; Supplemental Fig. S1) directly. Therefore, we cannot distinguish whether a reduction of disease susceptibility to pathogens in SFN-treated plants is due to defense priming, direct inhibition of the pathogen, or both these possibilities.
Here, we discovered a new role of SFN linking the glucosinolate pathway to defense priming. In the tritrophic interaction of crucifers, insects, and oomycetes, our work suggests that insect feeding on crucifers causes release of SFN from the glucosinolate glucoraphanin by myrosinase. SFN then imposes epigenetic modifications to histone H3 in the plant, associated with enhanced accessibility of DNA in defense gene regulatory regions. This specific chromatin environment seems to prepare defense genes for unprimed or primed transcription. By doing so, the plant may reduce the risk of microbial infection after insect feeding.
SFN combines insect deterrent (Halkier and Gershenzon, 2006), antimicrobial (Tierens et al., 2001; Supplemental Fig. S1), defense priming (Figs. 2A and 5A), and defense gene inducing (Fig. 5C) activity within a same molecule. In addition, SFN is a natural compound and is present in substantial quantities in human diet (Li and Zhang, 2013). Therefore, the isothiocyanate presents a promising candidate for the development of a novel, nonhazardous plant protectant with a dual mode of action.
MATERIALS AND METHODS
Parsley cell cultures and Arabidopsis (Arabidopsis thaliana) wild-type Col-0 and ein2-1 plants were grown, kept, and treated as described by Katz et al. (1998) and Beckers et al. (2009). BTH and WP were provided by Syngenta.
High-Throughput Screening for Defense Priming Compounds in Parsley Cell Cultures
At 3 d after subculture, 1 mL aliquots of parsley cell suspension were transferred to a 24-well CELLSTAR microtiter plate (Greiner). Cells were either left untreated (negative control), treated with 200 µm SA in DMSO (0.25%; positive priming control), or treated with the given concentration of test compound in DMSO. Cell suspensions were shaken at 100 rpm and at 25°C in the dark. After 24 h, 50 pm of custom-synthesized Pep13 (Thermo Fisher Scientific) was added to appropriate aliquots of cell suspension. Addition of a same volume of water served as a control for the Pep13 treatment. After another 24 h, relative furanocoumarin fluorescence in the supernatant of cell suspension was determined in a microtiter plate reader (Tecan) at 335 nm excitation and 398 nm emission.
Determination of Defense Priming in Arabidopsis
At 24 h after spraying plants with WP, BTH in WP, or SFN in WP, three leaves of half of the plants of each treatment were infiltrated with 200 nm custom-synthesized flg22 (Thermo Fisher Scientific) in water. The other half of plants was infiltrated with a same volume of water. Forty-five min after infiltration, leaves were harvested and snap-frozen in liquid nitrogen. RNA extraction and quantification of mRNA transcript accumulation by RT-qPCR was performed as described by Beckers et al. (2009) using gene-specific primers (Supplemental Table S1).
FAIRE-qPCR
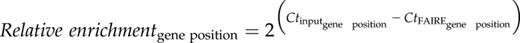
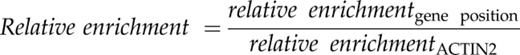
ChIP
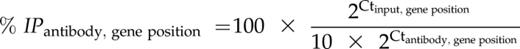

Determining Arabidopsis Susceptibility to Hpa
Two-to-three-week-old Arabidopsis plants were left untreated or treated with WP or a WP formulation of SFN. At 24 h after treatment, plants were inoculated by spraying with a conidiospore suspension of Hpa (5 × 104 spores per mL of water). Alternatively, conidiospores (5 × 104 spores per mL) were suspended in water, WP, or a WP formulation of SFN. Spore suspensions were thoroughly mixed on a bench shaker and used for spray inoculation of 2- to 3-week-old Arabidopsis plants. In both cases (pretreatment and cotreatment), inoculated plants were covered with a transparent lid to ensure high humidity and kept in short day condition. At 7 d after inoculation, the number of spores released by Hpa was determined as described (Schmitz et al., 2010).
Accession Numbers
The Arabidopsis Genome Initiative accession number for ein2-1 is At5g03280.
Supplemental Data
The following supplemental materials are available.
Supplemental Table S1. Gene-specific primers for measurement of mRNA transcript abundance by RT-qPCR.
Supplemental Table S2. Gene-specific primers used in FAIRE and ChIP.
Supplemental Figure S1. SFN inhibits P. pachyrhizi spore germination in vitro.
ACKNOWLEDGMENTS
We thank Gerold J. M. Beckers, Laura Buglioni, Michal R. Jaskiewicz, and Caspar J. Langenbach for discussions. G. J. M. Beckers and C. Langenbach are thanked for advice, and Stefan Kusch and Anja Reinstädler for help with plant infections. We thank Eva Reimer-Michalski for composing the VTOC chart.
LITERATURE CITED
Author notes
Address correspondence to [email protected].
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Uwe Conrath ([email protected]).
B.S. and U.C. conceived the research plan; B.S., C.B., and U.C. designed the experiments; B.S., I.J., A.B., and S.B. performed the experiments; B.S., I.J., and U.C. analyzed the data; U.C. wrote the manuscript; B.S. and I.J. composed the figures; and B.S., I.J., S.B., A.B., and C.B. revised the manuscript.
[OPEN]Articles can be viewed without a subscription.



