-
PDF
- Split View
-
Views
-
Cite
Cite
Sunok Moon, Sung-Ryul Kim, Guochao Zhao, Jakyung Yi, Youngchul Yoo, Ping Jin, Sang-Won Lee, Ki-hong Jung, Dabing Zhang, Gynheung An, Rice GLYCOSYLTRANSFERASE1 Encodes a Glycosyltransferase Essential for Pollen Wall Formation , Plant Physiology, Volume 161, Issue 2, February 2013, Pages 663–675, https://doi.org/10.1104/pp.112.210948
Close - Share Icon Share
Abstract
The pollen wall consists of an exine and an intine. The mechanism underlying its formation is not well understood. Glycosyltransferases catalyze the modification of biological molecules by attaching a single or multiple sugars and play key roles in a wide range of biological processes. We examined the role of GLYCOSYLTRANSFERASE1 (OsGT1) in pollen wall development in rice (Oryza sativa). This gene is highly expressed in mature pollen, and plants containing alleles caused by transfer DNA insertion do not produce homozygous progeny. Reciprocal crosses between OsGT1/osgt1 and the wild type indicated that the mutation leads to a male gametophyte defect. Microscopic analyses revealed that osgt1 pollen developed normally to the pollen mitosis stage but failed to produce mature grains. In osgt1 pollen, intine structure was disrupted. In addition, starch and protein levels were much lower in the mutant grains. Recombinant OsGT1 transferred glucose from UDP-glucose to the third and seventh positions of quercetin, a universal substrate of glycosyltransferases. Consistent with the role of OsGT1, an OsGT1-green fluorescent protein fusion protein was localized to the Golgi apparatus. Taken together, our results suggest that OsGT1 is a Golgi-localized glycosyltransferase essential for intine construction and pollen maturation, providing new insight into male reproductive development.
Glycosyltransferases constitute a large group of enzymes that transfer one or multiple molecules of sugar to a wide range of acceptor molecules such as lipids, proteins, hormones, secondary metabolites, and oligosaccharides (Rosenwald and Krag, 1990; Lerouge et al., 1998; Gachon et al., 2005; Tognetti et al., 2010; Luan et al., 2011). These enzymes participate in diverse biological processes including hormone homeostasis, flower and fruit pigmentation, and defense responses (Schiefelbein et al., 1988; Langlois-Meurinne et al., 2005; Ono et al., 2010; Tognetti et al., 2010; Yin et al., 2010). They are also involved in the biosynthesis of plant cell walls (Lao et al., 2003; Fangel et al., 2011). The major wall components are celluloses, hemicelluloses, pectin, and proteins (Heredia et al., 1995; Sato et al., 2001). Cellulose is synthesized at the plasma membrane from a d-Glc unit through β-1,4-glucosidic bonds by cellulose synthase, a member of the glycosyltransferase family (Roberts et al., 2002). Hemicellulose and pectin are synthesized in the Golgi complex by other glycosyltransferases and secreted at the cell surface, where they are cross linked with cellulose microfibrils (Bouton et al., 2002; Scheller and Ulvskov, 2010). The cell walls contain variable amounts of structural proteins including arabinogalactan proteins, Gly-rich proteins, and Pro-rich proteins (Yang et al., 2008). Among them, arabinogalactan proteins and Pro-rich proteins are glycosylated in the endoplasmic reticulum or Golgi apparatus.
Pollen has a characteristic wall with two layers: exine and intine (Blackmore et al., 2007). Both microspores and the tapetum contribute to pollen wall development. At the meiotic stage, callose is deposited between the plasma membrane and the primary cell wall of the meiocytes. After meiosis, primexine, mainly composed of cellulose, is formed between the plasma membrane and the callose wall (Ariizumi and Toriyama, 2011; Li and Zhang, 2010). The exine is then formed by sequential polymerization of sporopollenin, which consists of phenols and fatty acid derivatives (Morant et al., 2007; Ariizumi and Toriyama, 2011; Li and Zhang, 2010). During the tetrad stage, sporopollenin is deposited onto the primexine. Once that first layer forms, the callosic wall begins to break down. The intine that begins to develop has a composition similar to that of the primary wall of typical plant cells and includes cellulose, pectin, and various proteins (Noher de Halac et al., 2003; Li et al., 2010b). While the tapetum plays a pivotal role in exine formation, intine synthesis is largely under the control of the microspore (Nakamura et al., 2010; Yeung et al., 2011).
Several mutants in pollen wall synthesis have been reported. CALLOSE SYNTHASE5 (CALS5) encodes a glycosyltransferase that is responsible for callose synthesis in the meiocytes of Arabidopsis (Arabidopsis thaliana; Dong et al., 2005; Nishikawa et al., 2005). In cals5, pollen grains are severely deformed due to the lack of regular exine formation. NO EXINE FORMATION1 (NEF1), RUPTURED POLLEN GRAIN1 (RPG1), DEFECTIVE IN EXINE FORMATION1 (DEX1), and NO PRIMEXINE AND PLASMA MEMBRANE UNDULATION (NPU) are essential for primexine formation (Paxson-Sowders et al., 2001; Ariizumi et al., 2004; Guan et al., 2008; Chang et al., 2012). Mutations in those genes cause severe defects in male fertility in Arabidopsis because of aberrant primexine deposition that leads to a defective exine structure. NEF1 is predicted to be a membrane protein that maintains the envelope integrity in plastids. RPG1, DEX1, and NPU encode integral plasma membrane proteins. MALE STERILITY1 (MS1), a PHD finger motif-containing transcription factor, is required for regulating genes involved in primexine formation (Ito et al., 2007; Yang et al., 2007). Although viable pollen is not produced in a strong ms1 mutant allele, defective pollen with an abnormal exine structure has been observed in a mild ms1 mutant. Yang et al. (2007) performed microarray analysis with that mutant and selected 260 genes that may be associated with pollen wall and coat formation.
MS2, LESS ADHESIVE POLLEN5 (LAP5), LAP6, CYP703A2, and CYP704B1 participate in fatty acid modifications or the accumulation of sporopollenin in Arabidopsis (Ariizumi et al., 2003; Morant et al., 2007; Dobritsa et al., 2009, 2010; Chen et al., 2011). MS2 is predicted to encode a fatty acid reductase, converting the palmitoyl-acyl carrier protein to C16:0 alcohol, which is essential for pollen wall biosynthesis. A mutation in that gene is thought to cause a defect in the exine. MS2 protein is localized in plastids (Chen et al., 2011). An abnormal exine pattern has been observed in lap5 and lap6 mutants, whereas pollen from the double mutants lacks exine deposition. LAP5 and LAP6 encode chalcone synthase, a key flavonoid biosynthesis enzyme. Knockout lines of Arabidopsis CYP703A2 also lack the exine layer. The anther-specific cytochrome P450, CYP703A2, catalyzes the in-chain hydroxylation of lauric acid, an essential building block for sporopollenin synthesis. Mutation in CYP704B1 results in impaired pollen walls without a normal exine layer. CYP704B1 catalyzes ω-hydroxylation of long-chain fatty acids. The AtMYB103 protein, a MYB transcription factor, directly regulates the expression of MS2 (Higginson et al., 2003; Zhu et al., 2010). Most of the pollen grains from AtMYB103 are distorted in shape and have little or no cytoplasm. All of these reports indicate that fatty acids serve as essential compounds for sporopollenin formation. Among the 12 kaonashi (kns) mutants with defective exine formation, kns2 is caused by a loss-of-function mutation in glycosyltransferase (Suzuki et al., 2008). Through large-scale genetic screening of Arabidopsis, Dobritsa et al. (2011) identified 14 genes involved in exine formation. Two of them encode glycosyltransferases. However, the detailed roles for glycosyltransferase in male reproductive development are less understood.
Mutations in UDP-SUGAR PYROPHOSPHORYLASE (USP), REVERSIBLY GLYCOSYLATED POLYPEPTIDE1 (RGP1) and RGP2, and FASCICLIN-LIKE ARABINOGALACTAN PROTEIN3 (FLA3) cause defects in intine formation in Arabidopsis (Drakakaki et al., 2006; Schnurr et al., 2006; Li et al., 2010b). USP is involved in the synthesis of UDP-sugar, while RPGs assist in supplying it to either the endoplasmic reticulum or the Golgi. FLA3 is a highly glycosylated intine protein.
Glycosyltransferases participate in pollen wall formation, especially the exine (Dong et al., 2005, Suzuki et al., 2008, Dobritsa et al., 2011). Although some evidence has suggested that these enzymes are also involved in intine synthesis (Drakakaki et al., 2006; Schnurr et al., 2006; Li et al., 2010b), their exact role in intine wall development has not been elucidated. Here, we report that GLYCOSYLTRANSFERASE1 (OsGT1), a glycosyltransferase gene from rice (Oryza sativa), is essential for the production of viable pollen grains. In the pollen, abnormalities were detected after mitotic division, manifested as a defect in intine formation and the accumulation of cytosolic contents. We present a detailed description of its functions.
RESULTS
T-DNA Insertions in OsGT1 Cause a Defect in Male Gametophyte Development
We previously generated transfer DNA (T-DNA)-tagged lines of japonica rice (Jeon et al., 2000; Jeong et al., 2002) and determined flanking sequences for the insertion sites (An et al., 2003; Jeong et al., 2006). To identify genes essential for gametophyte development, we genotyped the T-DNA insertion lines; those with a segregation ratio close to 1:1:0 (wild type:heterozygote:homozygote) were selected. From 541 independent lines, we obtained eight with the segregation distortion phenotype. Here, we report detailed analyses of line 3C-00590, which carries a T-DNA insertion in the fifth intron of LOC_Os01g15780 (Fig. 1A). Because the gene encodes a protein in the glycosyltransferase family, we named it OsGT1. We found that another allele (osgt1-2) also harbors T-DNA in the fourth exon of OsGT1 (Fig. 1A). This line also exhibited a 1:1:0 segregation (Table I; Supplemental Fig. S1), confirming that this phenotype is due to the T-DNA insertion to OsGT1.
![Schematic diagrams of OsGT1 and aberrant pollen phenotype in osgt1 mutant. A, T-DNA insertion positions. Start and stop codons are represented as ATG and TGA, respectively; positions of insertion are shown with triangles. Shaded boxes indicate exons; lines connecting exons are introns. Primers for genotyping and expression analysis are marked with arrows. Scale bar = 100 nucleotides. B and C, Pollen grains from wild-type (B) and OsGT1/osgt1-1 (C) anthers. Bars = 20 μm. D, Defective pollen was separated from normal grains through Suc density gradient centrifugation. Bar = 500 mm. E to H, Representative pollen from each band. Bars = 10 μm. I, PCR products by two OsGT1 primers. J, PCR products by OsGT1 primer and T-DNA primer. [See online article for color version of this figure.]](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/plphys/161/2/10.1104_pp.112.210948/2/m_plphys_v161_2_663_f1.jpeg?Expires=1750182916&Signature=N5k4BGHjXXbk4GhkIfpwezbraczQQCGhi5-~m~jxq7GX892BqyOGpdfYxvfAKbBIxLORMXTU3g-txzX7sEcRVD6I2ErLNAoQd0zO2BAUfvy9CKDWQH3smYSYaDqEEJP7NoJqeTwCbNMO~mkZMVcDu1ISn6K~44AGjmiX1YK5X9dZYLF3N50wIWlMtAKKEk9h1~gEdJLFtDedO6BWQr30zpMh0L2aj2RHStFv5Aj8Q2Q3lipoeAy2FBJPsY~udDC~dXPQHWUDOGLDRZAymn0MiClUI4ieAKltbyq5q6PSPdDuz2~yFTeBWvW7wfYIYjpqvtU-4-5EeYS-GD3mGr-zCQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Schematic diagrams of OsGT1 and aberrant pollen phenotype in osgt1 mutant. A, T-DNA insertion positions. Start and stop codons are represented as ATG and TGA, respectively; positions of insertion are shown with triangles. Shaded boxes indicate exons; lines connecting exons are introns. Primers for genotyping and expression analysis are marked with arrows. Scale bar = 100 nucleotides. B and C, Pollen grains from wild-type (B) and OsGT1/osgt1-1 (C) anthers. Bars = 20 μm. D, Defective pollen was separated from normal grains through Suc density gradient centrifugation. Bar = 500 mm. E to H, Representative pollen from each band. Bars = 10 μm. I, PCR products by two OsGT1 primers. J, PCR products by OsGT1 primer and T-DNA primer. [See online article for color version of this figure.]
Segregation analysis of OsGT1/osgt1-1 and OsGT1/osgt1-2
The segregation ratio was analyzed in progeny of selfed OsGT1/osgt1 mutant lines. χ2 tests were performed to evaluate the relationship between the observed data and the predicted ratio of 1:1.
| Line . | Line No. . | Wild Type . | Heterozygote . | χ2 . |
|---|---|---|---|---|
| OsGT1/osgt1-1 | 3C-00590 | 57 | 50 | 0.458 (P < 0.05) |
| OsGT1/osgt1-2 | 3A-00186 | 64 | 57 | 0.409 (P < 0.05) |
| Line . | Line No. . | Wild Type . | Heterozygote . | χ2 . |
|---|---|---|---|---|
| OsGT1/osgt1-1 | 3C-00590 | 57 | 50 | 0.458 (P < 0.05) |
| OsGT1/osgt1-2 | 3A-00186 | 64 | 57 | 0.409 (P < 0.05) |
The segregation ratio was analyzed in progeny of selfed OsGT1/osgt1 mutant lines. χ2 tests were performed to evaluate the relationship between the observed data and the predicted ratio of 1:1.
| Line . | Line No. . | Wild Type . | Heterozygote . | χ2 . |
|---|---|---|---|---|
| OsGT1/osgt1-1 | 3C-00590 | 57 | 50 | 0.458 (P < 0.05) |
| OsGT1/osgt1-2 | 3A-00186 | 64 | 57 | 0.409 (P < 0.05) |
| Line . | Line No. . | Wild Type . | Heterozygote . | χ2 . |
|---|---|---|---|---|
| OsGT1/osgt1-1 | 3C-00590 | 57 | 50 | 0.458 (P < 0.05) |
| OsGT1/osgt1-2 | 3A-00186 | 64 | 57 | 0.409 (P < 0.05) |
These segregation phenotypes suggested that the defect results from a failure in gene transfer through either the male or female gamete. To clarify the function of OsGT1 in the gametophyte, we performed reciprocal crosses between heterozygotes (OsGT1/osgt1-1) and the wild type. When OsGT1/osgt1-1 was used as a pollen receiver (female), wild-type and heterozygous progeny were obtained at a similar frequency (11:13; Table II). However, when OsGT1/osgt1-1 served as a pollen donor (male), only wild-type progeny were obtained (32:0; Table II). Reciprocal crosses between OsGT1/osgt1-2 and wild-type plants produced similar results (Table II). These indicated that mutations in OsGT1 cause defects in the male gamete.
Genetic transmission analysis of OsGT1/osgt1-1 and OsGT1/osgt1-2 by reciprocal crossing
The segregation ratio was analyzed in F1 progeny of the represented crosses. The transmission efficiency represents the percentage of progeny that carried the mutant allele. HY, Rice ‘Hwayoung’ wild type.
| Genotype (Female × Male) . | Wild Type . | Heterozygote . | Transmission Efficiency . |
|---|---|---|---|
| OsGT1/osgt1-1 × HY | 11 | 13 | 54 |
| HY × OsGT1/osgt1-1 | 32 | 0 | 0 |
| OsGT1/osgt1-2 × HY | 33 | 27 | 45 |
| HY × OsGT1/osgt1-2 | 75 | 0 | 0 |
| Genotype (Female × Male) . | Wild Type . | Heterozygote . | Transmission Efficiency . |
|---|---|---|---|
| OsGT1/osgt1-1 × HY | 11 | 13 | 54 |
| HY × OsGT1/osgt1-1 | 32 | 0 | 0 |
| OsGT1/osgt1-2 × HY | 33 | 27 | 45 |
| HY × OsGT1/osgt1-2 | 75 | 0 | 0 |
The segregation ratio was analyzed in F1 progeny of the represented crosses. The transmission efficiency represents the percentage of progeny that carried the mutant allele. HY, Rice ‘Hwayoung’ wild type.
| Genotype (Female × Male) . | Wild Type . | Heterozygote . | Transmission Efficiency . |
|---|---|---|---|
| OsGT1/osgt1-1 × HY | 11 | 13 | 54 |
| HY × OsGT1/osgt1-1 | 32 | 0 | 0 |
| OsGT1/osgt1-2 × HY | 33 | 27 | 45 |
| HY × OsGT1/osgt1-2 | 75 | 0 | 0 |
| Genotype (Female × Male) . | Wild Type . | Heterozygote . | Transmission Efficiency . |
|---|---|---|---|
| OsGT1/osgt1-1 × HY | 11 | 13 | 54 |
| HY × OsGT1/osgt1-1 | 32 | 0 | 0 |
| OsGT1/osgt1-2 × HY | 33 | 27 | 45 |
| HY × OsGT1/osgt1-2 | 75 | 0 | 0 |
osgt1 Grains Are Defective at the Mature Pollen Stage
To examine further the morphological defects in male gametes of osgt1-1, we collected pollen grains from fully developed anthers and observed them with a bright-field microscope. Pollen from wild-type anthers was normal: only a small fraction (nine out of 1,393) was shrunken at stage 12 (Fig. 1B; Supplemental Fig. S2). However, anthers from the heterozygote produced shrunken grains at a 49.5% frequency (915 of 1,847; Fig. 1C; Supplemental Fig. S2), indicating that OsGT1 is required for male gamete development.
To determine whether those shrunken grains were caused by the T-DNA insertion, we used a Suc density gradient to separate them from normal grains (Bedinger and Edgerton, 1990; Fig. 1D). The gradient contained four discrete bands, two lower bands that contained normal pollen with a globular shape (Fig. 1, E and F) plus two upper bands with defective grains that were shrunken or underdeveloped (Fig. 1, G and H). To see whether this defect was due to the mutation, we prepared DNA from each band and performed PCR with gene-specific primers. This resulted in amplification of the OsGT1 fragment from the two lower bands but not from the upper bands (Fig. 1I). Amplification with a gene-specific primer (R2) and a T-DNA primer (L1) did not generate bands from any of the four samples, indicating that normal pollen in the lower bands did not carry T-DNA and that abnormal pollen in the upper bands lacked genomic DNA because they were dead (Fig. 1J).
To investigate the exact time when this mutant pollen began to show the phenotypic defect, we compared wild-type and osgt1 pollen at five stages of development (Fig. 2). At the young microspore stage (stage 9), cells from the wild type were released as free microspores from the tetrad (Fig. 2A). These gradually swelled and underwent vacuolation, causing the cytoplasm and nuclei to be distributed in the periphery (Fig. 2B). Afterward, the pollen started to divide mitotically (stage 11a; Fig. 2C; Zhang et al., 2011). Until this stage, all gametophytes in the OsGT1/osgt1-1 anthers appeared normal and were identical to those of the wild type (Fig. 2, F–H). However, at stage 11b, two different types of pollen were observed from the heterozygous anthers: normal grains showing even cytosolic staining and empty pollen with a lumped staining pattern (Fig. 2I). At the mature stage (stage 12), those differences were even more distinct. Whereas all of the pollen from wild-type anthers was normal (Fig. 2E), one-half of the grains from anthers of heterozygotes were shrunken and exhibited exiguous cytosolic contents (Fig. 2J).
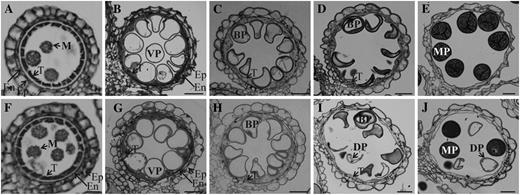
Light microscopy observation of anthers at different developmental stages. Cross sections are shown from segregating wild type (A–E) and OsGT1/osgt1-1 (F–J) at early microspore stage (A and F), vacuolated stage (B and G), mitotic division stage 11a (C and H), mitotic division stage 11b (D and I), and mature pollen stage (E and J). BP, Binuclear pollen; DP, defective pollen; En, endodermis; Ep, epidermis; M, microspore; MP, mature pollen; T, tapetum; VP, vacuolated pollen. Bars = 25 μm.
We tested pollen viability with fluorescein diacetate, which stains only live cells (Heslop-Harrison and Heslop-Harrison, 1970). Whereas globular pollen emitted a bright green signal, no signal was observed from the collapsed grains from mutant plants (Supplemental Fig. S3). These findings indicated that osgt1 pollen cannot form normal mature grains.
osgt1 Pollen Is Defective in Intine Formation
To investigate the defects in osgt1 pollen further, we stained the mature grains with auramine O, which binds to exine (Dobritsa et al., 2011). This revealed that the exine was not markedly different between mutant and wild-type pollen (Fig. 3, A–D; Supplemental Fig. S4). We also monitored for the presence of intine via calcofluor white staining. Whereas defective pollen from heterozygous plants exhibited a very weak signal, wild-type pollen emitted bright blue fluorescence, demonstrating that the mutant pollen was defective in intine (Fig. 3, E–H; Supplemental Fig. S4).
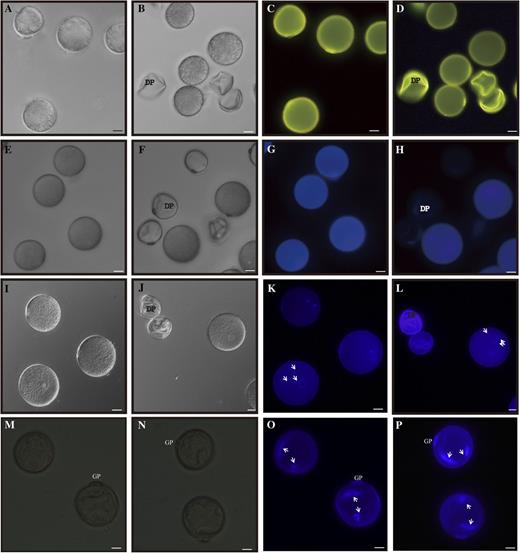
Phenotypes of pollen grains. A to D, Auramine O staining of grains from segregating wild-type (A and C) and OsGT1/osgt1-1 (B and D) plants at stage 12 observed under bright-field (A and B) and fluorescence (C and D) microscopy. E to H, Calcofluor white staining of pollen grains from segregating wild-type (E and G) and OsGT1/osgt1-1 (F and H) plants at stage 12 observed under bright-field (E and F) and fluorescence (G and H) microscopy. I to P, DAPI staining of pollen grains from segregating wild-type (I, K, M, and O) and OsGT1/osgt1-1 (J, L, N, and P) plants at stage 12 (I–L) and stage 11 (M–P) plants observed under bright-field (I, J, M, and N) and fluorescence (K, L, O, and P) microscopy. Arrows indicate nucleus. DP, Defective pollen. Bars = 20 μm.
Staining with 4′6-diamidino-2-phenylindole (DAPI) showed that the defective pollen did not carry any nuclei at stage 12, whereas wild-type grains contained one vegetative nucleus and two generative nuclei (Fig. 3, I–L). Because mitotic division precedes intine construction in rice (Lu et al., 2002; Lin et al., 2009), we performed DAPI staining at stage 11, when the binuclear grains are formed. All pollen from the heterozygous plants contained two nuclei (Fig. 3, N and P), suggesting that osgt1 pollen underwent normal mitosis I. Furthermore, amido black staining showed that osgt1 grains did not accumulate cytosolic contents such as proteins (Supplemental Fig. S5), supporting our belief that the pollen abnormality developed later in the mutant.
To understand the subcellular changes in osgt1 pollen, we performed transmission electron microscopy (TEM) analysis at stages 11a, 11b, and 12 (Zhang and Wilson, 2009). As revealed via bright-field microscopy, wild-type and osgt1 pollen did not differ significantly at stage 11a (Fig. 4, A–F). At the end of this stage, exine formation is generally considered complete (Lu et al., 2002; Lin et al., 2009). We noted a complete exine structure for both the wild type (Fig. 4, A–C) and osgt1 (Fig. 4, D–F) at stage 11a. However, differences were observed at stage 11b. Whereas wild-type grains had evenly stained cytoplasm (Fig. 4, G and H), mutant pollen showed severely degraded cytoplasm (Fig. 4, J and K). At higher magnification, intine accumulation was observed in wild-type pollen (Fig. 4I), but the intine was defective in osgt1 grains (Fig. 4L). At stage 12, these differences were more apparent. Whereas wild-type grains had accumulated starch granules as well as a thick intine (Fig. 4, M–O), approximately 50% (72 of 129) of the pollen from OSGT1/osgt1 anthers had an altered cytoplasm density and a severe defect in intine accumulation (Fig. 4, P–R). Because intine development precedes the accumulation of starch grains, proteins, and other inclusions in the cytosol (Lu et al., 2002; Lin et al., 2009), we speculated that OsGT1 plays a key role in the former process.
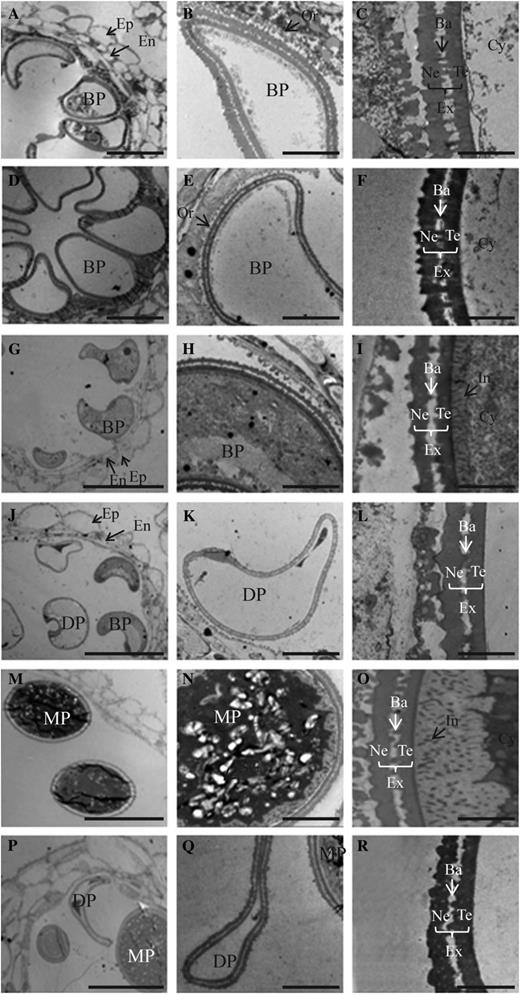
TEM analyses of developing anthers from wild-type (A–C, G–I, and M–O) and OsGT1/osgt1-1 (D–F, J–L, and P–R) plants at stage 11a (A–F), stage 11b (G–L), and stage 12 (M–R). Ba, Bacula; BP, binuclear pollen; Cy, cytosol; DP, defective microspores; En, endothecium; Ep, epidermis; Ex, exine; In, intine; MP, mature pollen; Ne, nexine; Or, orbicule; SG, starch granule; Te, tectum. Bars = 10 μm (A, D, G, J, M, and P), 2 μm (B, E, H, K, N, and Q), and 0.5 μm (C, F, I, L, O, and R).
Expression of OsGT1 Is High at Later Pollen Developmental Stages
To understand the role of OsGT1 in pollen development, we performed quantitative reverse transcription (RT)-PCR with gene-specific primers. Transcripts were weakly detected in the roots, shoots, and shoot apical meristems of seedlings and mature leaves as well as in reproductive organs such as inflorescences and developing seeds (Fig. 5A). During anther development, the OsGT1 expression signal increased at stage 9 (young microspore) and was high at stage 12 (mature pollen; Fig. 5A), suggesting that this latter developmental stage had the greatest requirement for OsGT1 activity.
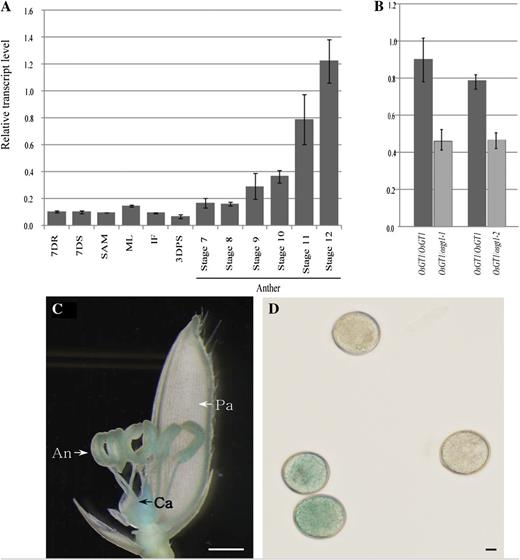
Expression pattern of OsGT1. A, Quantitative RT-PCR analyses in various organs. Transcript levels were normalized to UBIQUITIN and calculated by the comparative cycle threshold method. Error bars indicate sd. 7DR, Seven-day-old seedling roots; 7DS, 7-d-old seedling shoots; SAM, shoot apical meristem; ML, mature leaves; IF, immature panicles (2 cm long); 3DAPS, seeds at 3 d after pollination; stages 7 to 12, anthers at different developmental stages. B, Quantitative real-time RT-PCR analyses of OsGT1 in wild-type and heterozygous anthers at mitotic stage. C, GUS expression in a spikelet harboring an OsGT1 promoter::GUS construct. An, Anther; Ca, carpel; Pa, palea. Bar = 1 mm. D, GUS staining of pollen grains. Bar = 10 μm.
We used anthers from wild-type and heterozygous plants to measure OsGT1 expression, because separation of osgt1 pollen from wild-type grains at stage 11 by Suc density gradient centrifugation was unsuccessful. As expected, the transcript level in heterozygous plants was about one-half that measured from the wild type (Fig. 5B).
To explore the comprehensive expression patterns of OsGT1, we generated transgenic rice plants expressing the GUS reporter gene under the control of the OsGT1 promoter. Strong GUS activity was detected in the anthers and carpels (Fig. 5C). In mature anthers, activity was preferentially detected in the pollen. Only one-half of the mature grains were GUS positive, because we had used primary transgenic plants (Fig. 5D).
OsGT1 Encodes a Glycosyltransferase
To examine whether OsGT1 has glycosyltransferase activity, we purified a glutathione S-transferase (GST)-OsGT1 fusion protein after expression in Escherichia coli (Supplemental Fig. S6). Enzymatic activity was tested by measuring the conversion of quercetin to quercetin monoglucosides. HPLC analysis of the reaction product showed that quercetin was converted to quercetin-3-O-glucoside and quercetin-7-O-glucoside (Fig. 6F). Using AtGT-2 protein as a positive control, we found a similar HPLC pattern in which quercetin monoglucosides were produced from quercetin (Fig. 6E; Kim et al., 2006). By contrast, the negative-control GST protein did not produce monoglucosides (Fig. 6D). These results confirmed that OsGT1 is a newly discovered member of the glycosyltransferase family.
![Glycosyltransferase assay. A to C, Quercetin (A) and its glucosides, quercetin-3-O-glucoside (B) and quercetin-7-O-glucoside (C), were separated by HPLC. D and E, Chromatograms of assay products using GST protein as a negative control (D) and AtGT-2 protein as a positive control (E). F, Chromatogram of OsGT1 reaction products. Peak Q, Quercetin; peak 3, quercetin-3-O-glucoside; peak 7, quercetin-7-O-glucoside. [See online article for color version of this figure.]](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/plphys/161/2/10.1104_pp.112.210948/2/m_plphys_v161_2_663_f6.jpeg?Expires=1750182916&Signature=ifaqCZ2AwP6Ts3fQn3xrPEVXMN0LRP3G6~8852~lRkXLTYzYzWB~14aMCXTYOL3qEhI4eebppfdFTjAJqVEDVMqhSeNewwjNAwuBQL8-UUxFwRa3xXjpRuUmExnOkIB4uh7WeQtZxtaXctyDFq7X6Cm6pjrDZoG5kO9eeTsekBIaKK6qOrEuVx6p71bg-23ZWEQUIZDHk-xhE-d7adm6BuTlLvmwqwtYn9bTscGzOIilgU9u0Ms8UKO2KioHpAQHIkdVdG3acHxv5jt-GYC4QMsN1HMGAPLmrpY9hmGNlj5ZSsPbkIGheozff0kH6yWiMReqKMNc5OO1RNKIcDO74g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Glycosyltransferase assay. A to C, Quercetin (A) and its glucosides, quercetin-3-O-glucoside (B) and quercetin-7-O-glucoside (C), were separated by HPLC. D and E, Chromatograms of assay products using GST protein as a negative control (D) and AtGT-2 protein as a positive control (E). F, Chromatogram of OsGT1 reaction products. Peak Q, Quercetin; peak 3, quercetin-3-O-glucoside; peak 7, quercetin-7-O-glucoside. [See online article for color version of this figure.]
Our attempt to identify a specific target molecule for OsGT1 was unsuccessful. Identifying a specific target will be difficult because the GT4 family has both functional and sequence diversity (Kawai et al., 2011). Rice has 609 glycosyltransferases that are classified into 41 GT families (Cao et al., 2008). OsGT1 belongs to the GT4 family, members of which utilize diverse substrates such as proteins, lipids, and sugars (Dörmann et al., 1999; Silverstone et al., 2007). Moreover, OsGT1 is structurally distinct from other members in the family (http://ricephylogenomics.ucdavis.edu/cellwalls/gt/), suggesting that it has a unique role.
OsGT1 Is Localized in the Golgi Apparatus
Most glycosyltransferases act along a secretory pathway that includes the endoplasmic reticulum and the Golgi, where they transfer monosaccharide units to various receptor molecules (Priest et al., 2006; Yin et al., 2010). To investigate the subcellular localization of OsGT1, we generated a fusion construct of OsGT1 and GFP. This fusion molecule was placed under the control of the maize (Zea mays) UBIQUITIN (Ubi) promoter, and the chimeric molecule was transformed into mesophyll protoplasts. As a control, we cotransferred the MANNOSIDASE I (ManI)-RED FLUORESCENT PROTEIN (RFP) gene, which localizes at the Golgi (Kim et al., 2010). After incubating the transformed cells for 12 h, we monitored transient expression of the introduced molecules using a confocal microscope (Fig. 7, A and B). The GFP and RFP signals overlapped, as indicated by orange coloring, suggesting that OsGT1 is colocalized with ManI in the Golgi (Fig. 7C). To confirm this, we generated another OsGT1-GFP fusion construct under the control of the 35S promoter and introduced the molecule together with ManI-RFP into onion (Allium cepa) epidermal cells via bombardment. From the mesophyll cells, we observed an overlap of the green signal generated by OsGT-GFP and the red signal by ManI-RFP (Fig. 7, E–G). In contrast, we found green signals produced by GFP alone in the nuclei and cytoplasm of mesophyll protoplasts (Fig. 7I) and onion epidermal cells (Fig. 7J). These observations demonstrated that OsGT1 is localized to the Golgi apparatus.
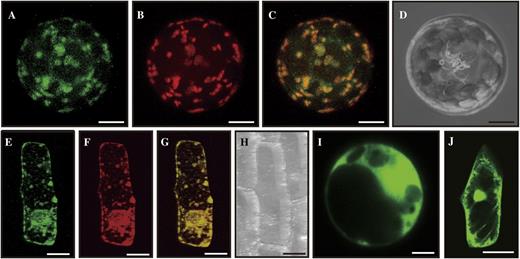
Subcellular localization. A to D, Mesophyll protoplasts prepared from young seedlings were cotransformed with fusion constructs Pubi::OsGT1-GFP and P35S::ManI-RFP. Images observed under GFP channel (A) and RFP channel (B) were merged (C); D is a bright-field image. E to H, Onion epidermal cells were cobombarded with fusion constructs. The GFP channel image (E) and RFP channel image (F) were merged (G); H is a bright-field image. I, GFP image of mesophyll cells expressing control construct Pubi::GFP. J, GFP image of onion epidermal cell expressing control construct P35S::GFP. Bars = 5 μm for mesophyll protoplasts and 50 μm for onion cells.
DISCUSSION
Identification of a Glycosyltransferase That Functions in Intine Formation
Glycosyltransferases participate in various cellular functions, including protein sorting, hormone modifications, and cell wall synthesis (Bowles et al., 2006; Tognetti et al., 2010; Tu and Banfield, 2010). In the work described here, we identified a new glycosyltransferase from rice. Mutations in the gene caused defects in intine development. Our conclusion that OsGT1 is located at the Golgi, where intine components are synthesized, supports our belief that the primary role of this protein is likely intine formation. OsGT1 exhibited a preferential expression in the pollen, with transcript levels peaking at the mature pollen stage. Temporal and spatial expression patterns showed a strong correlation with the formation of intine (Lu et al., 2002; Lin et al., 2009; Li and Zhang, 2010).
Male gametophyte development is a complex process that requires the coordinated participation of various cell and tissue types. Transcriptome profiling of developing rice anthers has revealed about 22,000 genes that are expressed during at least one developmental stage (Deveshwar et al., 2011). Functional analyses of these genes are important for furthering our understanding of the molecular mechanisms that control pollen development. Our finding of a glycosyl modification pathway for intine formation and pollen maturation by OsGT1 provides new insight into male reproductive development.
OsGT1 Functions at a Late Stage of Pollen Development
Recent genetic and biochemical investigations have uncovered several key genes required for pollen wall development in rice. For example, UDP-glucose pyrophosphorylase1 (UGP1) catalyzes the reversible production of Glc-1-P and UTP to UDP-Glc and pyrophosphate. In Ugp1-silenced plants, callose deposition is disrupted during meiosis. Consequently, the pollen mother cells begin to degenerate at the early meiosis stage, eventually resulting in complete pollen collapse (Chen et al., 2007). This indicates that Ugp1 plays a very early role (stage 6) in anther development (Fig. 8). TAPETUM DEGENERATION RETARDATION (TDR), GAMYB, PERSISTENT TAPETAL CELL1 (PTC1), CYP704B2, DEFECTIVE POLLEN WALL (DPW), POST-MEIOTIC-DEFICIENT ANTHER1 (PDA1), OsC6, and WAX-DEFICIENT ANTHER1 (WDA1) are critical for exine development (Jung et al., 2006; Zhang et al., 2008, 2010; Aya et al., 2009; Hu et al., 2010; Li et al., 2010a, 2011; Shi et al., 2011). In their mutants, microspores display a defective exine layer. TDR and GAMYB are transcription factors that control lipid biosynthesis and metabolism. PTC1 is orthologous to MS1 and regulates timely degradation of tapetal cells as well as pollen exine development. CYP704B2 catalyzes the production of ω-hydroxylated fatty acid with 16- and 18-carbon chains. DPW is a fatty acid reductase and produces 1-hexadecanol with higher specificity against the palmiltoyl-acyl carrier protein (Shi et al., 2011). OsC6 is a lipid transfer protein; expression of OsC6 is positively regulated by TDR. WDA1 is involved in the biosynthesis of cuticular waxes. Mutants defective in these genes start to show defects at stages 8 through 10 (Fig. 8). We observed that the defective phenotypes of osgt1 pollen began to appear at stage 11b, which is much later than the stages when the phenotypes became obvious in the exine-defective mutants. Mutations in RICE IMMATURE POLLEN1 (RIP1), which encodes a WD40 protein, result in defects at late stage 11b (Han et al., 2006). Because the rip1 mutant develops sperm cells normally, and the mutant pollen grains are nearly normal at the mature stage, we conclude that RIP1 functions downstream of OsGT1 (Fig. 8).
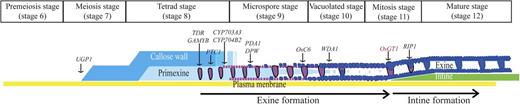
Schematic diagram of rice pollen wall development based on stages described by Li et al. (2010). Genes involved in this process are represented. Arrows indicate times when mutant phenotypes become visible.
The osgt1 Mutant Is Distinct from Previously Identified Intine Mutants
Because intine synthesis is largely under the control of the microspore (Nakamura et al., 2010; Yeung et al., 2011), most genes related to intine development have been isolated from segregation-distorted mutants. In Arabidopsis, several genes involved in intine formation have been reported, including USP, RGP1, RGP2, and FLA3 (Drakakaki et al., 2006; Schnurr et al., 2006; Li et al., 2010b). The USP protein is an enzyme engaged in the synthesis of nucleotide sugars. The product of this enzyme acts as a substrate for a glycosyltransferase to synthesize a glycosylated variant (Schnurr et al., 2006). Intine is not detectable in the pollen grains of T-DNA insertion mutants of USP (Schnurr et al., 2006). The RGP proteins participate in polysaccharide biosynthesis as nucleotide sugar carriers that bind to a nucleotide sugar in the cytosol and deliver it to an acceptor molecule at the Golgi surface (Saxena and Brown, 1999). Single-gene disruptions of RGP show no obvious morphological defects, whereas rpg1 rgp2 double mutant pollen walls have an irregular organization and poorly defined intine (Drakakaki et al., 2006). Arabinogalactan is a highly glycosylated cell wall protein (Gao and Showalter, 1999; MacMillan et al., 2010). Because it is a component of intine, the defective intine layer in pollen from FLA3-RNAi transgenic plants is thought to be caused by a deficiency in arabinogalactan (Li et al., 2010b). As observed in osgt1, pollen from these mutants had an abnormal and uneven intine layer that collapsed at the bicellular stage. Although exine formation is not always affected in all reported intine mutants, including osgt1, we found that our OsGT1 functioned differently from previously identified genes. Thus, to our knowledge, we are the first to identify this as a glycosyltransferase that functions in intine formation.
OsGT1 May Have a Role at the Vegetative Stage
Although OsGT1 was highly expressed in mature pollen grains, it was also expressed at low levels in various organs. To study its functional roles in vegetative tissues, we attempted to generate osgt1 plants through anther culture. Microspores from early uninucleus to first mitosis stage anthers are capable of developing into plants with a haploid genome (Chen, 1977). Because we first observed the defect after mitotic division of osgt1 pollen, we expected to obtain osgt1 haploid plants. However, all 60 plants produced from the anther culture were wild type. This indicated that the osgt1 plants were lethal. We also generated transgenic plants harboring an RNA interference construct. However, the level of endogenous OsGT1 transcripts was not reduced in all of them, suggesting that suppression of the gene was harmful. This observation supported our theory that OsGT1 may also play essential roles in vegetative tissues.
MATERIALS AND METHODS
Plant Growth and Genotyping
Lines 3C-00590 and 3A-00186 of rice (Oryza sativa), which carry a T-DNA insertion in Os01g15780 (OsGT1), were selected from our T-DNA insertion mutant population (An et al., 2003; Ryu et al., 2004; Jeong et al., 2006). Seedlings were grown in the greenhouse for 3 weeks and then transferred to a paddy field. DNA was prepared from leaves via the hexadecylotrimethylammonium method. Genotypes were determined by PCR using gene-specific primers and T-DNA primers (Supplemental Table S1).
Histochemical Analysis
Anthers were fixed in a formalin-acetic acid-alcohol solution for 8 h before dehydration. Samples were embedded in Technovit 8100 resin (Kulzer) and sectioned to a 5- to 10-mm thickness with a rotary microtome (model 2165; Leica Microsystems). After staining, the sections were observed with a BX61 microscope (Olympus).
TEM Analysis
Spikelets were fixed in 0.2 n sodium phosphate buffer (pH 7.0) containing 3% paraformaldehyde and 0.25% glutaraldehyde and were postfixed in 2% OsO4 in phosphate-buffered saline (pH 7.2). Following ethanol dehydration, samples were embedded in acrylic resin (London Resin). Ultrathin sections (50–70 nm) were double stained with 2% uranyl acetate and 2.6% lead citrate and examined at 80 kV with a JEM-1230 transmission electron microscope (JEOL).
RNA Isolation and RT-PCR Analysis
Total RNAs were extracted with Tri Reagent (Molecular Research Center) and then treated with DNase (Promega). For the synthesis of complementary DNA (cDNA), 1 mg of total RNA was reacted with Moloney murine leukemia virus reverse transcriptase (Promega), 2.5 mm deoxyribonucleotide triphosphate, and 10 ng of oligo(dT). PCR was performed as described previously using two gene-specific primers, RT F1 and RT R1 (Moon et al., 2008). To check the expression level of OsGT1 in mutant lines, we extracted RNAs from anthers at the mitotic stage. For real-time PCR analyses, primer sets RT F1 and RT R1 (for OsGT1/osgt1-1) or RT F2 and RT R2 (OsGT1/osgt1-2) were used (Supplemental Table S1).
Cytochemical Analysis
For staining with DAPI and calcofluor white, pollen grains were fixed in formalin-acetic acid-alcohol solution for 1 h at room temperature and then washed with Tris-HCl buffer (pH 7.5). To stain the nuclei, the grains were held for 1 h at 60°C in Tris-HCl buffer (pH 7.5) with 200 ng mL−1 DAPI. The intine was stained with 0.1% calcofluor white for 15 min. The stained pollen was monitored under UV light with an Olympus BX61 microscope. After staining with 0.001% auramine O in 17% Suc, the pollen was observed on the fluorescein isothiocyanate channel of the Olympus BX61 microscope.
Recombinant Protein Purification
A full-length OsGT1 cDNA was amplified with the primers OsGT Full F and OsGT Full R (Supplemental Table S1). To generate the GST-OsGT1 fusion construct, we placed the restriction enzyme sites EcoRI and NotI at the ends of the cDNA by PCR, using primers OsGT GST F and OsGT GST R (Supplemental Table S1). After the PCR product was inserted into the pGEM-T Easy vector (Promega), its sequence was determined. The insert was then cloned into the pGEX-5X vector for generating the GST-OsGT1 fusion and was transferred into Escherichia coli BL21. Strains harboring GST, GST-OsGT1, or GST-AtGT2 were cultured in Luria-Bertani medium at 28°C. At an optical density at 600 nm of 0.5, isopropylthiogalactopyranoside was added to a final concentration of 0.5 mm and the cultures were maintained for 6 h. The cells were pelleted and resuspended in STE buffer (50 mm Tris-HCl, 100 mm NaCl, and 1 mm EDTA). After sonication, the lysates were centrifuged. To purify the GST-containing proteins, we mixed each supernatant with a glutathione-agarose bead slurry and incubated it for 90 min. After washing twice with a buffer containing first 50 mm Tris-HCl/100 mm NaCl and then 50 mm Tris-HCl/300 mm NaCl, the GST fusion proteins were eluted with a solution containing 50 mm Tris-HCl (pH 8.0) and 10 mm reduced glutathione (Supplemental Fig. S6).
Assay of Glycosyltransferase Activity
The recombinant proteins (1 µg) were incubated at 30°C for 1 h in a solution containing 0.5 mm quercetin, 7 mm UDP-Glc, and 100 mm Tris-HCl (pH 7.0). Reactions were stopped by adding methanol. After filtration, quercetin, quercetin glucosides, and the reaction product were analyzed on an HPLC system (1200 series; Agilent Technologies) equipped with Eclipse XDB C18-RP columns. The filtrate was separated by a linear gradient of 20% to 60% acetonitrile. The flow rate was 1 mL min−1, and UV-visible detection was performed at 370 nm.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number NM_001049188.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Genetic transmission analysis.
Supplemental Figure S2. Pollen morphology of osgt1-2.
Supplemental Figure S3. FDA staining.
Supplemental Figure S4. Phenotype of pollen grain from osgt1-2.
Supplemental Figure S5. Protein staining of pollen grain from osgt1.
Supplemental Figure S6. Gel image of expressed proteins.
Supplemental Table S1. Primer sequences used.
ACKNOWLEDGMENTS
We thank Joong-Hoon Ahn and Inhwan Hwang for kindly providing the GST-AtGT2 and ManI-RFP constructs. We are grateful to Juyoung Yoon for lending us the Chemosensor to use for monitoring glycosyltransferase activity and to Priscilla Licht for critical reading of the manuscript.
Glossary
- DAPI
4′6-diamidino-2-phenylindole
- TEM
transmission electron microscopy
- RT
reverse transcription
- GST
glutathione S-transferase
- cDNA
complementary DNA
- T-DNA
transfer DNA
LITERATURE CITED
Author notes
This work was supported by the Next-Generation BioGreen 21 Program, Rural Development Administration, Republic of Korea (Plant Molecular Breeding Center grant no. PJ008128 to G.A.), the Basic Research Promotion Fund, Republic of Korea (grant no. KRF–2007–341–C00028 to G.A.), and Kyung Hee University (grant no. 20120227 to G.A.), and also by the National Key Basic Research Developments Program, Ministry of Science and Technology, China (grant no. 2013CB126902 to D.Z.), and the National Natural Science Foundation of China (grant nos. 31110103915, 31171518, and 30830014 to D.Z.).
Corresponding author; e-mail [email protected].
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Gynheung An ([email protected]).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.



