-
PDF
- Split View
-
Views
-
Cite
Cite
Sandra Goritschnig, Tabea Weihmann, Yuelin Zhang, Pierre Fobert, Peter McCourt, Xin Li, A Novel Role for Protein Farnesylation in Plant Innate Immunity , Plant Physiology, Volume 148, Issue 1, September 2008, Pages 348–357, https://doi.org/10.1104/pp.108.117663
Close - Share Icon Share
Abstract
Plants utilize tightly regulated mechanisms to defend themselves against pathogens. Initial recognition results in activation of specific Resistance (R) proteins that trigger downstream immune responses, in which the signaling networks remain largely unknown. A point mutation in SUPPRESSOR OF NPR1 CONSTITUTIVE1 (SNC1), a RESISTANCE TO PERONOSPORA PARASITICA4 R gene homolog, renders plants constitutively resistant to virulent pathogens. Genetic suppressors of snc1 may carry mutations in genes encoding novel signaling components downstream of activated R proteins. One such suppressor was identified as a novel loss-of-function allele of ENHANCED RESPONSE TO ABSCISIC ACID1 (ERA1), which encodes the β-subunit of protein farnesyltransferase. Protein farnesylation involves attachment of C15-prenyl residues to the carboxyl termini of specific target proteins. Mutant era1 plants display enhanced susceptibility to virulent bacterial and oomycete pathogens, implying a role for farnesylation in basal defense. In addition to its role in snc1-mediated resistance, era1 affects several other R-protein-mediated resistance responses against bacteria and oomycetes. ERA1 acts partly independent of abscisic acid and additively with the resistance regulator NON-EXPRESSOR OF PR GENES1 in the signaling network. Defects in geranylgeranyl transferase I, a protein modification similar to farnesylation, do not affect resistance responses, indicating that farnesylation is most likely specifically required in plant defense signaling. Taken together, we present a novel role for farnesyltransferase in plant-pathogen interactions, suggesting the importance of protein farnesylation, which contributes to the specificity and efficacy of signal transduction events.
Plant immunity to microbial pathogens requires an intricate signaling network, components of which are subjects of current investigation. An integral part of pathogen-specific defense is mediated by Resistance (R) proteins, which recognize pathogenic effector molecules or results of their pathogenic activity (Jones and Dangl, 2006). According to their structure, most known R proteins belong to the nucleotide binding site (NBS)-Leu-rich repeat (LRR) class, with carboxyl-terminal LRRs and a central NBS domain. NBS-LRR proteins are similar to mammalian receptor modules, the Toll-like receptors and NOD proteins, which recognize general pathogen-associated molecular patterns as a first step in the innate immune response (Ausubel, 2005). Activation of R proteins initiates discrete and overlapping signaling events, usually culminating in programmed death of cells at the site of infection (hypersensitive response [HR]) and containment of the invading pathogen (Nimchuk et al., 2003).
Proper localization of defense signaling components and interaction with other proteins are imperative for successful defense responses, and these often depend on posttranslational modifications. For example, membrane association has been demonstrated for the negative regulatory and avirulence target protein RPM1 INTERACTING PROTEIN4 (RIN4), which is tethered to the plasma membrane most likely via palmytoylation at its C terminus (Kim et al., 2005). RIN4 release from the membrane after proteolytic cleavage by the Pseudomonas syringae type III effector AvrRpt2 results in its degradation by the proteasome and activation of the associated R protein RESISTANCE TO PSEUDOMONAS SYRINGAE2 (RPS2; Kim et al., 2005).
Although many R genes have been cloned, the signaling events downstream of R-protein activation remain elusive. To search for additional components required for R-protein signaling, we took advantage of the plant autoimmune model suppressor of npr1 constitutive1 (snc1), a unique gain-of-function allele of a TIR-NBS-LRR R gene homologous to RESISTANCE TO PERONOSPORA PARASITICA4 (RPP4) and RPP5. Apart from constitutive resistance against virulent bacterial and oomycete pathogens, the snc1 mutant also displays increased levels of endogenous salicylic acid (SA) and constitutive expression of PR genes (Li et al., 2001; Zhang et al., 2003). snc1-mediated resistance completely depends on EDS1/PAD4 and involves several branches of a downstream signaling network, dependent or independent of SA and NON-EXPRESSOR OF PR GENES1 (NPR1), or both (Zhang et al., 2003). Components identified in the modifier of snc1 (MOS) screen unraveled the existence of a conserved signaling complex potentially involved in transcriptional control (Palma et al., 2007) and revealed the essential requirement of nucleocytoplasmic trafficking, RNA processing, and ubiquitination in basal and R-protein-mediated defense (Palma et al., 2005; Zhang et al., 2005; Zhang and Li, 2005; Goritschnig et al., 2007).
Here, we present mos8, an independent suppressor of snc1-mediated defense responses. mos8 is a novel allele of ENHANCED RESPONSE TO ABA1 (ERA1), which encodes the protein farnesyltransferase β-subunit and has been shown to be important in development and hormonal responses (Cutler et al., 1996; Yalovsky et al., 2000; Ziegelhoffer et al., 2000). mos8 affects basal resistance against virulent pathogens as well as some R-protein-mediated resistance responses. This novel function of farnesylation in response to biotic stresses adds another layer of complexity to the signaling network of plant innate immunity.
RESULTS
mos8 Suppresses Constitutive Resistance in snc1 npr1-1
![mos8 suppresses snc1-mediated resistance phenotypes. A, Morphology of 5-week-old soil-grown plants of indicated phenotypes. B, Semiquantitative reverse transcription-PCR of pathogenesis-related genes PR1 and PR2. Fragments were amplified from standardized cDNA in 30 cycles. Actin is included as normalization control. C and D, mos8 suppresses constitutive resistance to virulent bacteria and oomycetes. C, Five-week-old soil-grown plants were infected with P.s.m. ES4326 (OD600=0.0001) and quantified at 0 (white bars) and 3 (dark bars) dpi. D, Two-week-old seedlings were inoculated with H.p. Noco2 and conidia were quantified 7 dpi. E, mos8 reduces endogenous SA in snc1 npr1. Total SA was extracted and analyzed with HPLC. Bars represent the average of six (C) and four (D and E) biological replicates, error bars represent sd. All experiments were repeated at least twice with similar results. fw, Fresh weight. [See online article for color version of this figure.]](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/plphys/148/1/10.1104_pp.108.117663/2/m_plphys_v148_1_348_f1.jpeg?Expires=1750197053&Signature=L2yFIZCC9SAQC920JmA2hC-RQMfeBtEtKpwe8cP3~I4Li~hSvOTWeFCrfXqf8YFZwes4QjmZLfrQuzsk-pxCwt3swYcu9VLnz4lvfKQUlAMvYIaaN4Koak911VOH930b-7ar9wN47yV~9aljmBImbgYwwhjyiqkVwFk5xxOqFsmEBHDxW9xMiIoXScXMySErT~FeH1m5R9WK~dUuct58YY-3Qo1ANi6Eggz41Je0kuBzE0-wEMEylbZKSY3bHJo7L--LroEjiwtcoiDJLnYbZldxvTuPFsUri559jTP3PPsOyghAavfkKdm4O8o2xoQFyegIAu68vUpQCs40rO4BgA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
mos8 suppresses snc1-mediated resistance phenotypes. A, Morphology of 5-week-old soil-grown plants of indicated phenotypes. B, Semiquantitative reverse transcription-PCR of pathogenesis-related genes PR1 and PR2. Fragments were amplified from standardized cDNA in 30 cycles. Actin is included as normalization control. C and D, mos8 suppresses constitutive resistance to virulent bacteria and oomycetes. C, Five-week-old soil-grown plants were infected with P.s.m. ES4326 (OD600=0.0001) and quantified at 0 (white bars) and 3 (dark bars) dpi. D, Two-week-old seedlings were inoculated with H.p. Noco2 and conidia were quantified 7 dpi. E, mos8 reduces endogenous SA in snc1 npr1. Total SA was extracted and analyzed with HPLC. Bars represent the average of six (C) and four (D and E) biological replicates, error bars represent sd. All experiments were repeated at least twice with similar results. fw, Fresh weight. [See online article for color version of this figure.]
mos8 Contains a Mutation in a Farnesyltransferase Subunit
![mos8 is allelic to ERA1. A, Positional cloning of mos8. BAC clones and recombinants are indicated. A mutation (*) was identified in At5g40280/ERA1. B, Two additional alleles of era1 display the same late-flowering phenotype as the mos8 (era1-7) single mutant. Pictures were taken at 7 weeks after planting. [See online article for color version of this figure.]](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/plphys/148/1/10.1104_pp.108.117663/2/m_plphys_v148_1_348_f2.jpeg?Expires=1750197053&Signature=kqaRXXEyHgutet-fQC5cT-h5wY8B1OxDFYDtFjrOAP~~dM6Ws1TZ0E6fwSrPxl-fnitsNiZYEw6EgognKSChTrgPScZ7KYRk7~AijzRnSMw~Oe~Ifat4xwaE2hwaHkmF8fCuV8v0XUWV00AIGmOIzyiKEoe5guhHT0UQ7jx7CTlw28PGLu39IxSxQOc~DolPUbu1y9m1VSylVEVY2s4MLUDYvu8dvsy4VUAt~h9o99v5xJrKvXLv4Sa09iK0s1g89IyUz7ZjYDn~eAYNTUK6xG9D3mg4JlKFPfGDPFyF0s0hpz4sYLyQyvxXXO~jt8yV-DYivBQ2hfyzsl9MYicp5g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
mos8 is allelic to ERA1. A, Positional cloning of mos8. BAC clones and recombinants are indicated. A mutation (*) was identified in At5g40280/ERA1. B, Two additional alleles of era1 display the same late-flowering phenotype as the mos8 (era1-7) single mutant. Pictures were taken at 7 weeks after planting. [See online article for color version of this figure.]
![The mutation in mos8/era1-7 affects the ERA1 start codon. A, The G to C mutation in era1-7 (indicated by *) causes loss of the endogenous ATG start codon. Translation of the wild type and the mutant gene are indicated below and above the alignment, respectively. B, Complementation of era1-7 snc1 npr1-1 with ERA1 cDNA initiating at the start codon mutated in era1-7 restores snc1 morphology. Representative 5-week-old plants are shown among 20 transgenic T1 plants. [See online article for color version of this figure.]](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/plphys/148/1/10.1104_pp.108.117663/2/m_plphys_v148_1_348_f3.jpeg?Expires=1750197053&Signature=a6f354WPGRARCH68z7F8l~ABgSQR3FtDHBpF0gWcYBTB1rMFdskE61ceYGQM48slZ1N6q4iNJ0VcdzyLblRDmg9zIPJP6L5uWGHTW19r0aeVawpx5e2Mxk5XjYu-F3jLKZaZsbW1H~Uuo-RnWrN3hW6HKXs9ib8s5ZOOu~uTFU7bXVw12Jt4Yytz79CuxUu6w8UvL1BMpIfXgLVmcSkVHToLrYy1PxpR838-sljx0-H6RMwYFI8HU1zAAf7V9-TVlAU03stZgQqVJbscZB8tGrhA7Be3VKLq9HZUB4W35xfSPZUgTk6kw-jLL430qGsTPhHkPC-fW4lX0YUDjpWA2w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
The mutation in mos8/era1-7 affects the ERA1 start codon. A, The G to C mutation in era1-7 (indicated by *) causes loss of the endogenous ATG start codon. Translation of the wild type and the mutant gene are indicated below and above the alignment, respectively. B, Complementation of era1-7 snc1 npr1-1 with ERA1 cDNA initiating at the start codon mutated in era1-7 restores snc1 morphology. Representative 5-week-old plants are shown among 20 transgenic T1 plants. [See online article for color version of this figure.]
We crossed mos8 snc1 npr1-1 with several available alleles of era1 including Salk_110517, a T-DNA insertion allele in an exon of At5g40280 from the Arabidopsis Biological Resource Center (ABRC; Alonso et al., 2003), to test for complementation. The era1-2 allele contains a 7.5-kb deletion encompassing At5g40280 (Cutler et al., 1996). Point mutations in the wiggum alleles era1-4 and era1-5 cause premature stop codons, and in era1-6 an altered splice site results in truncated protein products (Ziegelhoffer et al., 2000). All era1 mutant alleles were unable to complement the mos8 mutation, confirming that MOS8 is allelic to ERA1 (Supplemental Table S1). Therefore, mos8 was subsequently referred to as era1-7, and Salk_110517 was renamed era1-8. Figure 2B shows the similarity in the morphological phenotypes of three era1 alleles, which are late flowering and have darker rosette leaves than the Col wild type. Moreover, era1-2 snc1 and era1-8 snc1 double mutants were obtained, and both alleles were able to suppress snc1-associated morphology and resistance (Supplemental Table S2; data not shown). All these data confirm that MOS8 is indeed ERA1.
The era1-7 Mutation Affects the Start Codon of ERA1
The phenotype of era1-7 is comparable to the deletion allele era1-2, suggesting that era1-7 is a complete loss-of-function allele. Based on the annotation of the published ERA1 open reading frame (Ziegelhoffer et al., 2000), the G to C point mutation in era1-7 changes a codon for Met to Ile 40 amino acids downstream of translation initiation (Fig. 3A). In accordance with the null-allele phenotype in era1-7, and also because ERA1 homologs in other organisms have start codons close to where the era1-7 mutation lies (Supplemental Fig. S1), we hypothesized that the mutation in era1-7 affects the actual start codon of ERA1, resulting in an aberrant protein product as the translation initiates at the next available ATG (Fig. 3A). To test this hypothesis, we transformed mos8 snc1 npr1-1 plants with the ERA1 cDNA beginning at the newly predicted start codon. Because we were using a hypothetical full-length cDNA, the cauliflower mosaic virus 35S promoter was used to simplify the experiment. Constitutive expression of 35S∷cERA1 fully complemented mos8 and restored typical snc1 morphology in all 20 T1 transgenic plants (Fig. 3B), indicating that the ERA1 protein may indeed be smaller than previously suggested. Complementation was also observed when era1-2 and era1-7 single mutant plants were transformed with 35S∷cERA1 (data not shown).
era1 Exhibits Enhanced Disease Susceptibility
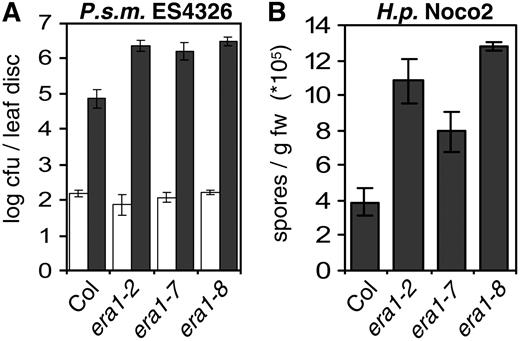
era1 confers enhanced susceptibility to virulent pathogens. A, Enhanced susceptibility to virulent P.s.m. ES4326 in era1. Bacterial growth was determined 0 (white bars) and 3 (dark bars) dpi. B, Several era1 alleles display enhanced susceptibility to virulent H.p. Noco2. Conidia were harvested and quantified 7 dpi. Bars represent the average of six (A) and four (B) biological replicates, error bars indicate sd. Experiments were repeated twice with similar results.
era1 Affects Resistance to Avirulent Pathogens
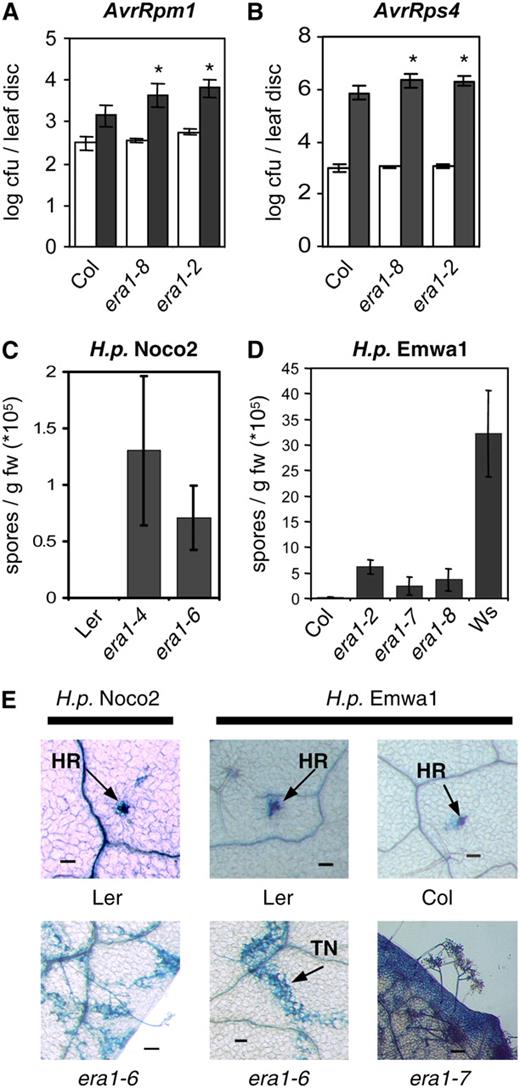
era1 affects R-protein-mediated resistance. A and B, Mutant era1 plants exhibit enhanced susceptibility to avirulent bacteria. Five-week-old plants were infected with P.s.t. DC3000 expressing AvrRpm1 (A) or AvrRps4 (B) at OD600=0.001 and bacterial growth quantified at 0 (white bars) and 3 dpi (dark bars). Susceptibility toward P.s.t. AvrRpm1 in the era1-8 mutant is significantly enhanced compared to Col wild type (P = 0.0149, t test). Other treatments show very significant differences compared to wild type (P < 0.0075, t test). C and D, Growth of H.p. isolates Noco2 and Emwa1 on era1 alleles in different ecotypes. H.p. Noco2 is avirulent on Ler ecotype (C), and H.p. Emwa is avirulent on Col (D). Wassilewskija (Ws) is included as a susceptible control. Conidiospores were harvested and quantified 7 dpi. Bars represent the average of six (A and B) or four (C and D) biological replicates, error bars indicate sd. Experiments were repeated at least twice with similar results. E, Visualization of oomycete growth by lactophenol trypan blue staining at 7 dpi. TN, Trailing necrosis. Bars in E represent 100 μm.
We also took advantage of the availability of era1 alleles in different genetic backgrounds to investigate their responses to avirulent oomycetes. H.p. Noco2 is a virulent pathogen on the Col ecotype but avirulent on Ler, which contains the RPP5 R gene. Infection assays with H.p. Noco2 on era1 alleles in different ecotypes can thus provide insight into the involvement of ERA1 in both compatible and incompatible interactions. era1 mutants in the Col genetic background showed significantly more growth of the oomycete pathogen (Fig. 4B). Inoculation of Ler plants induced a rapid HR, which is apparent in trypan blue staining (Fig. 5E), where the staining reveals hyphal structures and dead cells, whereas live cells are not stained. The era1-4 and era1-6 alleles in the Ler background suppress RPP5-mediated resistance toward H.p. Noco2, visualized by hyphal growth in infected tissues and the formation of conidiophores (Fig. 5, C and E).
When infected with H.p. Emwa1, which is recognized by RPP4 in Col, era1 mutant plants showed susceptibility (Fig. 5, D and E). Strong resistance toward H.p. Emwa1 in Ler conferred by RPP5 and RPP8 was also compromised in era1-6, as demonstrated by extensive trailing necrosis and occasional sporulation on the mutant plants (Fig. 5E). Taken together, these findings indicate that the farnesyltransferase encoded by ERA1 and therefore farnesylation is important in a subset of R-protein-mediated resistance responses to bacterial and oomycete pathogens.
ERA1 Acts Additively with NPR1 in Resistance Signaling
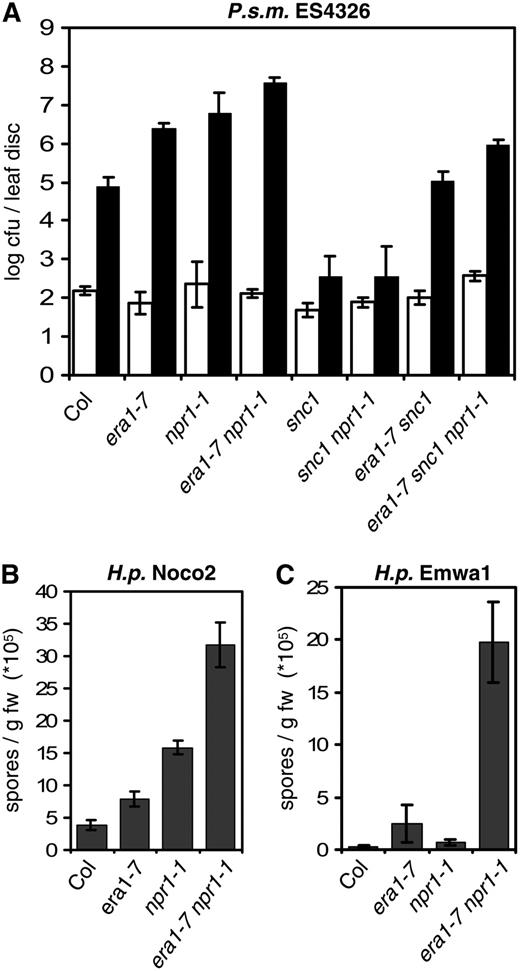
era1 and npr1 act additively in defense responses. A, Enhanced susceptibility to virulent P. s. m. ES4326 in era1 is potentiated by npr1-1. Bacterial growth was determined 0 (white bars) and 3 (dark bars) dpi. B and C, era1 and npr1 have additive effects in susceptibility to virulent H.p. Noco2 (B) and avirulent H.p. Emwa1 (C). Conidia were harvested and quantified 7 dpi. Bars represent the average of six (A) and four (B and C) biological replicates, error bars indicate sd. Experiments were repeated twice with similar results.
ABA Is Only Partially Responsible for Enhanced Susceptibility of era1 Mutant Plants
Another possible role of ERA1 in defense signaling could come from its involvement in ABA signaling. era1 mutants are known to be hypersensitive to ABA, showing enhanced responses to ABA during germination as well as in the guard cell response (Pei et al., 1998), thus implicating ERA1 in abiotic stress responses. Recent reports suggest additional roles of ABA in response to biotic stresses (Mauch-Mani and Mauch, 2005). Since era1 is hypersensitive to ABA and exhibits enhanced disease susceptibility, we were interested in investigating whether the increased susceptibility observed in era1 is caused by its defect in ABA signaling.
We first asked whether endogenous ABA levels are altered in snc1 similar to the SA levels, thus potentially contributing to its enhanced resistance phenotype. When ABA levels were measured in Col and snc1, we consistently observed slightly lower levels of ABA in snc1, and substantially lower levels of the major product of ABA catabolism, phaseic acid (Supplemental Fig. S3), suggesting that ABA could contribute negatively to the snc1-mediated resistance signaling. This is consistent with previous reports describing a negative correlation between ABA and susceptibility to biotrophic and necrotrophic pathogens (Audenaert et al., 2002; Mohr and Cahill, 2003).
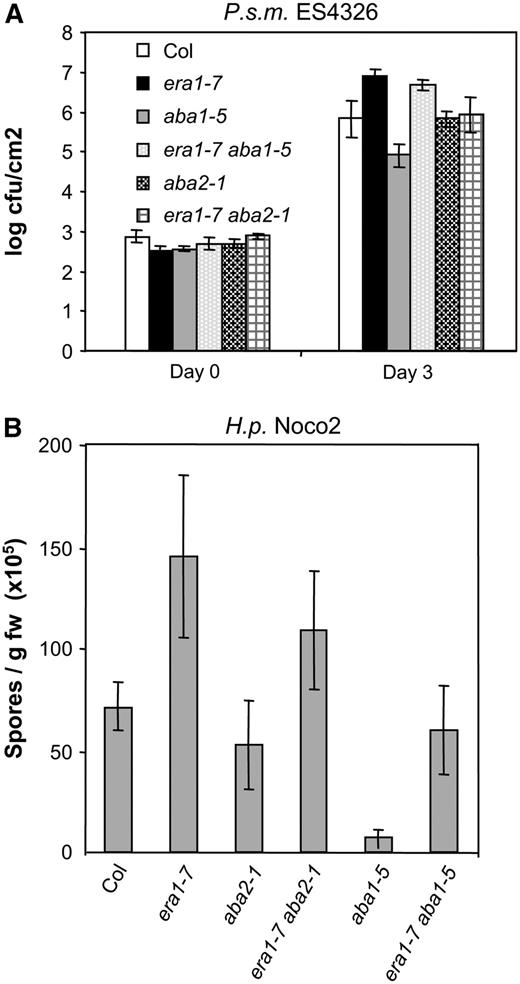
Enhanced susceptibility of era1-7 is only partially due to ABA. Plants were infected with virulent P.s.m. ES4326 (A) or virulent H.p. Noco2 (B) as described in Figure 4. Experiments were repeated at least twice with similar results. Bars represent the average of six biological replicates, error bars indicate sd.
Geranylgeranyltransferase 1 Is Not Required in Defense Responses
Protein farnesyltransferase is a modular enzyme in which the α-subunit forms a scaffold for the barrel-shaped β-subunit, which performs the addition reaction (Park et al., 1997). In an alternative prenylation pathway, protein geranylgeranyltransferase 1 utilizes the same α- but a distinct β-subunit to transfer geranylgeranyl units to different target proteins. The enhanced susceptibility of the era1 mutant toward virulent and avirulent pathogens prompted us to investigate a potential role of geranylgeranylation in defense responses. We obtained a T-DNA insertion line with a defect in At2g39550, the gene encoding the geranygeranyltransferase β-subunit (GGB), from the ABRC (Salk_040904; Alonso et al., 2003). The Salk_040904 line, representing the ggb-2 allele described previously (Johnson et al., 2005), carries the T-DNA insertion in the first exon. Although ggb-2 represents a null allele with no detectable GGB transcript, the ggb-2 mutant did not exhibit significant morphological differences compared with wild type (Johnson et al., 2005). However, the mutant has been described to affect several aspects of hormone signaling (Johnson et al., 2005).
![Involvement of geranylgeranylation in defense responses. Col wild type, ggb-2, and era1 plants were infected with virulent P.s.m. ES4326 (A), virulent H.p. Noco2 (B), and avirulent H.p. Emwa1 (C). Bacterial growth in A was determined 0 (white bars) and 3 (dark bars) dpi. Oomycete growth was determined by quantifying conidia (B) and trypan-blue staining of infected plant tissue 7 dpi. Experiments were repeated at least twice with similar results. Bars represent the average of six (A) and four (B and C) biological replicates, error bars indicate sd. Asterisks indicate statistically significant differences (t test). Bars in C represent 100 μm. [See online article for color version of this figure.]](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/plphys/148/1/10.1104_pp.108.117663/2/m_plphys_v148_1_348_f8.jpeg?Expires=1750197053&Signature=Ex3Hbe3GaJvsurjO8Yq2jzvXn-6k7bEtha~1TmkLl~Ex9vEavDDlJxqkYKqxk1B3-J9orySi~f9NIkNDfH2nHME6bKOgywl1YVMpySZufXezLEbTSnMmG3QIUCO3-ZYMSCfylisIZVqesMXyyC3QAuI2aLelOHz6uoyOsNeY5XsZizHkXWQylKxDEvMNIQ3hFoW0jIB7v24mxuqFFgrIMyzqUS2pUQZMw~HLpHopbej6n-jQv45FsCNIm54HEuL2aTKlgKW62Y-fqZ1zLwo~kh90GeEgy2AsbTbIfjNlr~gLG2mge8pSRJNJM-hFchs5AAF1RnUdDDP4iauqTJCTeA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Involvement of geranylgeranylation in defense responses. Col wild type, ggb-2, and era1 plants were infected with virulent P.s.m. ES4326 (A), virulent H.p. Noco2 (B), and avirulent H.p. Emwa1 (C). Bacterial growth in A was determined 0 (white bars) and 3 (dark bars) dpi. Oomycete growth was determined by quantifying conidia (B) and trypan-blue staining of infected plant tissue 7 dpi. Experiments were repeated at least twice with similar results. Bars represent the average of six (A) and four (B and C) biological replicates, error bars indicate sd. Asterisks indicate statistically significant differences (t test). Bars in C represent 100 μm. [See online article for color version of this figure.]
To test whether the ggb-2 mutation had an effect on snc1-mediated resistance, we generated the snc1 ggb-2 double mutant. We did not observe any suppression of snc1-associated morphological and disease phenotypes (data not shown). Taken together, our findings suggest that specifically farnesyltransferase, and not geranylgeranyltransferase 1, plays an important role in both basal and R protein defense signaling.
DISCUSSION
Proteins are frequently altered posttranslationally to modify their solubility, compartmentalization, or interaction with other proteins. The most common lipid modification, prenylation, involves the covalent attachment of farnesyl- or geranylgeranyl-diphosphate moieties to the C termini of a small group of target proteins, which contain a conserved CaaX motif (Galichet and Gruissem, 2003). Fatty acid acylation and prenylation of proteins has recently been shown to play important roles in trafficking and correct membrane targeting of signaling molecules (Resh, 2006). Unlike in yeast (Saccharomyces cerevisiae) and Drosophila, mutations in prenyltransferases are not lethal in Arabidopsis (Arabidopsis thaliana), suggesting that plants may have evolved alternative mechanisms to bypass the essential requirement for protein prenylation (Trueblood et al., 1993; Therrien et al., 1995).
Protein prenyltransferases are modular enzymes and mutations in several subunits have been described in Arabidopsis (Galichet and Gruissem, 2003; Running et al., 2004; Johnson et al., 2005). Pleiotropic phenotypes of mutants in the farnesyltransferase β-subunit ERA1 include hypersensitivity to ABA, increased size of the floral meristem, and delayed flowering (Cutler et al., 1996). Guard cell responses to ABA are affected in era1, resulting in enhanced drought resistance of the mutant plants (Pei et al., 1998). This phenomenon was used in the production of transgenic Brassica napus, in which silencing of endogenous ERA1 by an antisense construct results in increased drought resistance (Wang et al., 2005).
Here we show that protein farnesylation is not only important in development and abiotic stress responses, but also in biotic interactions. The enhanced susceptibility of era1 toward virulent bacterial and oomycete pathogens indicates the involvement of farnesylation in basal defense responses. In addition, signaling mediated by several R proteins relies on functional ERA1, further demonstrating the existence of divergent signaling events downstream of different R-protein classes.
Interestingly, a mutant in the alternative prenyltransferase β-subunit, ggb-2, displayed no defense-related phenotype and was unable to suppress constitutive resistance mediated by snc1 (Fig. 8). Geranylgeranyltransferase β (GGB) was previously shown to partially compensate a lack of farnesyltransferase activity and overexpression of GGB complemented the era1 phenotype (Running et al., 2004; Johnson et al., 2005). The double mutant ggb-2 era1-4 was shown to exhibit a phenotype similar to pluripetala (plp), a mutant in the α-subunit of prenyltransferase that exhibits an aggravated era1-like phenotype (Running et al., 2004; Johnson et al., 2005). However, in our cross between ggb-2 and era1-7, both in the Col ecotype background, we did not see the drastic increase of floral meristem size and the plants resembled era1-7 (data not shown). It has been shown previously for the wig mutants that backcrosses with Col increased the number of floral organs (Running et al., 1998). Hence, the plp-like phenotype observed in the ggb-2 era1-4 double mutant may be a result of the hybrid Col and Ler ecotype cross (since era1-4 is wig1 isolated in the Ler ecotype).
The differential requirement for farnesylation and geranylgeranylation in defense responses suggests that one or more targets of farnesyltransferase are involved in defense signaling. Farnesyl- and geranylgeranyltransferase target proteins differ with respect to their C-terminal CaaX consensus sequence, where C represents the prenylation target Cys and “a” indicates an aliphatic amino acid. While the “X” in farnesylated proteins could be Met, Ala, Gln, Ser, or Cys, the geranylgeranylated proteins usually have a Leu in this position (Rodriguez-Concepcion et al., 1999). Since completion of the Arabidopsis genome, more than 100 proteins with a protein prenyltransferase CaaX consensus sequence at the C terminus have been identified, roughly twice the number identified in the human genome (Galichet and Gruissem, 2003; Roskoski, 2003; W. Gruissem, personal communication). Among putative prenylated proteins with a CaaX consensus, cell cycle regulators, metal-binding proteins, and signaling proteins represent the majority (Galichet and Gruissem, 2003).
It is tempting to speculate about a requirement for specific farnesylated proteins in defense responses, and some prenylated proteins have been associated with plant-pathogen interactions. AVRRPT2 INDUCED GENE1 (AIG1) was identified as rapidly transcriptionally up-regulated in response to infection with P.s.m. ES4326 AvrRpt2 (Reuber and Ausubel, 1996), and the amino acid sequence of the predicted protein terminates in the geranylgeranylation consensus sequence CSIL. The Arabidopsis genome contains a small family of AIG1-like proteins clustered on chromosome 1, and their function is predicted to involve GTP binding. Other G proteins that contain the geranylgeranylation consensus sequence are members of the plant RAC/Rho of plants family, which have been implicated in the susceptible interaction between barley (Hordeum vulgare) and the powdery mildew Blumeria graminis f. sp. hordei (Bgh; Schultheiss et al., 2003). The functional analogs of RAC/Rho of plants proteins in animals are involved in cytoskeleton reorganization (Takai et al., 2001). Interestingly, one of the RAC proteins in barley may be involved in cell polarization and actin rearrangements in the interaction with Bgh (Opalski et al., 2005). Both of these examples concern geranylgeranylation targets involved in defense responses, leaving an open question as to why mutations in farnesyltransferase, but not geranylgeranyltransferase so severely affected resistance responses in our study. It remains to be determined whether the putative Rab geranylgeranyltransferase 2, which recognizes a distinct C-terminal motif, may be involved in defense signaling.
We investigated a number of potential farnesylated signaling components by a reverse genetics approach using available T-DNA insertion lines from the Arabidopsis stock center, but did not find any mutants showing an altered response to either virulent P.s.m. ES4326 or avirulent H.p. Emwa1 (Supplemental Table S3). However, one has to be cautious with the interpretation of these results, given that not all potential targets were tested due to unavailability of T-DNA insertions in a number of interesting genes (such as AIG1) or potential redundancies among protein family members. Also, among the mutants we tested, some may not be null knockout mutants, such as the ones with T-DNA insertions at 3′-untranslated region. Future in-depth investigation of all potential ERA1 targets may reveal which proteins are the ones regulated by ERA1 that signal in defense, and how their signaling activities are regulated by farnesylation.
It is widely accepted that ABA plays important and complex roles in both biotic and abiotic stress responses and has been the subject of recent reviews (Mauch-Mani and Mauch, 2005; Robert-Seilaniantz et al., 2007). ABA was shown to have complex roles in defense signaling, affecting defense responses on multiple levels. Prior to pathogen penetration, ABA induces guard cell closure, which contributes positively to prevent entry of the pathogen (Melotto et al., 2006). Infection with virulent P.s.t. strains induced ABA production in Arabidopsis, suggesting a positive correlation between increased ABA levels in Arabidopsis and susceptibility toward P.s.t. (Schmelz et al., 2003; de Torres-Zabala et al., 2007). de Torres-Zabala et al. (2007) suggest that ABA hormone homeostasis might constitute a target of at least one bacterial effector. At the same time, reduced levels of ABA in aba1-1 mutant plants correlated with increased resistance toward virulent and avirulent isolates of the biotrophic H.p., but the ABA insensitive mutant abi1-1 displayed wild-type susceptibility (Mohr and Cahill, 2003). ABA was also shown to suppress SA and lignin accumulation in an incompatible interaction (Mohr and Cahill, 2007). Yet on the other hand, ABA was found to act as positive regulator of defense responses in the interaction between Arabidopsis and the necrotrophic oomycete Phytium irregulare, since ABA deficient mutants were more susceptible to this pathogen (Adie et al., 2007; de Torres-Zabala et al., 2007). Our data that the enhanced susceptibility in era1 can be partially suppressed by ABA biosynthesis mutations support the role of ABA as a negative regulator of defense, while at the same time revealing the existence of other unknown regulators independent of ABA that need to be farnesylated by ERA1 in defense signaling.
Taken together, data presented in this study stress the importance of posttranslational lipid modifications during disease resistance signaling, and future work to search for farnesylation targets in innate immunity will reveal further details of the mystery.
MATERIALS AND METHODS
Plant Growth and Mutant Characterization
Plants were grown at 22°C under long-day conditions (16 h light/8 h dark). Seeds were surface sterilized using 5% hypochlorite solution and stratified for 7 d at 4°C before sowing. The screen for suppressors of the snc1 npr1-1 double mutant was described previously (Zhang and Li, 2005). pBGL2-GUS expression was determined by histochemical staining of 20-d-old seedlings grown on Murashige and Skoog following standard protocols. Measurements of endogenous SA levels were performed as described (Li et al., 1999). RNA extraction and semiquantitative reverse transcription-PCR were performed as described (Zhang et al., 2003).
Pathogen Assays
For Pseudomonas infections, 5-week-old soil-grown plants were infiltrated with a bacterial suspension in 10 mm MgCl2 at an optical density of OD600 = 0.0001 for virulent P.s.m. ES4326 or OD600 = 0.001 for avirulent P.s.m. and P.s.t. using a blunt syringe. Bacterial growth was measured 3 d postinfection (dpi) by harvesting leaf discs and determining colony forming units (cfu). H.p. isolates Noco2 and Emwa1 were inoculated on 2-week-old seedlings at a concentration of 5 × 105 conidia/mL. At 7 dpi, conidia were quantified by harvesting replicate samples of 15 plants, vortexing, and counting using a hemocytometer. Plant necrosis and hyphal growth were visualized using lactophenol trypan blue staining following a protocol by Koch and Slusarenko (1990). Responses to flagellin were tested as described (Gomez-Gomez et al., 1999).
Positional Cloning of mos8
The markers used to map mos8 were derived from the Ler/Col polymorphism database provided by Monsanto on the The Arabidopsis Information Resource homepage (Jander et al., 2002; http://www.arabidopsis.org/Cereon/). Marker MUL8 was amplified using primers MUL8-F (5′-aaggttaatagacctgtcgg-3′) and MUL8-R (5′-aagcacaagccatttgacca-3′), yielding PCR fragments of 285 and 174 bp in Col and Ler, respectively. Marker K1O13 was amplified using primers K1O13-F (5′-tgatcacaacttcaccattg-3′) and K1O13-R (5′-aatgtaaacaccaaagctgc-3′), yielding PCR fragments of 352 and 230 bp in Col and Ler, respectively. Marker MSN9-2 was amplified using primers MSN9-2F (5′-gtggagaagtgggtttatgg-3′) and MSN9-2R (5′-cgggaagatttgagagcagc-3′), yielding PCR fragments of 265 bp in Col and Ler, which were digested with HincII only in Ler. The marker MPO12-3 was amplified using primers MPO12-3F (5′-agacgtttatagcttcggag-3′) and MPO12-3R (5′-actggttggagatggaatcg-3′), yielding 397 bp PCR fragments in both ecotypes, which upon digestion with Taq1 generated four fragments in Col and three in Ler.
Complementation of mos8 with 35S∷cERA1
ERA1 cDNA as predicted was amplified using Platinum Pfx DNA polymerase (Invitrogen) with primers ERA1short_5′_EcoRI: 5′-agaattcatggaagagctttcaagcc-3′ and ERA1short_3′_NotI: 5′-ttttgcggccgctcatgctgctttaaagaagaac-3′, containing novel restriction sites (underlined). The PCR fragment was subcloned into pBluescript (Alting-Mees et al., 1992) using EcoRI and NotI, and sequenced. The fragment was subcloned into pBIN1.4 (Mindrinos et al., 1994) under the control of the cauliflower mosaic virus 35S promoter and transformed into mos8 snc1 npr1-1 using the Agrobacterium floral dip method (Clough and Bent, 1998). Transformants were identified based on kanamycin resistance. Twenty independent primary transformants that restored snc1 morphology were obtained.
Generating the era1-7 Single and Double Mutants
To generate the era1-7 single and the era1-7 snc1 and era1-7 npr1-1 double mutants, mos8 snc1 npr1-1 was crossed with Col carrying the pBGL2∷GUS reporter transgene. Mutant combinations were identified among the F2 progeny from selfed F1 plants based on morphological phenotypes and genotyping with PCR. Homozygosity of snc1 in the era1-7 snc1 double mutant was confirmed by backcrossing with snc1, all F1 progeny displayed snc1 morphology.
To generate the era1-7 aba2-1 and era1-7 aba1-5 double mutants, era1-7 plants were crossed with aba2-1 and aba1-5 homozygous plants obtained from Dr. J.-G. Chen and ABRC. F1 progeny displayed wild-type phenotype and were selfed. Among the F2 progeny, plants displaying a combination of typical mos8 and aba mutant morphology (late flowering, slender leaves) were identified as potential double mutants. Backcrossing with the respective parents was carried out to confirm the identity of the doubles.
Identification of Homozygous T-DNA Mutant Plants
The era1-8 allele was identified in Salk_110517 using the gene-specific primers Salk_110517-A (5′-agaacacaaggggctgcctg-3′) and Salk_110517-B (5′-tgcttccctcttccttgatg-3′). Homozygous ggb-2 mutant plants were identified in Salk_040904 using gene-specific primers Salk_040904-A (5′-tagtaggaaaggcctggaag-3′) and Salk_040904-B (5′-gatccaagtgtccttgaacg-3′). Presence of either T-DNA was verified using a combination of Lba1 and Salk_110517-A or Salk_040904-A.
ABA Measurement
The method for ABA and metabolite extraction and quantitative analysis by liquid chromatography-tandem mass spectrometry was as described (Feurtado et al., 2004). Three replicates of freeze-dried material (50 mg) from rosette leaves were analyzed for each genotype.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number ABR08910.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Alignment of farnesyltransferases from different plant species.
Supplemental Figure S2. Response of era1 alleles to flg22.
Supplemental Figure S3. Levels of ABA and its metabolites in leaves of Col and snc1.
Supplemental Figure S4. Contribution of ABA in era1-mediated enhanced pathogen susceptibility.
Supplemental Table S1. Allelism test between several era1 alleles and mos8 snc1 npr1-1.
Supplemental Table S2. Multiple era1 alleles suppress snc1.
Supplemental Table S3. Putative ERA1 targets tested in this study.
ACKNOWLEDGMENTS
We thank Yu Ti Cheng and Mark Tessaro for excellent technical assistance; Elliot Meyerowitz for seeds of era1 alleles; Jin-Gui Chen for the aba1-5 and aba2-1 seeds; Sue Abrams, Irina Zaharia, and the Plant Biotechnology Institute hormone profiling team for ABA analysis; Wilhelm Gruissem for sharing the list of predicted farnesylated proteins in Arabidopsis; and Bjoern Hamberger and Marcel Wiermer for critical reading of the manuscript.
LITERATURE CITED
Adie BA, Perez-Perez J, Perez-Perez MM, Godoy M, Sanchez-Serrano JJ, Schmelz EA, Solano R (
Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (
Alting-Mees MA, Sorge JA, Short JM (
Audenaert K, De Meyer GB, Hofte MM (
Ausubel FM (
Bonetta D, Bayliss P, Sun S, Sage T, McCourt P (
Clough SJ, Bent AF (
Cutler S, Ghassemian M, Bonetta D, Cooney S, McCourt P (
de Torres-Zabala M, Truman W, Bennett MH, Lafforgue G, Mansfield JW, Rodriguez Egea P, Bogre L, Grant M (
Feurtado JA, Ambrose SJ, Cutler AJ, Ross AR, Abrams SR, Kermode AR (
Galichet A, Gruissem W (
Gomez-Gomez L, Boller T (
Gomez-Gomez L, Felix G, Boller T (
Goritschnig S, Zhang Y, Li X (
Jander G, Norris SR, Rounsley SD, Bush DF, Levin IM, Last RL (
Johnson CD, Chary SN, Chernoff EA, Zeng Q, Running MP, Crowell DN (
Kim HS, Desveaux D, Singer AU, Patel P, Sondek J, Dangl JL (
Koch E, Slusarenko A (
Li X, Clarke JD, Zhang Y, Dong X (
Li X, Zhang Y, Clarke JD, Li Y, Dong X (
Mauch-Mani B, Mauch F (
Melotto M, Underwood W, Koczan J, Nomura K, He SY (
Mindrinos M, Katagiri F, Yu GL, Ausubel FM (
Mohr PG, Cahill DM (
Mohr PG, Cahill DM (
Nimchuk Z, Eulgem T, Holt BF III, Dangl JL (
Opalski KS, Schultheiss H, Kogel KH, Huckelhoven R (
Palma K, Zhang Y, Li X (
Palma K, Zhao Q, Cheng YT, Bi D, Monaghan J, Cheng W, Zhang Y, Li X (
Park HW, Boduluri SR, Moomaw JF, Casey PJ, Beese LS (
Pei ZM, Ghassemian M, Kwak CM, McCourt P, Schroeder JI (
Resh MD (
Reuber TL, Ausubel FM (
Robert-Seilaniantz A, Navarro L, Bari R, Jones JD (
Rodriguez-Concepcion M, Yalovsky S, Gruissem W (
Roskoski R Jr (
Running MP, Fletcher JC, Meyerowitz EM (
Running MP, Lavy M, Sternberg H, Galichet A, Gruissem W, Hake S, Ori N, Yalovsky S (
Schmelz EA, Engelberth J, Alborn HT, O'Donnell P, Sammons M, Toshima H, Tumlinson JH III (
Schultheiss H, Dechert C, Kogel KH, Huckelhoven R (
Takai Y, Sasaki T, Matozaki T (
Therrien M, Chang HC, Solomon NM, Karim FD, Wassarman DA, Rubin GM (
Trueblood CE, Ohya Y, Rine J (
Wang Y, Ying J, Kuzma M, Chalifoux M, Sample A, McArthur C, Uchacz T, Sarvas C, Wan J, Dennis DT, et al (
Yalovsky S, Kulukian A, Rodriguez-Concepcion M, Young CA, Gruissem W (
Zhang Y, Cheng YT, Bi D, Palma K, Li X (
Zhang Y, Goritschnig S, Dong X, Li X (
Zhang Y, Li X (
Ziegelhoffer EC, Medrano LJ, Meyerowitz EM (
Author notes
This work was supported by a doctoral fellowship from the Austrian Academy of Sciences and a University of British Columbia Graduate Fellowship to S.G., and by financial support from the Natural Sciences and Engineering Research Council of Canada, the Canadian Foundation for Innovation, the British Columbia Knowledge Development Fund, the University of British Columbia Blusson Fund, and the University of British Columbia Michael Smith Laboratories to X.L.
Present address: Department of Plant and Microbial Biology, 111 Koshland Hall, University of California, Berkeley, CA 94720–3102.
Corresponding author; e-mail [email protected].
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Xin Li ([email protected]).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.



