-
PDF
- Split View
-
Views
-
Cite
Cite
Xiaoying Zhao, Xuhong Yu, Eloise Foo, Gregory M. Symons, Javier Lopez, Krishnaprasad T. Bendehakkalu, Jing Xiang, James L. Weller, Xuanming Liu, James B. Reid, Chentao Lin, A Study of Gibberellin Homeostasis and Cryptochrome-Mediated Blue Light Inhibition of Hypocotyl Elongation, Plant Physiology, Volume 145, Issue 1, September 2007, Pages 106–118, https://doi.org/10.1104/pp.107.099838
Close - Share Icon Share
Abstract
Cryptochromes mediate blue light-dependent photomorphogenic responses, such as inhibition of hypocotyl elongation. To investigate the underlying mechanism, we analyzed a genetic suppressor, scc7-D (suppressors of cry1cry2), which suppressed the long-hypocotyl phenotype of the cry1cry2 (cryptochrome1/cryptochrome2) mutant in a light-dependent but wavelength-independent manner. scc7-D is a gain-of-expression allele of the GA2ox8 gene encoding a gibberellin (GA)-inactivating enzyme, GA 2-oxidase. Although scc7-D is hypersensitive to light, transgenic seedlings expressing GA2ox at a level higher than scc7-D showed a constitutive photomorphogenic phenotype, confirming a general role of GA2ox and GA in the suppression of hypocotyl elongation. Prompted by this result, we investigated blue light regulation of mRNA expression of the GA metabolic and catabolic genes. We demonstrated that cryptochromes are required for the blue light regulation of GA2ox1, GA20ox1, and GA3ox1 expression in transient induction, continuous illumination, and photoperiodic conditions. The kinetics of cryptochrome induction of GA2ox1 expression and cryptochrome suppression of GA20ox1 or GA3ox1 expression correlate with the cryptochrome-dependent transient reduction of GA4 in etiolated wild-type seedlings exposed to blue light. Therefore we propose that in deetiolating seedlings, cryptochromes mediate blue light regulation of GA catabolic/metabolic genes, which affect GA levels and hypocotyl elongation. Surprisingly, no significant change in the GA4 content was detected in the whole shoot samples of the wild-type or cry1cry2 seedlings grown in the dark or continuous blue light, suggesting that cryptochromes may also regulate GA responsiveness and/or trigger cell- or tissue-specific changes of the level of bioactive GAs.
Cryptochromes are blue light receptors that regulate various photomorphogenic responses in plants, including deetiolation and photoperiodic control of floral initiation (Cashmore, 2003; Lin and Shalitin, 2003). Arabidopsis (Arabidopsis thaliana) CRYPTOCHROME1 (CRY1) and CRY2 mediate blue light inhibition of hypocotyl elongation, although CRY1 plays a more prominent role in this response (Koornneef et al., 1980; Ahmad and Cashmore, 1993; Guo et al., 1998; Koornneef et al., 1998; Lin et al., 1998). Cryptochromes and phytochromes regulate many overlapping physiological and developmental responses, and they may do so by regulating similar genes (Fankhauser and Chory, 1997; Quail, 2002; Lin and Shalitin, 2003; Sullivan and Deng, 2003). Many hormones, including auxin, GA, brassinosteroids, ethylene, and cytokinin, are known to be involved in hypocotyl growth (Vandenbussche et al., 2005). In etiolated pea (Pisum sativum) seedlings exposed to light, the levels of indole-3-acetic acid, GA, and abscisic acid were found to change to various extents. A reduction of GA1 (the major bioactive GA in pea) was the first detected and most dramatically changed (Symons and Reid, 2003). Results of genome-wide expression profiling also indicate that many photoreceptor-regulated genes encode enzymes involved in the biosynthesis and catabolism of phytohormones, including GA (Ma et al., 2001; Folta et al., 2003; Ohgishi et al., 2004; Zimmermann et al., 2004; Jiao et al., 2005; X. Yu, D. Shalitin, and C. Lin, unpublished data). Those enzymes are often encoded by multiple-member gene families, but few of those gene families have been characterized in detail sufficient to assess the blue light effects on hormonal homeostasis and photomorphogenesis.
GAs are tetracyclic diterpenoid hormones that promote growth, such as hypocotyl elongation (Olszewski et al., 2002; Sun and Gubler, 2004). Only a few of the presently known 126 different GAs have been shown to be physiologically active, including GA1, GA3, GA4, and GA7 (Hedden and Phillips, 2000). GA4 is believed to be the major bioactive GA in Arabidopsis. Key enzymes involved in the metabolism and catabolism of bioactive GAs include ent-kaurene synthase, P450 monooxygenases, and dioxygenases. Two dioxygenases, GA 20-oxidase (GA20ox) and GA 3β-hydroxygenase (GA3ox), catalyze the last few steps in the synthesis of bioactive GAs (Hedden and Phillips, 2000; Reid et al., 2004). Another dioxygenase, GA 2-oxidase (GA2ox), catalyzes catabolism and inactivation of bioactive GAs or their precursors (Lester et al., 1999; Thomas et al., 1999; Hedden and Phillips, 2000; Schomburg et al., 2003). GA signal transduction involves DELLA proteins; it is believed that GA promotes degradation of DELLA proteins to release their suppression on the GA signaling pathway (Peng and Harberd, 1997; Silverstone et al., 1997; Peng et al., 1999; Olszewski et al., 2002; Sun and Gubler, 2004).
GA is well known for its involvement in phytochrome-regulated hypocotyl elongation (Kamiya and Garcia-Martinez, 1999; Hedden and Phillips, 2000; Garcia-Martinez and Gil, 2001; Halliday and Fankhauser, 2003; Vandenbussche et al., 2005). Phytochromes affect GA levels by regulating expression of the GA2ox and GA3ox genes (Reid et al., 2002). Phytochromes may also regulate GA responsiveness (Reed et al., 1996; Cao et al., 2005; Foo et al., 2006). It has been recently shown that phyA and phyB mediate light stabilization of the DELLA proteins, which may, at least partially, result from the phytochrome-dependent regulation of GA homeostasis (Achard et al., 2007). In comparison to the phytochrome-regulated responses, the relationship between cryptochromes and GA in the blue light responses is less clear in Arabidopsis. It has been found in pea that cry1 and phyA redundantly regulate GA2ox and GA3ox expression and GA signaling (Symons and Reid, 2003; Foo et al., 2006). Arabidopsis cry1 has been reported to regulate hypocotyl elongation via its effect on GA and auxin homeostasis or signaling (Folta et al., 2003).
Prompted by a study of the genetic suppressor of cry1cry2 that corresponds to the Arabidopsis GA2ox8 gene, we investigated the relationship between GA homeostasis and cryptochrome-mediated deetiolation. We showed that increased expression of a GA2ox gene caused hypersensitive or constitutive photomorphogenesis, depending on the relative levels of GA2ox overexpression. We further demonstrated that cryptochromes are required for the blue light induction of GA2ox1 expression and blue light suppression of GA20ox1 and GA3ox1 expression. Although all those observations are consistent with a simple hypothesis that cryptochromes may inhibit accumulation of bioactive GAs to suppress hypocotyl growth, our analyses of the GA4 content in whole shoot samples indicate the involvement of a more complex mode of regulation in the cryptochrome-mediated photomorphogenic responses.
RESULTS
Overexpression of GA2ox8 Suppresses the Long Hypocotyl Phenotype of the cry1cry2 Mutant
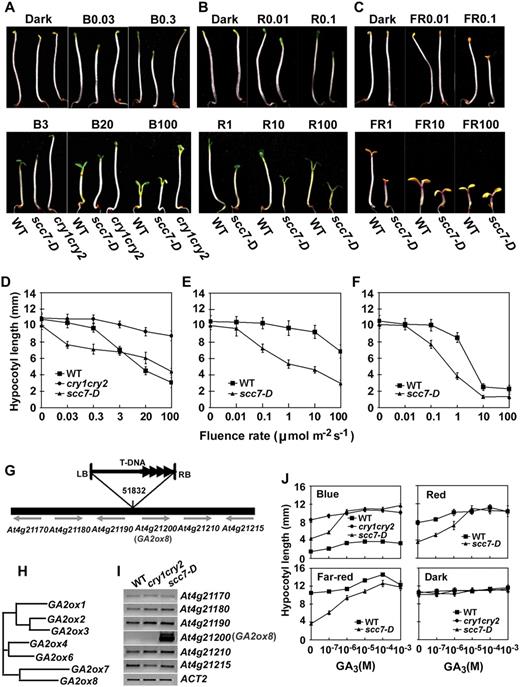
Characterization of the scc7-D mutant (A–F). Phenotypic analyses of scc7-D. Six-day-old seedlings of scc7-D, cry1cry2 mutant (cry1cry2), and wild type (WT) grown in dark or continuous blue (A and D), red (B and E), and FR (C and F) light with indicated fluence rates. Hypocotyl lengths (D–F) and representative seedlings grown under indicated light conditions are shown (A–C). The symbols of B0.03, B0.3, B3, B20, and B100 indicate that seedlings were grown under continuous blue light with fluence rates of 0.03, 0.3, 3, 20, and 100 μmol m−2 s−1, respectively. Similarly, R0.01 or FR0.01 symbolize red light or FR light, respectively, with fluence rates (μmol m−2 s−1) indicated by the numbers. G to J, Molecular characterization of scc7-D. G, Diagram depicting the scc7-D locus and the T-DNA insertion. H, A putative phylogenetic relationship of members of the GA2ox gene family as predicted using ClustalW (http://www.ebi.ac.uk/clustalw/). I, mRNA expression of genes flanking the T-DNA insert of scc7-D. J, Hypocotyl lengths of scc7-D seedlings grown in the dark or in continuous lights of different wavelength (100 μmol m−2 s−1 blue or red light, and 1 μmol m−2 s−1 FR light) in the presence of different concentrations of GA3.
The scc7-D locus contains a T-DNA inserted in an intergenic region, at 4,293 bp upstream from the start codon of GA2ox8 (Fig. 1, G–H), and GA2ox8 seems the only one overexpressed among the six T-DNA flanking genes tested (Fig. 1I). The light-hypersensitive phenotype of scc7-D can be rescued by exogenous GA3 (Fig. 1J). In addition, scc7-D also showed a dwarf and late-flowering phenotype (data not shown). Similar dominant alleles of the GA2ox8 gene have been reported previously, and it was shown that GA2ox8 encodes a GA2ox that catalyzes 2β-hydroxylation of C20-GAs (Schomburg et al., 2003). Importantly, plants overexpressing GA2ox8 contain reduced amounts of bioactive GAs and precursors, while loss-of-function ga2ox7 and ga2ox8 mutants exhibited a long hypocotyl phenotype (Schomburg et al., 2003). We concluded that the exaggerated light response in the scc7-D allele was also caused by the increased GA2ox8 expression and reduced accumulation of bioactive GAs.
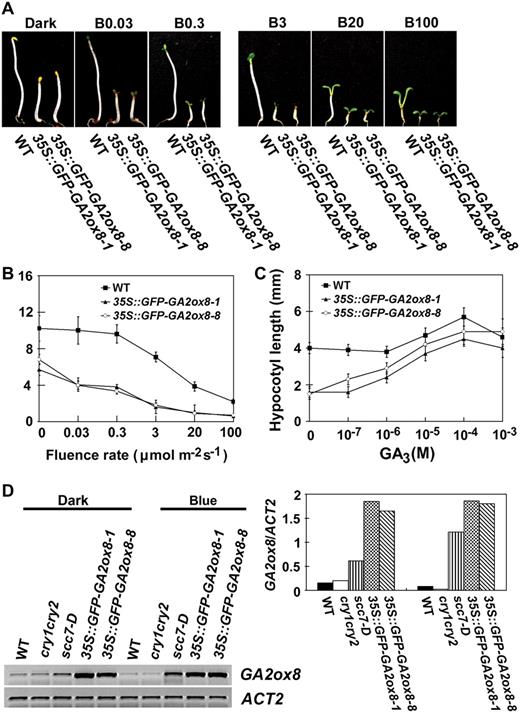
The 35S∷GFP-GA2ox8 transgenic plants are constitutively photomorphogenic (A–C). GA-rescuable constitutive photomorphogenic phenotype of two independent transgenic lines overexpressing the GFP-GA2ox8 protein. D, A markedly higher level of GFP-GA2ox mRNA in the 35S∷GFP-GA2ox8 transgenic plants than the level of GA2ox8 mRNA in scc7-D. Seedlings were grown in the dark or continuous blue light for 6 d.
Association between GA Homeostasis and Blue Light Inhibition of Hypocotyl Elongation
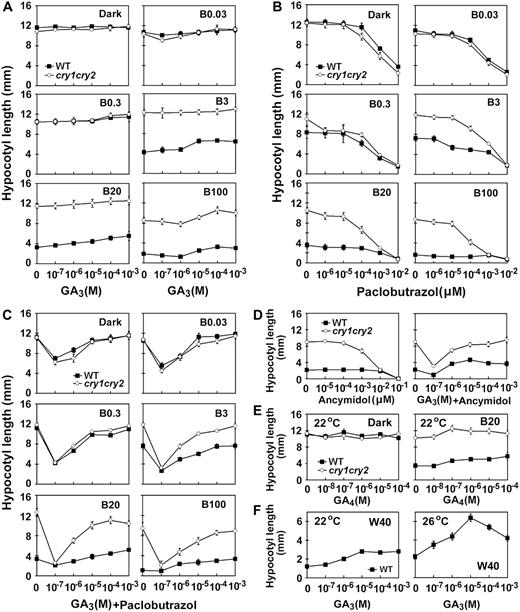
Effects of GAs or GA biosynthesis inhibitors on blue light inhibition of hypocotyl elongation. Seedlings were grown on Murashige and Skoog medium containing different concentrations of GA3 (A), GA4 (E), GA inhibitors paclobutrazol (B) or ancymidol (D), and GA inhibitors plus GA3 at different concentrations (C and D). The GA inhibitor used in C or D is paclobutrazol (0.02 μ m) or ancymidol (0.1 μ m), respectively. Hypocotyl lengths of 6-d-old seedlings grown under blue light of different fluence rates are shown. The symbols of B0.03, B0.3, B3, B20, and B100 indicate that seedlings were grown under continuous blue light with fluence rates of 0.03, 0.3, 3, 20, and 100 μmol m−2 s−1, respectively. Hypocotyl responses of seedlings grown on Murashige and Skoog medium containing different concentrations of GA3 under white light (W40, 40 μmol m−2 s−1) at two different temperatures are shown in F.
We next examined how the cry1cry2 mutant responded to GA biosynthesis inhibitors. The GA biosynthesis inhibitors, paclobutrazol (Fig. 3B) or ancymidol (Fig. 3D), can both rescue the long-hypocotyl phenotype of the cry1cry2 mutant, which is consistent with the observation of the cry1 mutant (Folta et al., 2003). These results suggest that GA is likely involved in the development of the long hypocotyl of cry1cry2, and that the level of bioactive GA is important for cryptochrome function. It is interesting that, when GA synthesis was inhibited, the cry1cry2 mutant seedlings grown in blue light with relatively high fluence rates exhibited greater hypocotyl elongation in response to GA3 than the wild-type seedlings (Fig. 3C; B3, B20, B100). These results indicate that cryptochromes may also be required to suppress GA response under blue light.
Cryptochromes Mediate Blue Light-Activated mRNA Expression of GA2ox Genes, Especially GA2ox1, under Dark-to-Light Transfer, Continuous Illumination, and Photoperiodic Conditions
The Arabidopsis genome encodes up to eight GA2ox-related sequences, referred to as GA2ox1 to GA2ox8 (see “Materials and Methods”). However, the mRNA expression of only six members (GA2ox1, GA2ox2, GA2ox4, GA2ox6, GA2ox7, and GA2ox8) was readily detected in our study. GA2ox5 seems to be a pseudogene (Hedden et al., 2001; Schomburg et al., 2003), whereas GA2ox3 is normally expressed at a low level in the absence of auxin induction (Frigerio et al., 2006). Results of various DNA microarray studies concerning the blue light effect on GA2ox expression were not always consistent. For example, the expression of GA2ox1 shows an 8-fold increase in response to blue light in the wild-type seedlings in one study (https://www.genevestigator.ethz.ch), but a 2.4-fold increase of GA2ox1 transcript was reported in the cry1 mutant in another study (Folta et al., 2003). We decided to systematically examine the blue light and cryptochrome effects on the expression of each member of the GA2ox gene family upon transfer from dark to blue light and under photoperiodic and continuous light conditions.

Blue light-induced change of mRNA expression of the GA2ox genes. Six-day-old etiolated wild-type or cry1cry2 mutant seedlings were exposed to 100 μmol m−2 s−1 blue light, and samples were collected at the time points indicated for RNA analyses. Levels of mRNA expression are shown as the RT-PCR gel images (A) and the relative signal intensities (B).
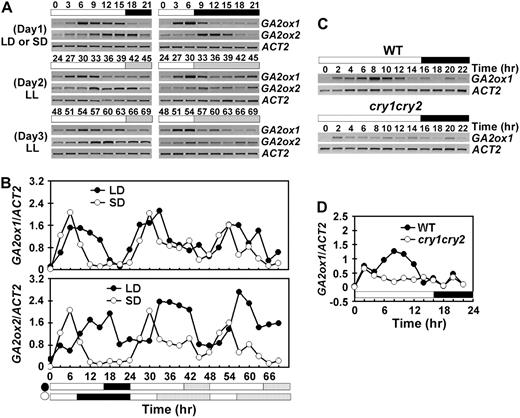
Cryptochromes control the circadian rhythm of GA2ox1 mRNA expression. mRNA expression of GA2ox1 and GA2ox2 genes in seedlings grown under long day (LD) or short day (SD) for 10 d and then transferred to continuous white light were examined using RT-PCR. Samples were collected every 3 h for 1 d in photoperiod and 2 d in continuous white light (A and B), or every 2 h for 1 d in long-day photoperiod (C and D). The white/black bars indicate light/dark phases, and the dashed bars indicate subjective night phase under continuous light. The time (hour) of light on of the first day of sample collection is set as zero.
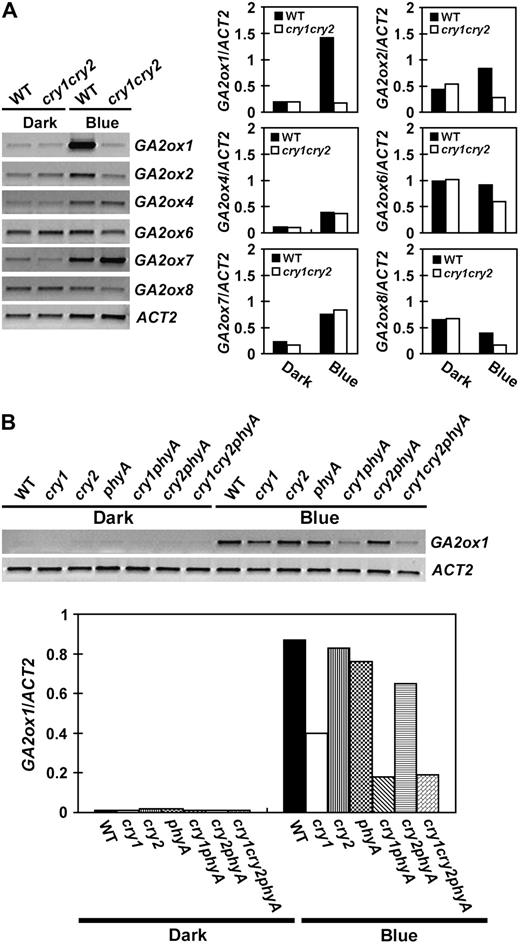
Comparison of the level of GA2ox mRNAs in seedlings grown in dark or continuous blue light. Six-day-old seedlings were grown in dark or continuous blue light (20 μmol m−2 s−1), the levels of mRNA expression are shown as the RT-PCR gel images (left) and the relative signal intensities (right). The mRNA expression of GA2ox genes in the wild-type or cry1cry2 mutant are shown in A. An independent experiment shows the levels of GA2ox1 mRNA expression in 6-day-old wild type and the indicated photoreceptor mutants grown in the dark or continuous blue light (B).
Cryptochromes Mediate Blue Light Suppression of GA20ox1 and GA3ox1 mRNA Expression
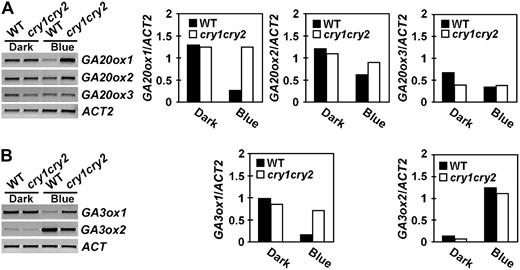
Expression of GA20ox and GA3ox genes in seedlings grown in the dark or continuous blue light. Six-day-old wild-type or cry1cry2 mutant seedlings grown in dark or continuous blue light (20 μmol m−2 s−1) were analyzed for the expression of the GA20ox genes (A) or the GA3ox genes (B). Levels of mRNA expression are shown as the RT-PCR gel images (left) or the relative signal intensities (right).
Cryptochromes Mediate Transient Blue Light Suppression of GA4 Accumulation
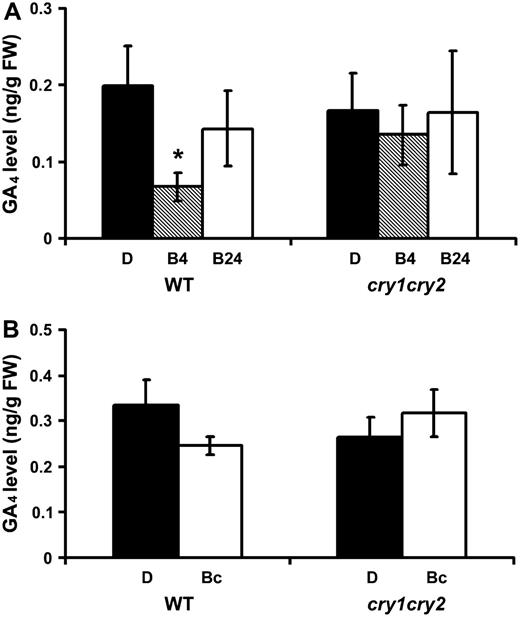
Cryptochromes mediate transient blue light suppression of GA4. The GA4 content of 6-d-old etiolated wild-type (WT) and cry1cry2 mutant seedlings exposed to blue light (60 μmol m−2 s−1) for 4 or 24 h are shown in A. The GA4 content of 5-d-old wild-type (WT) and cry1cry2 mutant seedlings grown in continuous dark or continuous blue light are shown in B. Asterisk indicates a significant difference (P < 0.05, two-way ANOVA). D, Dark; B4 and B24, etiolated seedlings exposed to blue light for 4 and 24 h, respectively; Bc, seedlings grown in continuous blue light. GA4 in whole seedling samples except roots was separated by HPLC and analyzed by gas chromatography-MS.
Finally, we compared GA4 levels in etiolated wild-type and cry1cry2 seedlings with those grown under continuous blue light. Interestingly, we detected no significant difference in GA4 level in those samples (Fig. 8B). However, because whole-shoot samples were used in our GA analyses, we cannot exclude the possibility that a localized or cell-specific change of GA4 content may occur in seedlings grown in continuous blue light.
DISCUSSION
In this study, we showed that increased expression of a GA2ox gene genetically suppressed the cry1cry2 mutant (Figs. 1 and 2). Although this observation by itself may have alternative interpretations, our follow-up photophysiological (Fig. 3) and gene expression studies (Figs. 4–5) demonstrate that cryptochromes are indeed positive regulators of GA2ox genes, especially GA2ox1. We showed that cryptochromes are required for the transient induction of GA2ox1 expression in etiolated seedlings exposed to blue light, for the sustained elevation of GA2ox1 expression in seedlings grown in continuous blue light, and for maintaining a high amplitude of the circadian rhythm of GA2ox1 expression in seedlings grown in long-day photoperiods. Consistent with the cryptochrome-mediated blue light stimulation of the expression of the GA catabolic gene GA2ox1, we also demonstrated that cryptochromes mediate blue light suppression of the expression of GA biosynthesis genes GA20ox1 and GA3ox1 (Fig. 7). We concluded that cryptochromes are positive regulators of GA2ox1 but negative regulators of GA20ox1 and GA3ox1. These cryptochrome-regulated gene expression changes may result in a blue light-dependent reduction of bioactive GAs. Given the well-established role of GA as a growth promoter, we propose that cryptochrome-regulated change in GA homeostasis is an important mechanism underlying blue light inhibition of hypocotyl elongation.
Consistent with this hypothesis, our analyses of GA4 content showed a cryptochrome-dependent transient reduction of GA4 in etiolated wild-type seedlings exposed to blue light (Fig. 8A). This result correlates with the transitory/rhythmic expression patterns of many GA metabolism/catabolism genes (Figs. 4 and 5; Supplemental Figs. S1 and S2). However, in contrast to the transient reduction in GA levels in deetiolating seedlings exposed to blue light, we did not detect a significant reduction of GA4 in the wild-type seedlings grown in continuous blue light (Fig. 8B) or white light (G.M. Symons and J.B. Reid, unpublished data). Neither did we detect a significant effect of the cry1cry2 mutation on the GA4 level in seedlings grown in continuous blue light (Fig. 8B). These results impose a significant challenge to the hypothesis that cryptochromes inhibits hypocotyl elongation solely by reducing GA4 levels. Similar observations have been previously reported in pea (O'Neill et al., 2000; Symons and Reid, 2003; Foo et al., 2006). In comparison to etiolated seedlings, the level of bioactive GA also showed a transient reduction in pea seedlings transferred from dark to blue light but not in seedlings grown under prolonged illumination (Reid et al., 2002; Foo et al., 2006). It was hypothesized that phyA and cry1 regulate not only GA homeostasis but also GA responsiveness to affect shoot elongation (Reid et al., 2002; Foo et al., 2006). Therefore, it is possible that light regulation of GA responsiveness or GA signal transduction may account for the inhibition of hypocotyl elongation in Arabidopsis plants grown under continuous blue light.
However, we cannot escape the question why continuous blue light caused markedly changed mRNA expression of the GA2ox1, GA20ox1, and GA3ox1 genes (Figs. 4–75 6 7) without a significant change in the level of GA4 (Fig. 8B). It would be interesting to examine whether those mRNA changes actually resulted in corresponding changes in the protein levels of the respective key enzymes in GA homeostasis. Alternatively, a localized change of GA4 levels in response to continuous blue light may provide another possible explanation of our puzzling observations. Specifically, a blue light-dependent reduction of GA4 may be limited to specific organs or cells. For example, light inhibits hypocotyl elongation but stimulates cotyledon expansion, suggesting that light may trigger a decrease or increase of GA4 in hypocotyls or cotyledons, respectively. Moreover, hypocotyl elongation is accomplished mostly by a limited number of cells in the elongation zone (Vandenbussche et al., 2005). Therefore, it is conceivable that light may suppress GA4 accumulation only in cells located in the elongation zone, but not in cells located in other regions of the same hypocotyl. Such localized changes of GA4 homeostasis may not be readily discernable when the whole shoot samples were examined as in this study. The localized change of hormone homeostasis is not uncommon in plants. For example, differential distribution of GA1 was previously reported in pea seedlings (O'Neill et al., 2000). Developmental regulation by cell-specific auxin and cytokinin biosynthesis have also been reported recently in Arabidopsis and rice (Oryza sativa), respectively (Cheng et al., 2006; Kurakawa et al., 2007). A cell-specific change of GA levels in response to blue light may be regulated by a posttranslational mechanism specific to the respective cells, because the blue light-dependent changes of mRNA expression of GA metabolism/catabolism genes were readily detectable in the whole shoot samples (Figs. 4–75 6 7). Whether cryptochromes differentially regulate changes of GA4 levels in specific cells and what molecular mechanism(s) may be responsible for such changes remain to be further investigated.
MATERIALS AND METHODS
Plant Materials
Arabidopsis (Arabidopsis thaliana) mutants cry1, cry2, cry1cry2, phyA, and cry1cry2phyA used in this study are in the Columbia background as described previously (Mockler et al., 2003).
To prepare an activation-tagging population, the cry1cry2 plants were transformed using the binary vector pSKI015 and Agrobacterium strain GV3101 as described (Weigel et al., 2000). The T1 seeds were harvested in pools and each pool contains seeds harvested from approximately 50 T0 plants. T1 seeds were germinated in white light and grown on compound soil, submerged in the herbicide Basta (approximately 0.006% ammonium glufosinate), and approximately 250,000 Basta-resistant T1 individuals were obtained. The herbicide-resistant individuals that showed hypocotyl length shorter than that of cry1cry2 parent were selected as putative scc-D mutants, and they were separated from the rest of the mutant populations. Those scc-D lines that showed short hypocotyls in the T2 generation grown in continuous blue light were subject to further genetic analysis. Genomic sequence flanking the T-DNA insert was identified using plasmid rescue (Weigel et al., 2000) or the Tail-PCR method (Liu et al., 1995). Hypocotyl lengths were measured manually for 6-d-old or 7-d-old seedlings (Lin et al., 1998). The sample size is larger than 20 seedlings, and the sds are calculated.
To investigate the response of hypocotyl elongation to exogenous GA3, paclobutrazol, or ancymidol, seeds were surface sterilized for 30 s in 70% ethanol, placed in 0.1% Hgcl2 for 8 min, and rinsed five times with sterile, distilled water. About 100 seeds were placed in Murashige and Skoog agar growth medium. All hormone and inhibitor stocks were dissolved in 70% (V/V) ethanol at a concentration 500 times greater than the final concentration used. GA3 (Shanghai Solvent), GA4 (Sigma), and/or GA biosynthesis inhibitors ancymidol (Sigma) or paclobutrazol (J&K Chemical Ltd), were added into the Murashige and Skoog medium to the final concentrations indicated in the respective figures. Seeds were placed in the dark at 4°C for 4 d, exposed to white light for 12 h to enhance germination before transferring to temperature-controlled growth chambers, and grown under continuous blue, red, or FR light or in the dark at 22°C unless it is indicated otherwise (i.e. 26°C).
For studies of light- or clock-regulated gene expression, about 300 sterile seeds were sown on Murashige and Skoog agar medium, cold treated at 4°C for 4 d, exposed to white light for 12 h, and grown in the dark for 6 d before transfer to various light treatments. Alternatively, seedlings were grown under white light (20 ± 3 μmol m−2 s−1) with long-day (16-h light/8-h dark) or short-day (8-h light/16-h dark) photoperiod for 10 d, and some petri dishes were transferred to continuous white light for 2 d. At the end of treatment, petri dishes were dipped in liquid nitrogen, and tissues (mostly shoot devoid of roots) were harvested by gentle scraping and stored at −80°C for RNA extraction.
Light Sources
In addition to light sources reported previously (Shalitin et al., 2002; Yu et al., 2007): LED-B (peak: 470 nm, half band width: 30 nm), LED-R (peak: 660 nm, half band width: 20 nm), and LED-FR (peak: 740 nm, half band width: 25 nm) were also used. Fluence rates of white, red, and blue light were measured using a Li-250 quantum photometer (LI-COR). The approximate fluence rates of FR light were estimated by plotting the relative fluence rates measured with a Li-250 quantum photometer to a near-linear standard curve of fluence rates measured with a spectroradiometer (T. Mockler and C. Lin, unpublished data).
GA Analysis
Whole shoot (all tissues except root) samples were used to analyze the GA content. For inductive light experiments, seeds were sown thickly on pots filled with potting mix, covered with a fine mesh, and placed in weak fluorescent light at 4°C for 4 d. Plants were transferred to dark at 22°C for 6 d and then transferred to 60 μmol m−2 s−1 blue light at 22°C using light source described (Platten et al., 2005).
For GA measurement, 3 to 4 g whole shoot samples were harvested, placed in ice-cold 80% methanol, homogenized, and the extract was filtered as described (Symons and Reid, 2003). Samples were concentrated to approximately 1 mL and loaded onto a preconditioned Sep-pak C18 cartridge in 0.4% acetic acid. GAs were eluted in 80% methanol in 0.4% acetic acid, dried, and fractionated using the reverse-phase C18 HPLC system (Jager et al., 2005). HPLC fractions corresponding to retention times of the relevant GAs were pooled, dried, methylated, and further purified by solvent partitioning as described (Jones et al., 2005). A total of 10 μL dry pyridine and 40 μL N,O-bis(trimethylsilyl) trifluoroacetamide were added to each vial and samples were heated at 80°C for 20 min. The samples were then dried before the addition of 15 μL N,O-bis(trimethylsilyl) trifluoroacetamide, incubated at 80°C for 15 min, dried under nitrogen gas, resuspended in 20 μL of chloroform, and analyzed by gas chromatography/tandem mass spectrometry (MS/MS) on a Varian 1200 triple quadrupole mass spectrometer (Jones et al., 2005). Injections of 1.5 μL were made in splitless mode at an injection temperature of 260°C onto a Varian VF5-ms column (30 m × 0.25 mm × 0.25 micron). The oven temperature was held at 50°C for 2 min, ramped to 230°C at 30°C per min, increased to 270°C at 5°C per min, and held at 270°C for 3 min. The transfer line temperature was 290°C, and the ion source was held at 220°C. The electron multiplier gain was 1,950 V. Carrier gas was helium at 1.2 mL per min. The MS was operated in selected reaction monitoring mode, with a Q1 peak width of 1.5 mass-to-charge ratio (m/z) units, and a Q3 peak width of 2.0 m/z units. The collision energy was −12 V, with the collision gas being argon at 1 mTorr. Prior to analyses instrument tuning was manually optimized in MS/MS mode rather than using the autotune feature. Based on preliminary full scan MS/MS experiments of standards, for endogenous GA4 (methyl ester TMS ether) the selected precursor ion (Q1) was m/z 284 and the selected product ion (Q3) was m/z 224. For 2H2 GA4 (methyl ester TMS ether) the ions were 286 (Q1) and 226 (Q3). The amount of endogenous GA4 was calculated from the peak areas. The internal standards ([2H2]GA4, [2H2]GA34, and [2H2]GA9) were supplied by Professor L.N. Mander (Research School of Chemistry, Australian National University, Canberra).
Although the transient reduction in GA4 level may result from reduced conversion of the precursor GA9 to GA4 and/or increased inactivation of GA4 to the 2β-hydroxylated GA34, we detected no significant changes in the GA9 or GA34 levels. These discrepancies may be due to the fact that GA9 level (less than 0.0025 ng g−1) was below our detection limit, whereas GA34 levels were approximately 10-fold higher than that of GA4, resulting in technical difficulties to detect relatively small or transient changes in the GA34 level under our experimental conditions. To compare GA content in etiolated seedlings with that grown in continuous blue light, 5-d-old whole shoot samples were used.
RNA Analyses
The accession numbers of genes discussed in this report are: GA2ox1 (At1g78440), GA2ox2 (At1g30040), GA2ox3 (At2g34555, mRNA not detectable), GA2ox4 (At1g47990), GA2ox5 (pseudogene), GA2ox6 (At1g02400), GA2ox7 (At1g50960), GA2ox8 (At4g21200), GA20ox1 (At4g25420), GA20ox2 (At5g51580), GA20ox3 (At5g07200), GA3ox1 (At1g15550), and GA3ox2 (At1g80340).
Total RNA was isolated using Puprep RNAeasy mini kit (Ambiogen Life Tech Ltd). DNA-free RNA was obtained by RQ1 DNase I treatment according to the manufacturer's instructions (Promega). The amount of mRNA was analyzed using semiquantitative reverse transcription (RT)-PCR as decribed (Mockler et al., 2003). cDNA was prepared from 2 μg of total RNA by using Moloney murine leukemia virus reverse transcriptase according to the manufacturer's instructions (Promega). The cDNA was generally diluted 10-fold, and 1 μL of diluted cDNA was used in a 20 μL PCR reaction. DNA sequences of the PCR primers used in this study are the following: ACT2F (5′-CACTGTGCCAATCTACGAGGGT-3′), ACT2R (5′-CACAAACGAGGGCTGGAACAAG-3′); GA2ox1F (5′-CACTATCCACCATGTCCTCTTA-3′), GA2ox1R (5′-CAGACCAAGTAAACTCCTCGTA-3′); GA2ox2F (5′-AGAGGCGGAGAAGATGGTGAA-3′), GA2ox2R (5′-GACAAGGCATGGCAATGGTGC-3′); GA2ox4F (5′-CCGATCAATTCTTTGGTGAAG-3′), GA2ox4R (5′-AATGTTTGGTACAACCGTGGC-3′), GA2ox6F (5′-ATGATTACATACGCACGGTTAG-3′), GA2ox6R (5′-ACATACGTGGCTTCTTTGCTG-3′); GA2ox7F (5′-GGGAAACAAGTGAACGTGAGT-3′), GA2ox7R (5′-GAGAAACCTGGACAAGCCTAC-3′); GA2ox8F (5′-CGGAATCAGAGGCATTAGC-3′), GA2ox8R (5′-CCACCTTTGGGTTCGTCAT-3′); A20ox1F (5′-CAGCCATTTGGGAAGGTGTATC-3′), GA20ox1R (5′-CAAGCAGCTCTTGTATCTATCGT-3′); GA20ox2F (5′-TCAATATTGGTGACACTTTCAT-3′), GA20ox2R (5′-GATGGGATTGTTGTTGGTAATA-3′); GA20ox3F (5′-AAAATGGGCGATGGATACGAAG-3′), GA20ox3R (5′-CGAAAGCGTGAGGGTTAGGAG-3′), GA3ox1F (5′-CCGAAGGTTTCACCATCACTG-3′), GA3ox1R (5′-GAGGCGATTCAACGGGACTAAC-3′); GA3ox2F (5′-CCAGCCACCACCTCAAATAC-3′), GA3ox2R (5′-GTGAAGCACGCTCGGGAAGA-3′).
PCR was generally performed with a 5 min denaturation at 95°C followed by 24 to 35 cycles with each cycle composed of 95°C for 30 s, 55 to 60°C for 30 s, and 72°C for 30 s. PCR products were analyzed using 1.5% agarose gel eletrophoresis. RT-PCR reactions for each experiment were repeated at least three times, and the representative gel images were shown. The expression level of the ACTIN2 gene was used as the internal control to normalize and calculate relative expression levels of genes tested using ImageJ (http://rsb.info.nih.gov/ij/).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers At1g78440, At1g30040, At2g34555, At1g47990, At1g02400, At1g50960, At4g21200, At4g25420, At5g51580, At5g07200, At1g15550, and At1g80340.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Blue light-reduced mRNA expression of members of the GA20ox and GA3ox genes.
Supplemental Figure S2. The circadian rhythm of the expression of GA20ox genes.
ACKNOWLEDGMENTS
The authors thank Detlef Weigel for providing the activation-tagging vectors, Professor L.N. Mander for the GA standards, and John Klejnot for critical reading of the manuscript.
LITERATURE CITED
Achard P, Liao L, Jiang C, Desnos T, Bartlett J, Fu X, Harberd NP (
Ahmad M, Cashmore AR (
Alabadi D, Gil J, Blazquez MA, Garcia-Martinez JL (
Blazquez MA, Weigel D (
Cao D, Hussain A, Cheng H, Peng J (
Cashmore AR (
Cheng Y, Dai X, Zhao Y (
Collett CE, Harberd NP, Leyser O (
Eriksson S, Bohlenius H, Moritz T, Nilsson O (
Fankhauser C, Chory J (
Folta KM, Pontin MA, Karlin-Neumann G, Bottini R, Spalding EP (
Foo EJ, Platten D, Weller JL, Reid JB (
Frigerio M, Alabadi D, Perez-Gomez J, Garcia-Carcel L, Phillips AL, Hedden P, Blazquez MA (
Garcia-Martinez JL, Gil J (
Gray WM, Ostin A, Sandberg G, Romano CP, Estelle M (
Guo H, Yang H, Mockler TC, Lin C (
Halliday KJ, Fankhauser C (
Hedden P, Phillips AL (
Hedden P, Phillips AL, Rojas MC, Carrera E, Tudzynski B (
Jager CE, Symons GM, Ross JJ, Smith JJ, Reid JB (
Jiao Y, Ma L, Strickland E, Deng XW (
Jones SE, Demeo JS, Davies NW, Noonan SE, Ross JJ (
Kamiya Y, Garcia-Martinez JL (
Koornneef M, Alonso-Blanco C, Peeters AJM, Soppe W (
Koornneef M, Rolff E, Spruit CJP (
Kurakawa T, Ueda N, Maekawa M, Kobayashi K, Kojima M, Nagato Y, Sakakibara H, Kyozuka J (
Lester DR, Ross JJ, Smith JJ, Elliott RC, Reid JB (
Lin C, Shalitin D (
Lin C, Yang H, Guo H, Mockler T, Chen J, Cashmore AR (
Liu YG, Mitsukawa N, Oosumi T, Whittier RF (
Ma L, Li J, Qu L, Hager J, Chen Z, Zhao H, Deng XW (
Mockler T, Yang H, Yu X, Parikh D, Cheng YC, Dolan S, Lin C (
Mockler TC, Guo H, Yang H, Duong H, Lin C (
O'Neill DP, Ross JJ, Reid JB (
Ohgishi M, Saji K, Okada K, Sakai T (
Olszewski N, Sun TP, Gubler F (
Peng J, Harberd NP (
Peng J, Richards DE, Moritz T, Cano-Delgado A, Harberd NP (
Platten JD, Foo E, Elliott RC, Hecht V, Reid JB, Weller JL (
Reed JW, Foster KR, Morgan PW, Chory J (
Reid JB, Botwright NA, Smith JJ, O'Neill DP, Kerckhoffs LH (
Reid JB, Symons GM, Ross JJ (
Schomburg FM, Bizzell CM, Lee DJ, Zeevaart JA, Amasino RM (
Shalitin D, Yang H, Mockler TC, Maymon M, Guo H, Whitelam GC, Lin C (
Silverstone AL, Mak PY, Martinez EC, Sun TP (
Stavang JA, Lindgard B, Erntsen A, Lid SE, Moe R, Olsen JE (
Sullivan JA, Deng XW (
Sun T, Goodman HM, Ausubel FM (
Sun TP, Gubler F (
Symons GM, Reid JB (
Thomas SG, Phillips AL, Hedden P (
Vandenbussche F, Verbelen JP, Van Der Straeten D (
Weigel D, Ahn JH, Blazquez MA, Borevitz JO, Christensen SK, Fankhauser C, Ferrandiz C, Kardailsky I, Malancharuvil EJ, Neff MM, et al (
Yamaguchi S, Smith MW, Brown RG, Kamiya Y, Sun T (
Yu X, Klejnot J, Lin C (
Yu X, Shalitin D, Liu X, Maymon M, Klejnot J, Yang H, Lopez J, Zhao X, Bendehakkalu KT, Lin C (
Zhao XY, Yu XH, Liu XM, Lin C (
Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W (
Author notes
This work was supported by the National Institutes of Health (grant no. GM56265 to C.L.), Changjiang scholarship (to C.L.), and 985 higher education enhancement fund to Hunan University. J.L. and K.B. were partially supported by UC MEXUS-CONACYT fellowship from the University of California and the BOYSCAST award from India, respectively.
These authors contributed equally to the article.
Present address: Department of Plant Physiology, College of Agriculture, Vellayani, Thiruvananthapuram–695, India.
These authors contributed equally to the article.
Corresponding author; e-mail [email protected].
The authors responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) are: Xuanming Liu ([email protected]) and Chentao Lin ([email protected]).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.



