-
PDF
- Split View
-
Views
-
Cite
Cite
Claudia-Anahí Pérez-Torres, José López-Bucio, Alfredo Cruz-Ramírez, Enrique Ibarra-Laclette, Sunethra Dharmasiri, Mark Estelle, Luis Herrera-Estrella, Phosphate Availability Alters Lateral Root Development in Arabidopsis by Modulating Auxin Sensitivity via a Mechanism Involving the TIR1 Auxin Receptor, The Plant Cell, Volume 20, Issue 12, December 2008, Pages 3258–3272, https://doi.org/10.1105/tpc.108.058719
Close - Share Icon Share
Abstract
The survival of plants, as sessile organisms, depends on a series of postembryonic developmental events that determine the final architecture of plants and allow them to contend with a continuously changing environment. Modulation of cell differentiation and organ formation by environmental signals has not been studied in detail. Here, we report that alterations in the pattern of lateral root (LR) formation and emergence in response to phosphate (Pi) availability is mediated by changes in auxin sensitivity in Arabidopsis thaliana roots. These changes alter the expression of auxin-responsive genes and stimulate pericycle cells to proliferate. Modulation of auxin sensitivity by Pi was found to depend on the auxin receptor TRANSPORT INHIBITOR RESPONSE1 (TIR1) and the transcription factor AUXIN RESPONSE FACTOR19 (ARF19). We determined that Pi deprivation increases the expression of TIR1 in Arabidopsis seedlings and causes AUXIN/INDOLE-3-ACETIC ACID (AUX/IAA) auxin response repressors to be degraded. Based on our results, we propose a model in which auxin sensitivity is enhanced in Pi-deprived plants by an increased expression of TIR1, which accelerates the degradation of AUX/IAA proteins, thereby unshackling ARF transcription factors that activate/repress genes involved in LR formation and emergence.
Lateral root formation is stimulated in plants deprived of phosphate. Here, phosphate-deprived Arabidopsis seedlings are shown to have an increased sensitivity to auxin, which alters the expression of auxin-responsive genes and promotes lateral root formation.
INTRODUCTION
Root branching is a major determinant of plant root architecture, in which lateral root (LR) development makes a considerable contribution to the spatial configuration of the root system in the soil and substantially determines the ability of a plant to secure anchorage and absorb water and nutrients. Root system architecture depends on both genetic determinants and postembryonic developmental processes that are under the influence of environmental factors, including water and nutrient availability (Malamy, 2005).
Phosphorus (P) is one of the most limiting nutrients for plant growth in many natural and agricultural ecosystems. P acquisition by plants is usually a constraint for plant productivity because phosphate (Pi), the inorganic form of P that is taken up by roots, is unevenly distributed and relatively immobile in soil due to its affinity for cations and its conversion to organic forms (Holford, 1997). In plants, a general strategy to cope with low Pi availability has been described, which involves three fundamental mechanisms: (1) the release and uptake of Pi from external organic and inorganic sources (Baldwin et al., 2001; Karthikeyan et al., 2002), (2) the optimization of Pi use by a wide range of metabolic alterations and the mobilization of internal Pi (Raghothama, 1999; Vance et al., 2003; Cruz-Ramírez et al., 2006), and (3) an increase in the exploratory capacity of the root and the absorptive surface area by altering the root system architecture (Bates and Lynch, 1996; López-Bucio et al., 2002; Sánchez-Calderón et al., 2005).
In terms of changes in root system architecture, plants typically respond to Pi deficiency by allocating more carbon to roots, thereby increasing their root-to-shoot ratio (Hermans et al., 2006). In addition, low Pi availability dramatically alters the spatial configuration of the root system by increasing root hair density and length and promoting LR formation and elongation (Föhse et al., 1991; Bates and Lynch, 1996; López-Bucio et al., 2002). Such plastic root alterations are believed to play a crucial role in enabling the plant to search increased volumes of soil for Pi-rich patches (Neumann and Martinoia, 2002; López-Bucio et al., 2003). Changes in root system architecture caused by Pi starvation have been well characterized in Arabidopsis thaliana. In low Pi conditions, Arabidopsis seedlings have a characteristic short primary root with a high density of LRs and an abundance of root hairs (Williamson et al., 2001; López-Bucio et al., 2002). The reduction in primary root growth of Pi-deprived seedlings involves a local signaling mechanism that operates in the root tip and triggers a gradual decrease in proliferative activity of root meristematic cells in response to low Pi (Sánchez-Calderón et al., 2006; Franco-Zorrilla et al., 2007). Prior to the arrest of primary root growth, primary and higher-order LR formation is stimulated (Williamson et al., 2001; López-Bucio et al., 2002, 2005; Ticconi and Abel, 2004; Sánchez-Calderón et al., 2006; Jain et al., 2007).
Available evidence suggests that phytohormones, particularly auxin, play an important role in mediating the Pi starvation effects on root system architecture (López-Bucio et al., 2002; Al Ghazi et al., 2003; Nacry et al., 2005; Jain et al., 2007). The roots of Pi-deprived seedlings are more responsive, in terms of LR formation, to exogenous auxins than are the roots of nondeprived seedlings (Gilbert et al., 2000; López-Bucio et al., 2002). The role of auxin in mediating root developmental responses to low Pi availability seems to be rather complex. Recently, López-Bucio et al. (2005) proposed that primary root growth inhibition in low Pi conditions was independent of polar auxin transport, whereas enhanced root branching was likely to be an auxin-dependent process. However, to date, it is not clear whether changes in auxin synthesis, distribution, or sensitivity are responsible for the increase in LR formation observed under Pi limiting conditions.
Auxin signaling plays a major role in LR development, as shown by the findings that several auxin-related mutants have altered LR formation (reviewed in Woodward and Bartel, 2005). Treatment with exogenous auxin induces LR formation (Blakely et al., 1988; Laskowski et al., 1995), and treatment with auxin transport inhibitors drastically decreases LR formation (Reed et al., 1998; Casimiro et al., 2001). Auxin is required at several stages of LR development, initially to establish a population of rapidly dividing pericycle cells and later for the emergence of LRs (Himanen et al., 2002). Transient changes in auxin concentration have been proposed to provide the basis for LR patterning, in which increases of auxin above a threshold level prompt xylem pole pericycle cells to become founder cells committed to LR formation (Casimiro et al., 2001; Ivanchenko et al., 2006). Therefore, the establishment of an LR primordium and the emergence of an LR are primarily determined by auxin; however, LR formation/emergence can also be modulated by environmental cues through as yet unknown mechanisms that could involve increased synthesis, transport, or sensitivity to auxin (Malamy, 2005).
Auxin responses are regulated by two large protein families: the auxin response factors (ARFs) and the AUXIN/INDOLE-3-ACETIC ACID (AUX/IAA) proteins. ARFs function as transcriptional activators/repressors of auxin-responsive genes, whereas AUX/IAA proteins are repressors of auxin responses that bind ARFs and prevent their transcriptional activity (for a review, see Guilfoyle, 2007). Auxin activates transcription by promoting the degradation of AUX/IAA repressors, thereby allowing ARFs to regulate the expression of auxin-responsive genes involved in growth and development (Dharmasiri and Estelle, 2004). Genetic and biochemical analyses showed that the ubiquitin protein ligase complex SCFTIR1 mediates AUX/IAA degradation. Recently, it was shown that TRANSPORT INHIBITOR RESPONSE1 (TIR1), the F-box subunit of SCFTIR1, is an auxin receptor that binds to AUX/IAA proteins in the presence of auxin and promotes their ubiquitination and degradation by the 26S proteosome (Dharmasiri et al., 2005a; Kepinski and Leyser, 2005). It is not known whether Pi availability modulates auxin signaling by altering the expression or function of the auxin receptors, ARF transcriptional activators, or AUX/IAA repressors.
Here, we show that the increase in LR formation in Pi-deprived Arabidopsis seedlings is, at least in part, mediated by an increase in auxin sensitivity of root cells and that Pi availability modulates the expression of the TIR1 auxin receptor. Furthermore, we provide genetic and molecular evidence showing that a SCFTIR1-dependent signaling mechanism plays an important role in root architectural modifications caused by low Pi availability.
RESULTS
Effect of Pi Availability on LR Formation
To determine the kinetics of LR primordia (LRP) formation and LR emergence in response to Pi availability, Arabidopsis (Columbia [Col-0]) seeds were germinated on vertically oriented Petri dishes containing 0.1× solid Murashige and Skoog (MS) media with sufficient (1 mM) or limiting (10 μM) Pi concentrations, and the number of LRP at different developmental stages and LRs were quantified at different time points. The developmental stage of each LR primordium was classified according to Zhang et al. (1999): stage A, up to three cell layers; stage B, unemerged LR, of more than three cell layers; stage C, emerged LR of <0.5 mm in length; stage D, LR longer than 0.5 mm.

Effect of Pi Availability on LR Formation.
(A) Wild-type (Col-0) seedlings were grown on the surface of agar plates containing 0.1× MS medium under P+ (1 mM) or P− (10 μM) for the indicated days after germination. Data are presented for LRP formation and emerged LRs (LRs) at different time points after germination. Values shown represent the mean of three groups of 15 seedlings ± se.
(B) Micrographs showing the LRPs in seedlings grown in P+ or P− at 1 and 2 dpg. Bar = 50 μm.
(C) Kinetic assay of primary root growth. Mean values were plotted at the indicated days after seed germination (n = 15). Different letters in (A) are used to indicate means that differ significantly (P < 0.05). [See online article for color version of this figure.]
These results show that low Pi availability accelerates the formation of LRP and LR emergence. Moreover, the position, absolute number, and density of LRP suggest that low Pi availability increases the formation and emergence of LRs, starting at early stages of seedling development.
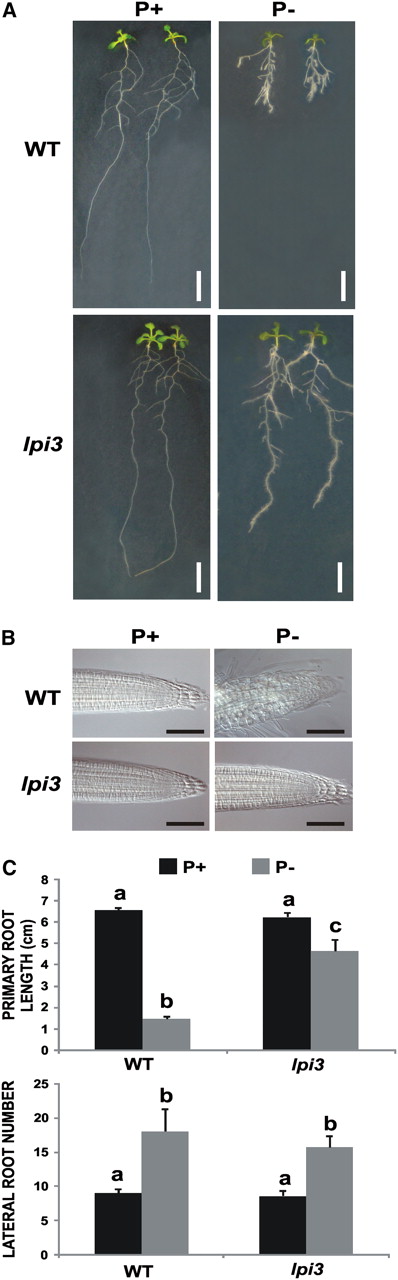
Effect of Pi Availability on LR Formation in the Phosphate-Insensitive lpi3 Arabidopsis Mutant.
Wild-type and lpi3 seedlings were grown for 13 d on vertically oriented agar plates.
(A) Photograph of seedlings growing on P+ (left) or P− (right). Bar = 1 cm.
(B) Nomarski images of the meristems of wild-type (Col-0) and lpi3 plants growing in P+ (left) or P− (right). Bar = 50 μm.
(C) Mean values (±se) of primary root length and LR number are presented (n = 15 seedlings). Different letters are used to indicate means that are significantly different (P < 0.05). [See online article for color version of this figure.]
It has been shown that LR formation and emergence are processes that can be genetically dissected and that both require auxin (Casimiro et al., 2001). To examine whether Pi availability affects LR formation by modulating the accumulation of auxin in the root, we measured the concentration of free IAA in the root and shoot of seedlings grown in P+ or P−. We found that P− seedlings had, in both the shoot and the root, similar concentrations of free IAA to those observed in P+ seedlings (Table 1).
. | pg free IAA mg−1 fw . | . | |
|---|---|---|---|
. | P+ . | P− . | |
| Shoot | 28.89 ± 1.37 | 28.22 ± 3.06 | |
| Root | 23.96 ± 3.22 | 22.63 ± 2.50 | |
. | pg free IAA mg−1 fw . | . | |
|---|---|---|---|
. | P+ . | P− . | |
| Shoot | 28.89 ± 1.37 | 28.22 ± 3.06 | |
| Root | 23.96 ± 3.22 | 22.63 ± 2.50 | |
Wild-type (Col-0) Arabidopsis seedlings were grown for 6 d on P+ (1 mM) and P− (10 μM) media. Roots and shoots were excised at the root-shoot junction, and the free IAA content was determined. Values are means ± se from three independent experiments (n = 1000). fw, fresh weight.
. | pg free IAA mg−1 fw . | . | |
|---|---|---|---|
. | P+ . | P− . | |
| Shoot | 28.89 ± 1.37 | 28.22 ± 3.06 | |
| Root | 23.96 ± 3.22 | 22.63 ± 2.50 | |
. | pg free IAA mg−1 fw . | . | |
|---|---|---|---|
. | P+ . | P− . | |
| Shoot | 28.89 ± 1.37 | 28.22 ± 3.06 | |
| Root | 23.96 ± 3.22 | 22.63 ± 2.50 | |
Wild-type (Col-0) Arabidopsis seedlings were grown for 6 d on P+ (1 mM) and P− (10 μM) media. Roots and shoots were excised at the root-shoot junction, and the free IAA content was determined. Values are means ± se from three independent experiments (n = 1000). fw, fresh weight.
Pericycle Cells of P− Seedlings Are More Responsive to Exogenous Auxin
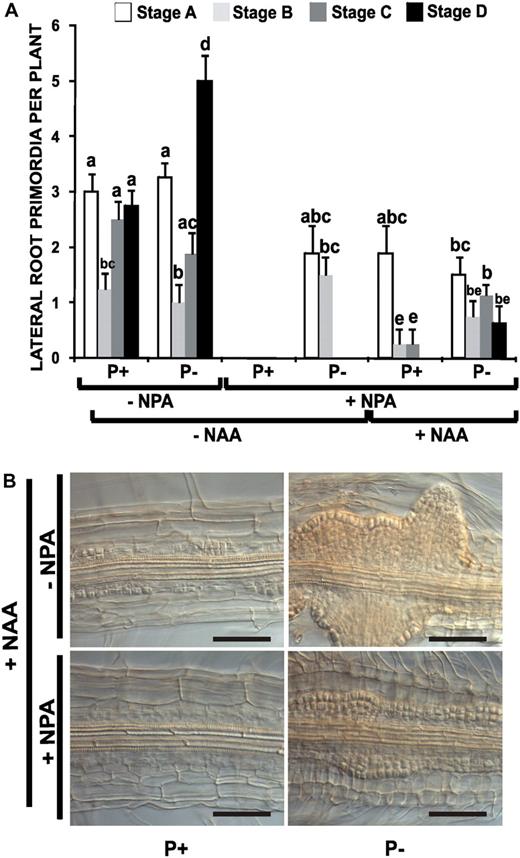
Effect of Auxin Transport Inhibition on LR Development in Arabidopsis Seedlings.
(A) Arabidopsis (Col-0) seedlings were grown for 4 d on P+ and P− with 1 μM of NPA and transferred to medium with or without 1 μM NPA or to medium containing 1 μM NPA with or without 10−8 M NAA for 48 h. The seedlings were cleared and the number and stage of LRP recorded according to Zhang et al. (1999). The data represent the mean ± se of the number of LR primordia per plant (n = 10). Different letters indicate that the means differ significantly (P < 0.05).
(B) Arabidopsis (Col-0) seedlings were grown for 4 d on P+ or P− with 1 μM of NPA and transferred to medium with (+) or without (−) NPA and 10−6 M NAA for 48 h. The seedlings were cleared, and the division and cellular proliferation in the pericycle were analyzed. Nomarski micrographs are representative of at least 10 plants analyzed. In all cases, the data represent three independent experiments. Bar = 50 μm. [See online article for color version of this figure.]
Continuous treatment of P+ seedlings with 1 μM NPA resulted in total absence of LRP and emerged LRs (Figure 3A). Interestingly, although P− seedlings treated with NPA did not form emerged LRs, they were still capable of producing LRP at stages A and B (Figure 3A), suggesting that pericycle cells of P− seedlings are more sensitive than P+ seedlings to the reduced amount of auxin transported in the presence of 1 μM NPA. To determine whether the roots of P− seedlings are more sensitive to auxin than those of P+ seedlings, we examined the effect of low concentrations (10−8 M) of exogenous auxin on the formation of LRP in NPA-treated seedlings. We used the auxin naphthalene acetic acid (NAA) because it is known to enter root cells without requiring an auxin uptake transport system; thus, its effect should not be altered by changes in the level or activity of the auxin influx carrier. Therefore, using NPA to block polar auxin transport and NAA, which does not require an influx carrier, would reveal changes in auxin sensitivity rather than in the transport of internal or exogenous auxin (Delbarre et al., 1996, Bennett et al., 1998). We observed that P+ seedlings treated with NAA and NPA formed approximately the same number of LR primordia as NPA-treated P seedlings in the absence of exogenous auxin (Figure 3A). In P− seedlings, the addition of low concentrations of exogenous auxin resulted in the emergence of LRs (Figure 3A). These results support the notion that the root pericycle of Pi-deprived seedlings is more sensitive to auxin than that of nondeprived seedlings and that this sensitivity affects both the formation and emergence of LRP.
To determine whether low Pi availability not only affects LR emergence, but also the capacity of pericycle cells to proliferate, we examined the ability of a high concentration of auxin to promote cell proliferation in seedlings grown in P+ and in P−. Arabidopsis seedlings were germinated and grown in medium with NPA for 4 d and then transferred to medium with 10−6 M NAA in the presence or absence of 1 μM NPA and for 48 h. Transfer of NPA-treated P+ seedlings to medium with 10−6 M NAA in the absence of NPA induced cell proliferation of pericycle cells, which led to the formation of several visible LRP in the primary root (Figure 3B). The same treatment of P− seedlings resulted in a dramatic increase in cell proliferation and consequently in the formation of a large number of LRP, some of which rapidly formed emerged LRs (Figure 3B). Transfer of P+ seedlings to medium containing NPA and NAA induced cell proliferation of pericycle cells along the root, but no LRP were clearly visible (Figure 3B). By contrast, in similarly treated P− seedlings, a high level of cell proliferation and LRP formation was observed in spite of the presence of the auxin transport inhibitor NPA (Figure 3B). The increased cell proliferation and LRP formation observed in P− seedlings treated with NPA and high auxin concentrations resulted in the formation of a high number of visible LR 4 d after the treatment (see Supplemental Figure 3 online). These results show that the pericycle cells of Pi-deprived seedlings are more sensitive to auxin than are the cells of nondeprived seedlings and that this activates cell proliferation and causes LRs to form and emerge.
Pi Availability Alters the Expression of Auxin-Responsive Genes
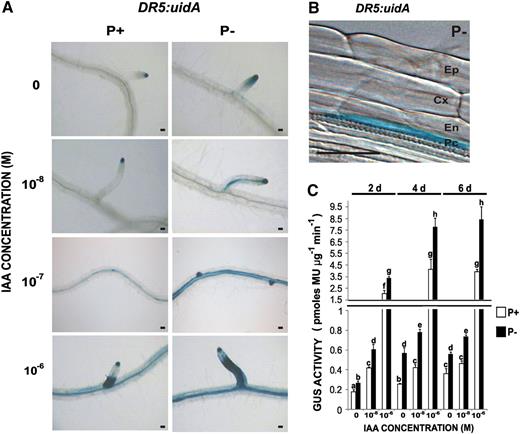
Effect of Pi Availability on the Temporal and Spatial Expression Patterns of the Auxin-Responsive Gene Marker DR5:uidA in Arabidopsis Roots.
(A) DR5:uidA seedlings grown for 5 d in P+ (right) or P− (left), immediately transferred to liquid media with various concentrations of auxin (IAA) for 6 h, and then subjected to GUS staining or quantification. Panels for 0, 10−8, and 10−6 M IAA correspond to the root region where LR have emerged, and that for 10−7 M illustrates changes in DR5:uidA expression in the root region where LRP are being formed. Photographs are representative of at least 15 stained plants. Bar = 50 μm.
(B) Close-up Normarski image of 5-d-old seedlings showing the expression pattern of DR5:uidA in pericycle cells. Micrograph is representative of at least 15 stained plants. Bar = 50 μm.
(C) Fluorometric analysis of the expression of the GUS reporter gene, DR5:uidA. Bars represent the average ± se of three independent experiments (n = 15). Different letters indicate that the means differ significantly (P < 0.05). GUS activity is presented as pmol methylumbelliferone per microgram of protein per minute and was measured 2, 4, and 6 dpg, as indicated.
To test whether Pi deprivation affects the expression of auxin-regulated promoters other than DR5:uidA, we examined the pattern of expression of the auxin-responsive gene marker BA3:uidA (Oono et al., 1998). We found that GUS expression was essentially absent in all root tissues of seedlings grown in P+ either without auxin or supplied with 10−8 M IAA (see Supplemental Figures 4A and 4B online), while expression of BA3:uidA was induced in the vascular tissue and elongation zone of the primary root when these seedlings were treated with 10−6 IAA (see Supplemental Figures 4A and 4B online). By contrast, in P− seedlings, BA3:uidA expression was detected in the vascular tissue even in the absence of exogenous IAA (see Supplemental Figures 4A and 4B online). When P− seedlings were treated with 10−6 IAA, no major changes in staining were observed in the vascular tissue; however, a stronger induction of BA3:uidA was observed in the primary root elongation zone as compared with P+ seedlings (see Supplemental Figures 4A and 4B online). Quantitative flourometric GUS assays showed that BA3:uidA expression was higher in 2 and 4 dpg P− seedlings grown in the absence of IAA and in 2, 4, and 6 dpg seedlings treated with 10−6 and 10−8 M IAA than in similarly treated P+ seedlings (see Supplemental Figure 4C online).
These results show that Pi deprivation induces the expression of auxin-responsive markers in the central cylinder of the root and that Pi-deprived roots are more responsive to the addition of exogenous IAA than are seedlings grown under sufficient levels of Pi, suggesting that Pi deprivation increases the perception or sensitivity of Arabidopsis roots to endogenous and exogenous auxins.
The Auxin Receptor TIR1 Is Required for the Modulation of LR Formation Due to Pi Availability
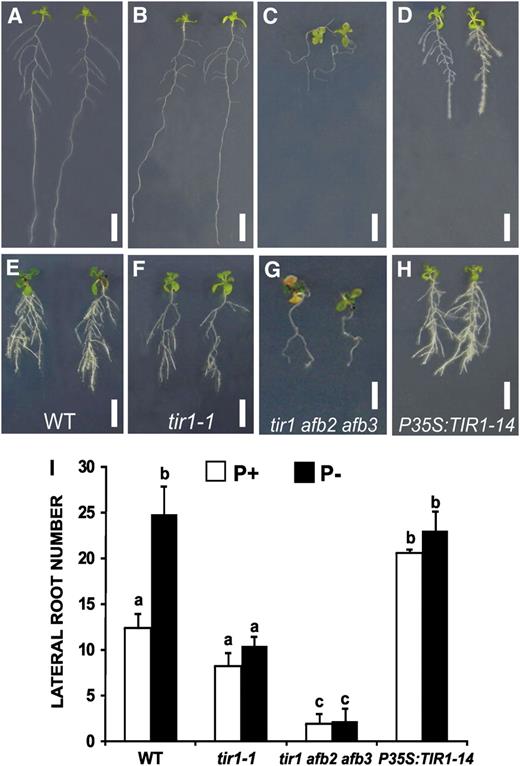
Effects of the tir1-1 Mutation and TIR1 Overexpression on LR Formation in Response to Pi Availability.
Seedlings of Arabidopsis Col-0, the tir1-1 and tir1-1 afb2 afb3 mutants, and P35S:TIR1-14 were grown in P+ or P−. Seedlings were photographed 13 dpg.
(A) to (H) Changes in root system morphology and LR number of seedlings grown in P+ ([A] to [D]) and P− ([E] to [H]). Photographs are representative of at least 15 plants analyzed. Bar = 1 cm.
(I) LR number in the wild type, tir1, and the TIR1 overexpressor at 13 dpg. Values shown represent the mean of 15 seedlings ± se. Letters represent statistically different means (P < 0.05). [See online article for color version of this figure.]
In Arabidopsis, TIR1 and its closest paralogs, the Auxin Signaling F-box proteins 1 to 5 (AFB1 to AFB5), belong to the C3 subfamily of leucine-rich-repeat–containing F-box proteins (Dharmasiri et al., 2005b). Together with TIR1, AFB1 to AFB3 have been found to function as redundant auxin receptors, collectively mediating auxin-regulated responses throughout plant growth and development (Dharmasiri et al., 2005b). To determine whether other members of the TIR1 family of auxin receptors are involved in Pi deprivation root responses, we analyzed LR formation during Pi deprivation in single afb1, afb2, and afb3 Arabidopsis mutants. No alterations in LR formation were observed in single afb mutants grown in either P+ or P−. However, when a triple tir1 afb2 afb3 mutant was analyzed, it was found that, in P+, LR formation was drastically reduced to one LR per seedling (Figures 5C and 5I). Interestingly, LR formation was not stimulated by Pi deprivation in the tir1 afb2 afb3 triple mutant (Figures 5G and 5I). These results suggest that, although TIR1 plays the most important role in LR formation and the response to Pi deprivation, other members of the TIR1 family of receptors play a partially redundant role both in LR formation and the root response to Pi deprivation.
Pi Availability Stress Modulates the Expression of TIR1
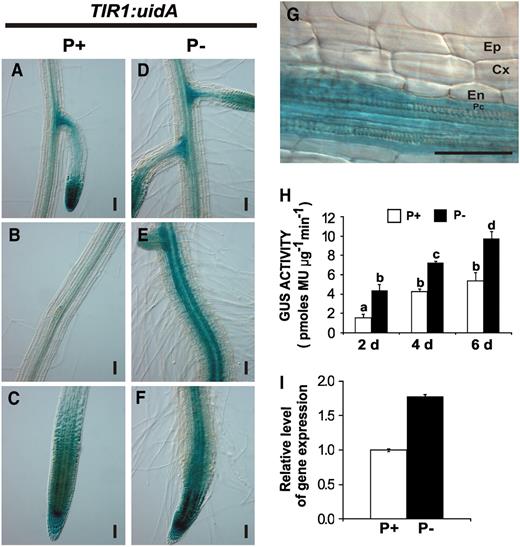
Effect of Pi Availability on the TIR1 Expression Pattern.
(A) to (F) Expression patterns of TIR1:uidA in 6-d-old seedlings grown in P+ ([A] to [C]) or in P− ([D] to [F]). Photographs are representative of at least 15 stained plants. Bars = 50 μm.
(G) Nomarski optics close-up of the central cylinder of seedlings grown in P− in the same root region presented in (F).
(H) GUS activity of TIR1:uidA seedlings grown in P+ or P− for the indicated times. Letters represent statistically different means (P < 0.05).
(I) Real time-PCR analysis of TIR1 in total RNA of roots isolated from 6-d-old seedlings. White bars represent growth in P+, and black bars represent growth in P−.
Overexpression of TIR1 Produces a Phenotype Similar to That Observed in Pi-Deprived Seedlings
The results presented above suggest that TIR1 expression is increased in seedlings exposed to low Pi availability and that this in turn increases the capacity of roots to initiate LR formation. To determine whether an increase in TIR1 expression alters LR formation in response to Pi availability, transgenic Arabidopsis lines that overexpress TIR1 under the control of the 35S promoter were produced. Sixteen independent P35S:TIR1 transgenic lines were isolated and analyzed. qRT-PCR analysis showed that only four of the P35S:TIR1 lines had an increased level of TIR1 transcript, ranging from 1.9- to 2.3-fold the TIR1 transcript level in untransformed seedlings (see Supplemental Figure 8 online). We selected one of the highest expressing lines (P35S:TIR1-14) for further analysis (see Supplemental Figure 8 online). We observed that P+ seedlings of the P35S:TIR1-14 line had a reduction in primary root growth and a 75% increase in the number of LR when compared with wild-type seedlings (Figures 5A, 5D, and 5I). In contrast with wild-type seedlings grown in P−, which showed an increase of >100% in the number of LRs, P35S:TIR1-14 seedlings grown in P− showed only a small increase in the number of LRs that was not statistically significant (Figures 5H and 5I). These results show that TIR1 is indeed a limiting factor in determining auxin sensitivity and LR formation and that small changes in its transcription level can have profound effects on root development.
Interestingly, overexpression of TIR1 also led to shorter primary roots in P+ seedlings, indicating that the level of expression of this F-box protein also played a role in the auxin sensitivity that regulates root elongation (Figure 5D). Although the roots of the P35S:TIR1-14 line were shorter than those of the wild type grown in P+, the process of meristem exhaustion, which has been documented for Pi-deprived seedlings (Sánchez-Calderón et al., 2005), was not observed for these lines grown in P+ media (see Supplemental Figure 9 online), showing that not all of the morphological changes observed in Pi-deprived seedlings are TIR1 dependent and that increased LR formation in TIR1-overexpressing lines is not linked to meristem exhaustion.
Phosphate Limitation Induces AUX/IAA Degradation via the SCFTIR1 Complex
It has been shown that auxin promotes SCFTIR1-dependent degradation of AUX/IAA repressors, leading to the activation or repression of gene expression by ARF transcription factors (Ouellet et al., 2001; Tiwari et al., 2001; Zenser et al., 2001; Dharmasiri and Estelle, 2004). Our results show that Pi deficiency induced an increase in the formation of LRP and the emergence of LRs by enhancing the expression of TIR1 in pericycle cells.
To examine whether the increase in expression of TIR1 in Pi-deficient seedlings mediates an increase in the degradation of AUX/IAA proteins, we analyzed the kinetics of auxin-mediated degradation of AUX/IAA proteins using the transgenic Arabidopsis line HS:AXR3NT-GUS, which harbors the protein fusion between the coding sequences of the auxin resistant 3 (AXR3/IAA17) and GUS genes under the control of a heat shock promoter. The HS:AXR3NT-GUS line has been previously used to assess proteasome-mediated degradation of AUX/IAA proteins, since the protein is only produced during a heat shock treatment and its degradation can be followed when the seedlings are returned to normal temperature (Gray et al., 2001). HS:AXR3NT-GUS seedlings were grown in P+ or P−, exposed to high temperature for 120 min, treated with NAA for 20 min after the end of the heat shock period, and assayed for GUS activity at 20-min intervals thereafter.
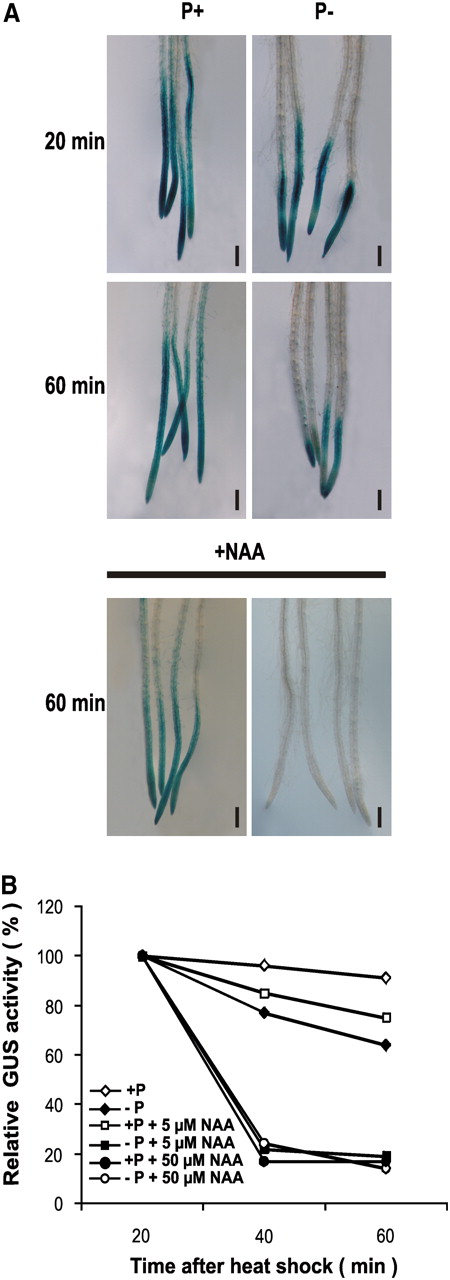
Effect of Pi Availability on AXR3 Degradation.
Four-day-old HS:AXR3NT-GUS seedlings grown in P+ or P− media were heat shocked for 2 h at 37°C and then transferred for 20 min to liquid media containing various concentrations of NAA.
(A) HS:AXR3NT-GUS seedlings were stained for histochemical GUS activity for the indicated periods of time. Bars = 200 μm.
(B) Fluorometric quantification of GUS activity. Relative activity is expressed as a percentage of the 20-min level. Each data point shows the mean of three independent experiments.
When GUS activity was quantified, it was found that AXR3NT-GUS is more rapidly degraded in P− than in P+ seedlings, with a 10% degradation in P+ seedlings and a 35% degradation in P− seedlings 60 min after the heat shock (Figure 7B). When seedlings were exposed to NAA treatment, a more rapid degradation of AXR3NT:GUS was observed for P− compared with P+ seedlings. At low (5 μM) NAA concentrations, P+ seedlings showed a moderate increase in AXR3NT:GUS degradation compared with seedlings that were not treated with auxin, showing a 25% decrease in GUS activity, whereas the same NAA concentration in P− seedlings showed an 85% reduction in GUS activity. At a high (50 μM) NAA concentration, no further increase in AXR3 degradation was observed in P− seedlings, while in P+ seedlings, the degradation of AXR3NT:GUS was increased to a level similar to that observed in P− plants at low auxin concentrations (Figure 7B). These data support the notion that Pi availability modulates auxin sensitivity by altering the kinetics of AUX/IAA degradation.
The Transcription Factor ARF19 Is Required for Increased LR Formation in Response to Low Pi Availability
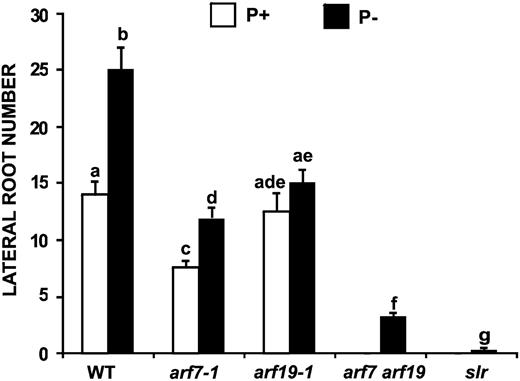
Effect of Pi Availability on LR Formation in Auxin Response Mutants.
LR number for 13-d-old wild-type (Col-0), arf7-1, arf19-1, arf7 arf19, and slr Arabidopsis seedlings was determined. The data represent the means ± se of seedlings (n = 15) grown in P+ (white bars) and P− (black bars). Different letters are used to indicate means that differ significantly (P < 0.05).
The solitary root mutant (slr) is a gain-of-function mutant of Aux/IAA14 that is insensitive to SCFTIR1-mediated degradation and has been proposed to impair LR formation by permanently binding to ARF7 and ARF19 (Fukaki et al., 2002; Okushima et al., 2005; Wilmoth et al., 2005). To determine whether slr has an effect on LR formation induced by Pi deprivation, we examined the LR number in slr grown in P+ and P−. It was observed that LR formation in the slr mutant was severely affected under both of these conditions (Figure 8).
DISCUSSION
Plants are endowed with nutrient sensing mechanisms that allow them to respond and adapt their growth and development to conditions of limited nutrient supply. These sensing mechanisms and responses are nutrient specific and involve biochemical and developmental changes that improve acquisition and efficient use of the limiting nutrient. In agreement with the importance of Pi for plants, the control of Pi deficiency responses involves a highly elaborate regulatory mechanism, whose details are just beginning to emerge. LR development is crucial for maximizing the ability of the root to absorb Pi from the soil. The formation of a highly branched root system in response to low Pi availability is believed to assist in the acquisition of Pi by exploring a greater soil volume and by increasing the absorptive surface of the root system.
Two controversial aspects of the mechanisms that trigger the increase in LR formation in response to low Pi availability needed to be addressed: (1) since physical and biological ablation of the primary root meristem induces LR formation, it remained to be determined whether the meristem exhaustion process observed in P− seedlings was responsible for the observed increase in LR formation; and (2) independently of whether the increased LR formation in Pi-deprived seedlings is related or not to the loss of meristematic activity in the primary root of P− seedlings, it remained to be determined whether this process is mediated by an increase in the synthesis and/or transport of auxins or by an increase in auxin sensitivity. Regarding the first aspect, we provide two lines of evidence that show that an increase in LR formation is independent of the meristem exhaustion process: (1) an increase in LR formation is observed prior to any detectable reduction of primary root growth (Figure 1), and (2) the lpi3 mutant that does not undergo the meristem exhaustion process (Sánchez-Calderón et al., 2005; Figure 2) is still capable of increasing LR formation in response to low Pi availability. The second aspect is discussed in detail below.
Several studies have addressed the role played by phytohormones in root architectural changes due to low Pi availability. These studies have shown that the signaling role of Pi in regulating primary root growth and LR formation is modulated by sugars, cytokinins, ethylene, and auxins (López Bucio et al., 2002, 2005; Nacry et al., 2005; Jain et al., 2007). A primary role for auxin sensitivity in LR induction by Pi limitation was inferred from three lines of evidence: (1) the roots of Pi-deprived seedlings are 10- to 100-fold more responsive to exogenous auxin in terms of LR formation (López-Bucio et al., 2002), (2) the tir3/big/low phosphate response 1 Arabidopsis mutant, which has a reduced auxin transport and is defective in LR formation, has increased LR formation under Pi limiting conditions (López-Bucio et al., 2005), and (3) it was previously reported that auxin transport and the concentration of free auxin in the roots of P+ and P− seedlings is quite similar (Jain et al., 2007). However, in spite of the available data, it was still unclear whether altered auxin transport or sensitivity was the critical factor in promoting LR formation under Pi limitation. In this work, we show that auxin sensitivity in pericycle cells plays a critical role in LR induction under low Pi availability.
Low Pi Availability Promotes LR Formation and Increases Auxin Sensitivity in Pericycle Cells
In Arabidopsis, LRs are initiated by the auxin-dependent local activation of pericycle cells at the xylem poles (Casimiro et al., 2001, 2003). Auxin is required at several stages of LR development, initially to establish a population of rapidly dividing pericycle cells and later for the emergence of LRs. Therefore, the control of LR formation by auxin involves the complex regulation of biosynthesis, transport, and the ability of cells to respond to this phytohormone in an appropriate manner (Okushima et al., 2007). Our results showed that seedlings grown in P− exhibited a twofold increase in LR number compared with seedlings grown in P+ and that this increase initiates at an early stage of seedling development (Figure 1). The increase in LR formation in Pi-deprived seedlings was found to correlate with an enhanced expression of the DR5:uidA and BA3:uidA auxin-responsive gene markers. These results suggest that auxin distribution or sensitivity could be involved in the activation of auxin-responsive genes involved in root architectural changes activated by Pi deficiency. In this regard, Nacry et al. (2005) suggested that low Pi availability modifies local auxin concentrations within the root system through changes in auxin transport rather than auxin synthesis. However, the finding that the expression directed by the DR5 promoter (Figure 4) and cell proliferation of pericycle cells are also more responsive to the addition of exogenous auxin in Pi-deprived seedlings suggests that an increase in auxin sensitivity rather than an increase in auxin synthesis is involved in changes in root architecture. Previous reports showing that mutants with reduced (tir3 and pgp19) or elevated (transparent testa mutant tt4-2) auxin transport are still able to enhance LR formation under Pi limiting conditions argue against an auxin transport–dependent mechanism for the increased formation of LRs in Pi-deprived seedlings (López-Bucio et al., 2002, 2005; Jain et al., 2007). Moreover, it was determined that the free auxin content in P− seedlings is quite similar to that present in P+ seedlings (Table 1). These results are similar to those previously reported by Jain et al. (2007). Our results provide two additional lines of evidence supporting an auxin sensitivity mechanism: (1) P− seedlings grown in the presence of the auxin transport inhibitor NPA were capable of forming LRP, whereas P+ seedlings grown in the presence of NPA did not (Figure 3), and (2) in P− seedlings transferred to medium with NPA and exogenous auxin, an increased proliferation of LRP was observed compared with plants grown in P+ and transferred to P+ medium supplemented with the same concentrations of NPA and auxin. Taken together, these results show that in low Pi conditions, pericycle cells are more responsive to auxin, accelerating the formation of LRP and their further development to mature LRs.
The SCFTIR1 Complex Is Involved in the LR Response to Pi Deficiency
Auxins regulate diverse aspects of plant growth and development by promoting the degradation of transcriptional repressors called Aux/IAA proteins through the action of the ubiquitin protein ligase SCFTIR1. In recent work, the F-box protein subunit of SCFTIR1, a protein called TIR1, was shown to function as an auxin receptor (Dharmasiri et al., 2005a; Kepinski and Leyser, 2005). The finding that tir1-1 greatly reduced the increase of LR formation in response to low Pi availability (Figure 3) suggests that this process requires a functional TIR1 to occur. TIR1 is a member of a small family of five genes (AFBs) within the large F-box gene family, of which at least four have been shown to be involved in auxin signaling (Dharmasiri et al., 2005b). Our results showing that tir1-1 is impaired in the capacity to respond to low Pi in terms of LR formation but is still capable of forming LRs and that the triple mutant tir1 abf2 abf3 is almost unable to form LRs suggest that the other members of the AFB family play a minor role in the Pi deprivation response but have a functional role in normal LR formation.
Interestingly, we found that the expression of TIR1 increases in Pi-deprived seedlings, particularly in the pericycle of the primary root, prior to the increase in LR formation (Figure 6; see Supplemental Figure 5 online). The increase in TIR1 expression in Pi-deprived seedlings together with the finding that TIR1-overexpressing lines are no longer able to increase LR formation in response to Pi limiting conditions shows that TIR1 is a limiting component of the auxin signaling pathway leading to LR formation and provides compelling evidence that changes in TIR1 expression in response to environmental factors alter the sensitivity of pericycle cells to auxin, resulting in changes in the postembryonic developmental program of the Arabidopsis root system. Therefore, we propose that the increase in the expression of TIR1 is responsible for the changes observed in LR formation in response to Pi deficiency. Recent evidence showed that the priming of pericycle cells for LR initiation takes place in the basal meristem (De Smet et al., 2007). We observed that the increase in expression of TIR1 takes place mainly in the basal part of the root, suggesting that a higher rate of pericycle cell priming might be occurring under low Pi conditions.
It has been reported that a microRNA (miR393) negatively regulates the level of TIR1, AFB2, and AFB3 mRNA during Pseudomonas syringae infection (Navarro et al., 2006). However, in the case of Pi-deprived seedlings, the increased expression of TIR1 appears to occur primarily at the transcriptional level, since not only the level of TIR1 transcript but also the expression of a reporter gene driven by the TIR1 promoter increases in Pi-derived seedlings (Figure 6; see Supplemental Figure 5 online). Moreover, preliminary results indicate that the expression of miR393 is not altered by Pi availability (Pérez-Torres, A., and Herrera-Estrella, L., unpublished results), providing further support for a mechanism in which TIR1 transcriptional activation, rather than posttranscriptional events, is involved in LR formation during Pi deprivation.
It has been previously reported that a developmental process that leads to primary root meristem exhaustion takes place in Pi-deprived seedlings (Sánchez-Calderón et al., 2005). Interestingly, we found that although P35S:TIR1-14 seedlings have shorter primary roots and an increased number of LRs than wild-type seedlings, the process of meristem exhaustion does not take place (see Supplemental Figure 9 online). This finding suggests that there are root developmental processes in response to Pi availability that are independent of TIR1. Recently, it was proposed that ethylene is part of a signaling pathway that modulates cell division in the quiescent center during postembryonic development of the root system (Ortega-Martínez et al., 2007). Since a number of genes involved in ethylene signaling are regulated by Pi availability (Misson et al., 2005), it is possible that the root meristem exhaustion observed in Pi-deprived seedlings is mediated by ethylene or by some factor other than auxin. Ethylene could negatively regulate the expression of LPR1, a gene encoding a multicopper oxidase, which was recently shown to be responsible for a major quantitative trait locus involved in the reduction of root growth induced by Pi deprivation (Svistoonoff et al., 2007), or it could be due to a possible toxic effect of an increased Fe uptake in Pi-deprived roots, as recently suggested by Ward et al. (2008). Nevertheless, further studies are needed to determine the role of ethylene, auxin, and other plant hormones in the meristem exhaustion process observed in plants under Pi deficiency.
Role of ARF7 and ARF19 in the LR Response to Pi Deficiency
The current model of auxin signaling postulates that Aux/IAA-ARF complexes bind to auxin-responsive elements (AuxREs) present in the promoter of auxin-responsive genes and actively repress transcription. The auxin receptor TIR1 stimulates degradation of Aux/IAA proteins, allowing ARF proteins already bound to AuxREs motifs to activate the transcription of genes involved in LR formation (Guilfoyle, 2007). Although the ARF gene family is composed of 29 members and could potentially have overlapping functions in LR development, it has recently been shown that ARF7 and ARF19 are critical for LR formation (Fukaki et al., 2002; Okushima et al., 2005). Analysis of arf7 and arf19 in contrasting conditions of Pi availability showed that ARF19 plays an important role in LR formation in response to Pi deprivation and suggests that this transcription factor participates in the TIR1-mediated activation of pericycle cells to form LRP during the low Pi response (Figure 8). However, the observations that Pi-deprived arf19 seedlings are still able to increase LR formation and that the double mutant arf7 arf19 forms LRs under Pi limiting conditions suggest that an additional ARF is involved in the response to Pi deprivation.
Based on the results obtained in this work, we propose a model for the increased formation of LRs under Pi-deprived conditions (see Supplemental Figure 10 online) in which an increased expression of TIR1 in pericycle cells leads to a higher degradation rate of AUX/IAA repressors. The increased degradation of AUX/IAA allows ARF19, and probably other ARFs, already bound to the AuxREs to activate or repress the auxin-responsive genes that promote pericycle cell division. This Pi deficiency response makes pericycle cells more sensitive to auxins, leading to an enhanced LR formation and emergence without the need for an increase in auxin transport or synthesis (see Supplemental Figure 10 online). Under these conditions, a higher number of pericycle cells would become founder cells committed to organogenesis. This model is supported by two additional important findings: (1) slr, a mutation that makes Aux/IAA14 (the putative molecular partner that represses the activity of ARF7 and ARF19) insensitive to the SCFTIR1-mediated degradation, is unable to increase LR formation during Pi deficiency; and (2) the degradation of AXR3, another member of the AUX/IAA protein family, is increased under P− compared with P+ and is more susceptible to treatment with exogenous auxin in Pi-deprived seedlings (Figure 7).
Hormones act as chemical messengers in the regulation of physiological, biochemical, and molecular processes underlying growth and development. To survive, plants rely heavily on the proper physiological and developmental adjustments that determine their ability to secure edaphic resources. Therefore, hormones probably serve as essential integrators of developmental processes with environmental signals. The finding that Pi availability regulates auxin sensitivity by modulating the expression of the auxin receptor gene TIR1 is a clear illustration of how the interaction of hormone signaling and environmental cues impact processes of postembryonic development. This molecular framework is an attractive model to explain how environmental signals can regulate postembryonic developmental processes by modulating the expression or activity of different components of hormone signaling during the plant life cycle.
METHODS
Plant Material and Growth Conditions
Arabidopsis thaliana ecotype Col-0, the transgenic lines DR5:uidA (Ulmasov et al., 1997), BA3:uidA (Oono et al., 1998), TIR1:uidA (Gray et al., 1999), and HS:AXR3NTGUS (Gray et al., 2001), and the mutant lines tir1-1 (Ruegger et al., 1998), tir1 afb2 afb3 (Dharmasiri et al., 2005b), arf7-1, arf19-1, arf7 arf19 (Okushima et al., 2005), and slr/iaa14 (Fukaki et al., 2002) were used in various experiments. Seeds were surface sterilized with 95% (v/v) ethanol for 5 min and 20% (v/v) bleach for 7 min. After five washes in distilled water, seeds were germinated and grown on Petri dishes containing sterile modified MS medium (pH 5.7, 0.5% [w/v] Suc, and 1% [w/v] agar) under P− (10 μM KH2PO4) or P+ (1 mM KH2PO4). The basic modified medium contained 2.0 mM NH4NO3, 1.9 mM KNO3, 0.3 mM CaCl2·2H20, 0.15 mM MgSO4·7H20, 5 μM KI, 25 μM H3BO3, 0.1 mM MnSO4·H2O, 0.3 mM ZnSO4·7H20, 1 μM Na2MoO4·2H20, 0.1 μM CuSO4·5H20, 0.1 μM CoCl2·6H2O, 0.1 mM FeSO4·7H20, 0.1 mM Na2EDTA·2H20, 10 mg L−1 inositol, and 0.2 mg L−1 Gly.
Seeds were grown in Petri dishes under a photoperiod of 16 h of light and 8 h of darkness and a temperature of 22°C using a plant growth cabinet (Percival Scientific). Plates were placed vertically at an angle of 65° to allow root growth along the surface of the agar and to allow the unimpeded growth of the hypocotyl into the air.
Hormone Treatments
Nutrient medium with P− (10 μM KH2PO4) or P+ (1 mM KH2PO4) was supplemented with IAA (10−8 to 10−6 M), NAA (5 and 50 μM), or NPA (1 μM). Filter-sterilized compounds were added to cooled (50°C) molten medium and poured into plates.
Histochemical Analysis
For histochemical analysis of GUS activity, Arabidopsis seedlings were incubated overnight at 37°C in a GUS reaction buffer (0.5 mg/mL of 5-bromo-4-chloro-3-indolyl-β-d-glucuronide in 100 mM sodium phosphate, pH 7), and the stained seedlings were cleared by the method described by Malamy and Benfey (1997). For each marker line and for each treatment, at least 15 transgenic plants were analyzed. A representative plant was chosen for each Pi treatment and imaged using Nomarski optics on a Leica DMR microscope.
Analysis of Root Architecture Traits
The Arabidopsis root system was analyzed with an AFX-II-A stereomicroscope (Nikon). All emerged secondary roots that were clearly visible using a ×3 objective were taken into account when counting the number of LRs. For all experiments, the overall data were statistically analyzed in the SPSS 10 program as described by López-Bucio et al. (2002). Roots of Arabidopsis seedlings were cleared and observed by microscopy to classify the stage of LR development. For quantification purposes, the developmental stage of each LR primordium was classified according to Zhang et al. (1999) because it became difficult to classify LRP according to Malamy and Benfey (1997), particularly when the LRP were out of the focal plane of the microscope. However, it is helpful to make a comparison between the two methods for understanding the relationship between both development criteria. The classification of Zhang considers four stages that comprise one or several of the LRP developmental stages as classified by Malamy and Benfey (1997): stage A, LRP of up to three cell layers, corresponds to stages A, B, and C as described by Malamy and Benfey (1997); stage B, unemerged LRP of more than three cell layers, corresponds to stages D to H; emerged LRs of <0.5 mm in length corresponds to stages D, J, and K, and LRs longer than 0.5 mm correspond to stage L.
Free IAA Determination
Seedlings grown under different nutrient conditions for 6 d were harvested, cotyledons were excised at the root-shoot junction, and free IAA was quantified as described by Edlund et al. (1995).
Expression Analysis
For RT-PCR analysis, total RNA was extracted from the root tissue of 6-d-old wild-type plants using the Concert Plant RNA Reagent from Invitrogen according to the manufacturer's instructions. cDNA was first synthesized using 10 μg of total RNA with SuperscriptIII Reverse Transcriptase (Invitrogen) according to the manufacturer's instructions. For TIR1 transcript detection, we used the following specific primers: TIR1-RTF (5′-CGACGACGGTTTGGGGAG-3′) and TIR1-RTR (5′-CCCAAGCTGTGTAGCCAC-3′). The amplification reactions were performed under the following conditions: 52°C for 30 min, 94°C for 2 min, 35 cycles of 94°C for 20 s, 55°C for 30 s, and a final extension step at 72°C for 1min. We followed the same protocol and conditions for control reactions using specific primers for the Arabidopsis ACTIN2 gene AC2-RTF (5′-GTACAACCGGTATTGTGCTGGAT-3′) and AC2-RTR (5′-GCTTGGTGCAAGT GCTGTGATTTC-3′).
For qRT-PCR, the 7500 Real Time PCR System (Applied Biosystems) was used. qRT-PCR of ACTIN2 (ACT2) was performed for normalization. SYBR Green PCR Master Mix was used for the PCRs according to the manufacturer's protocol. Gene expression was normalized to that of the control ACT2 gene by subtracting the CT value of ACT2 from the CT value of the gene of interest (TIR1). P− to P+ average expression ratios were obtained from the equation (1 + E)ΔΔC T, where ΔΔCT represents ΔCT (P−) − ΔCT (P+), and E is the PCR efficiency according to the protocol reported by Czechowski et al. (2004).
Overexpression of TIR1
Transgenic plants overexpressing the TIR1 protein (P35S:TIR1) under the control of the 35S promoter were generated by amplifying the complete ORF sequence of this gene from genomic Arabidopsis DNA by PCR using the following primers: FWDAttB1TIR1 (5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTAAggagtaccattcacaaac-3′) and REVAttB2TIR1 (5′-GGGGACCACTTTGTACAAGAAAGCTGGGTAccgtcgacatcgattaac-3′). The PCR product was cloned in the vector pDONR221 and transferred to pB7WG2D by recombination using Gateway BP and LR Clonase enzyme mixes, respectively, and then introduced into the Arabidopsis genome via the Agrobacterium tumefaciens–mediated transformation system using the protocol reported by Martínez-Trujillo et al. (2004).
Heat Induction of HS:AXR3NTGUS
The HS:AXR3NTGUS seedlings were submerged in MS liquid and heat shocked for 2 h at 37°C. Plants were sampled at 20, 40, 60, and 80 min thereafter and stored in liquid nitrogen until protein extraction, or in the case of histochemical reactions, assayed immediately. Auxin treatments were performed by adding NAA 20 min after completion of the heat shock period. GUS activity was measured as described previously (Gallagher, 1992).
For fluorometric assays, seedlings were collected and ground in GUS extraction buffer. Total protein extracts were centrifuged at 12,000g for 10 min at 4°C, and protein content was quantified using Bradford reagent (Bio-Rad Laboratories). For each assay, 5 μg of protein were used, and the fluorometric assays were performed by incubating sample extracts in 2 mM MUG (4-methylumbelliferyl-β-d-glucoronide), 50 mM KPO4, pH 7.0, 0.1% Sarkosyl (BDH), 0.1% Triton X-100, 10 mM β-mercaptoethanol, and 10 mM EDTA for 16 h, followed by analysis with a TKO 100 fluorometer (Hoefer Scientific Instruments). GUS activity is reported as picomoles of 4-methylumbelliferone per microgram of protein per minute, although for means of comparison, it is expressed as relative activity in the figures.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: TIR1 (At3g62980), AFB2 (At3g26810), AFB3 (At1g12820), AXR3 (At1g04250), ARF7 (At5g20730), ARF19 (At1g19220), IAA14 (At4g14550), and CYCB1;1 (At1g34460).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Pattern of LRP Formation in Seedlings Grown in P+ and P−.
Supplemental Figure 2. Effect of Pi Availability on Arabidopsis Root Architecture.
Supplemental Figure 3. Effect of NPA and Auxin on LR Formation in Response to Pi Availability.
Supplemental Figure 4. Pattern of Expression of the Auxin-Responsive Gene Marker BA3:uidA in Response to Pi Availability.
Supplemental Figure 5. Close-Up of 4-d-Old TIR1:uidA Seedlings in P+ or P− Showing GUS Expression in the Pericycle of the Primary Root.
Supplemental Figure 6. TIR1 Expression Pattern during LR Formation in Response to Pi Availability.
Supplemental Figure 7. Effect of Rxogenous IAA on TIR1 Rxpression Pattern.
Supplemental Figure 8. Analysis of TIR1 Rxpression in the P35S:TIR1 Overexpressing Lines.
Supplemental Figure 9. Meristem Morphology of tir1 Mutants and P35S:TIR1-14 Overexpressing Seedlings Grown in P+ or P−.
Supplemental Figure 10. Schematic Representation of the Model Depicting the Molecular Mechanisms by Which TIR1 Could Modulate Auxin Sensitivity in Response to Pi Availability.
ACKNOWLEDGMENTS
We thank Enrique Ramírez-Chávez and Jorge Molina for their support in the auxin quantification experiments. We thank June Simpson for valuable help in reviewing the manuscript. We also thank Hidehiro Fukaki and The Arabidopsis Information Resource for providing the slr mutant and arf19 and arf7 mutants, respectively. A.P.-T. is indebted to CONACyT (Mexico) for a PhD fellowship. This work was supported in part by grants from the Howard Hughes Medical Institute (Grant 55005946) and CONACyT (299/43979) to L.H.-E. We also thank the anonymous reviewers for their positive and relevant comments, which improved the quality of this manuscript.
REFERENCES
Al-Ghazi, Y., Muller, B., Pinloche, S., Tranbarger, T.J., Nacry, P., Rossignol, M., Tardieu, F., and Doumas, P. (
Baldwin, J.C., Karthikeyan, A.S., and Raghothama, K.G. (
Bates, T., and Lynch, J.P. (
Bennett, M.J., Marchant, A., May, T.S., and Swarup, R. (
Blakely, L.M., Blakely, R.M., Colowit, P.M., and Elliott, D.S. (
Casimiro, I., Beeckman, T., Graham, N., Bhalerao, R., Zhang, H., Casero, P., Sandberg, G., and Bennett, M.J. (
Casimiro, I., Merchant, A., Bhalerao, R.P., Beeckman, T., Dhooge, S., Swarup, R., Graham, N., Inzé, D., Sandberg, G., Casero, P.J., and Bennett, M. (
Cruz-Ramírez, A., Oropeza-Aburto, A., Razo-Hernández, F., Ramírez-Chávez, E., and Herrera-Estrella, L. (
Czechowski, T., Bari, R.P., Stitt, M., Scheible, W.R., and Udvardi, M.K. (
Delbarre, A., Muller, P., Imhoff, V., and Guern, J. (
De Smet, I., et al. (
Dharmasiri, N., Dharmasiri, S., and Estelle, M. (
Dharmasiri, N., Dharmasiri, S., Weijers, D., Lechner, E., Yamada, M., Hobbie, L., Ehrismann, J., Jürgens, G., and Estelle, M. (
Dharmasiri, N., and Estelle, M. (
Föhse, D., Claassen, N., and Jungk, A. (
Edlund, A., Eklöf, S., Sundberg, B., Moritz, T., and Sanberg, G. (
Franco-Zorrilla, J.M., Valli, A., Todesco, M., Mateos, I., Puga, M.I., Rubio-Somoza, I., Leyva, A., Weigel, D., García, J.A., and Paz-Ares,J. (
Fukaki, H., Tameda, S., Masuda, H., and Tasaka, M. (
Gallagher, S.R. (
Gilbert, G.A., Knight, J.D., Vance, C.P., and Allan, D.L. (
Gray, W., del Pozo, J., Walker, L., Hobbie, L., Risseeuw, E., Banks, T., Crosby, W., Yang, M., Ma, H., and Estelle, M. (
Gray, W.M., Kepinski, S., Rouse, D., Leyser, O., and Estelle, M. (
Hermans, C., Hammond, J.P., White, P.J., and Verbruggen, N. (
Himanen, K., Boucheron, E., Vanneste, S., de Almeida, J., Inzé, D., and Beeckman, T. (
Holford, I.C.R. (
Ivanchenko, M.G., Coffeen, W.C., Lomax, T.L., and Dubrovsky, J.G. (
Jain, A., Poling, M.D., Karthikeyan, A.S., Blakeslee, J.J., Peer, W.A., Titapiwatanakun, B., Murphy, A.S., and Raghothama, K.G. (
Karthikeyan, A.S., Varadarajan, D.K., Mukatira, U.T., D'Urzo, M.P., Damsz, B., and Raghothama, K.G. (
Kepinski, S., and Leyser, O. (
Laskowski, M.J., Williams, M.E., Nusbaum, H.C., and Sussex, I.M. (
López-Bucio, J., Cruz-Ramírez, A., and Herrera-Estrella, L. (
López-Bucio, J., Hernández-Abreu, E., Sánchez-Calderón, L., Nieto-Jacobo, M.F., Simpson, J., and Herrera-Estrella, L. (
López-Bucio, J., Hernández-Abreu, E., Sánchez-Calderón, L., Pérez-Torres, A., Rampey, R., Bartel, B., and Herrera-Estrella, L. (
Malamy, J.E. (
Malamy, J.E., and Benfey, P.N. (
Martínez-Trujillo, M., Limones-Briones, V., Cabrera-Ponce, J.L., and Herrera-Estrella, L. (
Misson, J., et al. (
Nacry, P., Canivenc, G., Muller, B., Azmi, A., Van Onckelen, H., Rossignol, M., and Doumas, P. (
Navarro, L., Dunoyer, P., Jay, F., Arnold, B., Dharmasiri, N., Estelle, M., Voinnet, O., and Jones, J. (
Neumann, G., and Martinoia, E. (
Okushima, Y., Fukaki, H., Onoda, M., Theologis, A., and Tasakaa, M. (
Okushima, Y., et al. (
Oono, Y., Chen, Q.G., Overvoorde, P.J., Köhler, C., and Theologis, A. (
Ortega-Martínez, O., Pernas, M., Carol, R.J., and Dolan, L. (
Ouellet, F., Overvoorde, P.J., and Theologis, A. (
Raghothama, K.G. (
Reed, R.C., Brady, S.R., and Muday, G.R. (
Ruegger, M., Dewey, E., Gray, W.M., Hobbie, L., Turner, J., and Estelle, M. (
Sánchez-Calderón, L., López-Bucio, J., Chacón-López, A., Cruz-Ramírez, A., Nieto-Jacobo, F., Dubrovsky, J.G., and Herrera-Estrella, L. (
Sánchez-Calderón, L., López-Bucio, J., Chacón-López, A., Gutiérrez-Ortega, A., Hernández-Abreu, E., and Herrera-Estrella, L. (
Svistoonoff, S., Creff, A., Reymond, M., Sigoillot-Claude, C., Ricaud, L., Blanchet, A., Nussaume, L., and Desnos, T. (
Ticconi, C.A., and Abel, S. (
Tiwari, S.B., Wang, X.J., Hagen, G., and Guilfoyle, T.J. (
Torrey, J.G. (
Tsugeki, R., and Fedoroff, N.V. (
Ulmasov, T., Murfett, J., Hagen, G., and Guilfoyle, T.J. (
Vance, C.P., Uhde-Stone, C., and Allan, D.L. (
Ward, J.T., Lahner, B., Yakubova, E., Salt, D.E., and Raghothama, K.G. (
Williamson, L.C., Ribrioux, S.P., Fitter, A.H., and Leyser, H.M. (
Wilmoth, J.C., Wang, S., Tiwari, S.B., Joshi, A.D., Hagen, G., Guilfoyle, T.J., Alonso, J.M., Ecker, J.R., and Reed, J.W. (
Woodward, A.W., and Bartel, B. (
Zenser, N., Ellsmore, A., Leasure, C., and Callis, J. (
Zhang, H., Jennings, A., Barlow, P.W., and Forde, G.B. (
Author notes
Current address: Instituto de Investigaciones Químico-Biológicas, Universidad Michoacana de San Nicolás de Hidalgo, Ciudad Universitaria, CP 58030 Morelia, Michoacán, Mexico.
Current address: Department of Biology, Faculty of Science, Utrecht University, Padualaan 8, 3584 CH Utrecht, The Netherlands.
Current address: Department of Biology, Texas State University, 601 University Drive, San Marcos, TX 78666.
Address correspondence to [email protected].
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Mark Estelle ([email protected]).
Some figures in this article are displayed in color online but in black and white in the print edition.

Online version contains Web-only data.

Open Access articles can be viewed online without a subscription.






