-
PDF
- Split View
-
Views
-
Cite
Cite
T P Sarun, Jagadish S Patil, First report of the rare tintinnid genus Stelidiella Kofoid and Campbell 1929 in the Indian Ocean, Journal of Plankton Research, Volume 46, Issue 2, March/April 2024, Pages 224–227, https://doi.org/10.1093/plankt/fbae001
Close - Share Icon Share
Abstract
This study provides detailed information on the morphometry and distribution of some rare tintinnids species found in the Indian Ocean. The morphological features (scabbard-shaped with a fenestrated collar) reported here resemble those of the genus Stelidiella. Here, lorica morphology (fenestra arrangement and oral margin (OM) pattern) was the sole criteria for Stelidiella species identification. The dimensions and morphology (presence of two layers of fenestrae rings with smooth OMs) resemble the original description of Stelidiella fenestrata, a warm-water species. We found both small (260 μm long) and large S. fenestrata (309 μm long) with smaller and larger bowls in the former and latter, respectively. In the Indian Ocean, S. fenestrata was more abundant (i) between 12oN and 18oS (particularly, central Indian Ocean) similar to that reported in the Pacific Ocean; (ii) in the mesopelagic zone (100–1000 m) than the epipelagic zone (0–100 m) and (iii) in the winter season of December 2021–January 2022 than in spring inter-monsoon of March–May 2021. The S. fenestrata, despite being in low abundance (2–26 individuals 10 m−3), is widespread in the region, and their role in the ecosystem merits further investigation.
INTRODUCTION
Tintinnids are planktonic ciliates belonging to the phylum Ciliophora, Class Spirotrichea (Lynn, 2008). As part of microzooplankton (20–200 μm), tintinnids mainly feed on smaller plankton (picoplankton and nanoplankton), making them an important pathway of energy transfer to higher trophic levels (Dolan, 2013). The order Tintinnida currently comprises 77 genera and 14 families, which include marine, estuarine and freshwater planktonic ciliates (Santoferrara and McManus, 2020). The main characteristic of tintinnids is the presence of a lorica, which also forms the basis for the identification of tintinnid groups (Dolan, 2000) despite its frequent intraspecific variability and interspecific similarity (Santoferrara et al., 2016). The lorica is thought to provide protection from predators, aids in sinking and filter feeding (Agatha et al., 2013). Over 900 species are described to date, and biogeographies of tintinnids have been described (Pierce and Turner, 1993; Dolan and Pierce, 2013).
While many species records exist on oceanic tintinnids, information on species belonging to the tintinnid genera Stelidiella is very scarce. The first report of Stelidiella was recorded by the German scientist Richard Biedermann in 1893 during the Plankton Expedition from the North Equatorial Current of the Atlantic. He described a species as Tintinnus stelidium, member of a genus which is no longer accepted. A few decades later, Kofoid and Campbell (1929, 1939) published the plankton data collected during the Agassiz expedition (1904–1905), wherein a new subfamily Stelidiellineae was placed in the Tintinnidae Claparède and Lachmann 1858 family. Stelidiellineae contained three genera, viz. Ormosella, Brandtiella and Stelidiella. The genus Stelidiella was described as containing four species (S. fenestrata, Stelidiella phialia, Stelidiella simplex and Stelidiella stelidium). To date, the first three species (S. fenestrata, S. phialia and S. simplex) have been found only by Kofoid and Campbell in the Pacific Ocean (Kofoid and Campbell, 1939) and S. stelidium has been found in the Atlantic Ocean only by Biedermann (1893) and Brandt (1907). Here, for the first time, the characterization (Fig. 1) and distribution (Fig. 2) of the extremely rare tintinnid species, S. fenestrata, in the Indian Ocean are documented.
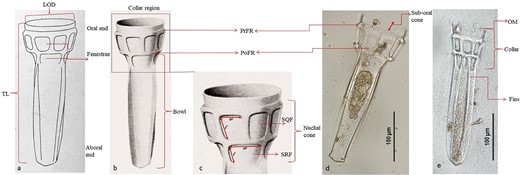
(a) Stelidiella fenestrata from Kofoid and Campbell (1929); (b) S. fenestrata from Kofoid and Campbell (1939); (c) expanded view of S. fenestrata Collar region (Kofoid and Campbell, 1939) showing length (l) and width (w) of fenestral windows, i.e. sub-quadrangular fenestra (SQF); sub-rectangular fenestra (SRF) as well as Nuchal cone; (d) S. fenestrata from the current study showing sub-oral cone; (e) S. fenestrata showing OM, collar and fins.
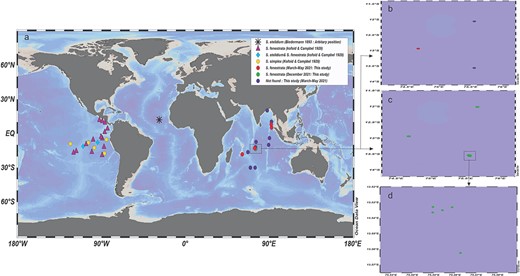
(a) Occurrences of Stelidiella species in world oceans based on literature and observations in the Indian Ocean. (b) Distribution of Stelidiella during March–May 2021. (c) Distribution of S. fenestrata during the December 2021–January 2022 cruise. (d) Elaborating the nearby stations during the December 2021–January 2022 cruise.
METHOD
The present study is based on the plankton samples collected during two cruises (March–May 2021 and December 2021–January 2022) onboard R.V. Sindhu Sadhana from the Indian Ocean (Fig. 2). In the first and second cruises, 14 (representing different regions) and 7 (close-by stations in the central region) stations were sampled in the Indian Ocean, respectively. The plankton sample was collected from various layers (up to five) between the surface to the near bottom (Supplementary Table S1) by using a Hydrobios multi-plankton net (100-μ mesh size; 0.25 m2 mouth area). Collected samples were preserved with 4% formalin in a 500-mL plastic container. A known volume of samples (5 mL in duplicates) was analyzed using an inverted microscope (Olympus IX 73; magnification: 100×–400×) equipped with a digital camera. The species identification was based on lorica descriptions found in the literature (Biedermann,1893; Brandt, 1907; Kofoid and Campbell, 1929, 1939). The abundance was estimated using the volume filtered to the number of individuals counted. Documentation of lorica morphology and morphometry is paramount for tintinnid diversity studies. In view of this, a total of 18 individuals Stelidiella (n = 18), of which 8, and 10 individuals from March to May 2021 and from December 2021 to January 2022 samplings, were selected for morphometric measurements. The morphometry details of each individual along with micrographs of some individual specimens are provided in Supplementary Fig. S1 and Supplementary Table S2 for reference.
RESULTS AND DISCUSSION
Morphology and morphometry
The morphological features of the tintinnid reported here resemble that of the description of marine tintinnid belonging to the genus Stelidiella described by Kofoid and Campbell (1929, 1939). Stelidiella is a scabbard-shaped and hyaline-walled loricate with an elongated body that is wider at the oral end and narrower aborally (Fig. 1a and b). The collar is truncated and consists of sub-oral and nuchal cones with single or double rows of latticed fenestra. Oral margin (OM) is with or without any serration. The bowl is constructed with four longitudinal fins. Genus Stelidiella differs from other genera of the subfamily Stelidiellineae (Prostelidiella, Brandtiella and Ormosella) by the presence of the latticed fenestra and the same was evident in the tintinnid specimen reported here. All three reported Stelidiella species (S. stelidium, S. simplex and S. fenestrata) can be easily differentiated under the microscope by looking at key lorica morphological characteristics, i.e. the arrangement of fenestra and the presence of serration in the OM (Kofoid and Campbell, 1939). The S. stelidium has a single row of eight quadrangular fenestra and oral serration. The S. simplex has a single row of fenestra without any oral serration. The presence of two layers of fenestrae rings with smooth OMs is an important morphological feature for S. fenestrata identification and was the basis for species identification in this study (Fig. 1c). Recently, updated procedures (using morphological, molecular and ecological data) for Tintinnid identification have been suggested, but adopting this is challenging particularly for the low abundance of rare tintinnids in oceanic samples (Santoferrara et al., 2016) as in this study. Given this limitation, lorica morphology will be a crucial criterion and was straightforward in identifying Stelidilella species in this study.
The S. fenestrata presented here is wider at the oral end and narrower aborally (Fig. 1a). The OM is free of any oral serrations. Nuchal-cone has two rows of latticed fenestrae, the upper row with eight sub-quadrangular windows and the lower row with four rectangular windows (Fig. 1b and c). Vertical frames separated each fenestra. The diameter of the pre-fenestral ring (PrFR) is larger than the post-fenestral ring (PoFR) (Fig. 1b and d). Two pairs of fins in opposite directions connect continuously across the aboral end, which starts from the PoFR (Fig. 1e). The lower bowl is four-sided, with a pair of wider faces and a pair of narrow faces (Fig. 1b). The aboral end is rounded in a wider face and recessed concavely in a thinner face (Fig. 1a and b). These observations support that the current specimen’s morphology is in accordance with the original description (Fig. 1a–e).
The morphometric study revealed that the average dimensions of the current specimen, such as total length (TL): 288.47 ± 13.10 μm, lorica oral diameter (LOD): 94.72 ± 2.51 μm and bowl length: 219.73 ± 11.08 μm as well as diameters of PrFR and PoFR: 102.06 ± 3.39 and 85.84 ± 2.77 μm, respectively, were also similar to the original description (Supplementary Table S2).
Lorica morphology not only aids in tintinnid identification but also provides information on its ecology. For instance, the LOD in tintinnids is considered as a conservative taxonomic characteristic (Laval-Peuto and Brownlee, 1986) and is related to the preferred prey size ingested i.e. 45% (largest size) and 25% (preferred size) of the LOD (Dolan et al., 2002). The average LOD and the average TL are 94.72 and 288.47, respectively. Given this, the largest and preferred (removed at maximum rates) prey ingested by S. fenestrata, in the central Indian Ocean, are inferred to be ~44 and ~24.5 μm, respectively. Interestingly, this study reports the smallest (260.30 μm) and largest S. fenestrata (309.90 μm) due to the presence of smaller and larger bowls in the former and latter, respectively. The previous size range for S. fenestrata TL was 274–303 μm (Kofoid and Campbell, 1939). In addition to the above similarity with the original description, the current specimen showed some minor differences with certain measurements (e.g. sub-oral cone) from the original description. For instance, the current specimen sub-oral cone is smaller (20.45 ± 1.03 μm) than the original description (24.3 μm) (Fig. 1d). Further, the dimensions of additional taxonomical characteristics, which are not documented in the original description, such as fenestral windows—sub-quadrangular window length: 32.36 ± 3.20 μm; width: 25.87 ± 2.62 μm; sub-rectangular window length: 24.49 ± 2.22 μm; width: 37.60 ± 1.70 μm; collar: 96.58 ± 4.88 μm; nuchal cone: 77.13 ± 4.35 μm; distance between PrFR and PoFR: 43.65 ± 3.06 μm; the widths of the broad and narrow sides of the bowl: 76.47 ± 3.71 and 61.70 ± 3.55 μm, respectively, are included in this study for future reference (Fig. 1b, c and e). Further, the overall morphometric variations between individuals showed larger variability during December 2021–January 2022 than during March–May 2021, and this was due to the presence of the smallest and largest individuals in the former period (Supplementary Table S2).
Distribution
In this study, S. fenestrata showed distinct spatial, depth and temporal variations in the occurrences. For instance: (i) spatial—of the 14 stations sampled, S. fenestrata occurred in only 4 stations, i.e. 2 each in the central and near north equatorial Indian Ocean, respectively (during March–May 2021 cruise) with the abundance being higher in the latter than the former; (ii) depthwise—S. fenestrata was recorded in both epipelagic, i.e. surface–100 m depth (only during the winter season) and mesopelagic zones, i.e. 100–1000 m depth (during both seasons), but the abundance was significantly higher in the latter than the former; and (iii) temporal—in the central Indian Ocean, the frequency of occurrences and the abundance of S. fenestrata was much higher in the winter season (December 2021–January 2022) than in the spring inter-monsoon (March–May 2021) irrespective of the regions (including north equatorial region).
The genus Stelidiella has been reported in three oceans (Atlantic Ocean, Eastern-Pacific Ocean and Indian Ocean; Fig. 2). The first report of S. stelidium is from the Atlantic Ocean, but the location, number of individuals and sampling depth were not mentioned (Biedermann,1893). From the Pacific Ocean, 26 Stelidiella individuals from 0 to 548 m and 0 to 1463 m depths were recorded; among them, S. fenestrata was dominant (19 individuals from 18 stations), followed by S. simplex (5 individuals from 5 stations) and S. stelidium (2 individuals from 2 stations) (Kofoid and Campbell, 1939). Here, 2–26 individuals 10 m−3 of S. fenestrata were recorded from stations at various depths (Supplementary Table S1). Interestingly, S. fenestrata was generally found in mesopelagic(100–1000 m) during March–May 2021, but in one station i.e. 12.93oS 74.67°E (also known for polymetallic nodules occurrences), it was recorded at epipelagic zone (0–100 m) from December 2021 to January 2022, indicating distinct seasonal variation.
Tintinnids are among the few protist groups that have clear biogeographical patterns (cosmopolitan, neritic, warm-water, boreal and austral) at the genus level (Dolan and Pierce, 2013). However, the Stelidiella species are classified as warm types (Pierce and Turner, 1993), and their distribution in the Pacific Ocean is restricted to 12oN–18oS. In the Indian Ocean, S. fenestrata was found in the stations between 7oN and 18oS (Fig. 2), and the sea-surface temperature ranged between 27.9 and 29.8°C. Despite several studies on tintinnid biogeography, Stelidiella species have not been documented from the Indian Ocean (Pierce and Turner, 1993; Liu et al., 2012; Dolan and Pierce, 2013; Asha Devi et al., 2018, 2020; Sai Elangovan and Gauns, 2018). A couple of Tintinnid community studies exist between 7oN and 18oS, but Stelidiella species are not recorded (Modigh et al., 2003; Zhang et al., 2017). Under-sampling strategy (i.e. sampling only from the upper water column or sampling a small volume for enumeration) could be one of the reasons for the lack of records of Stelidiella.
CONCLUSION
The S. fenestrata, which was recorded for the first time in the Indian Ocean, showed similar morphological characteristics and latitudinal distribution to that reported in the Pacific Ocean. This study provides detailed information on the description, morphometry and distribution of S. fenestrata found in the Indian Ocean. The study highlights the importance of vertical sampling of large volumes for the enumeration of rare tintinnids such as Stelidiella from the Indian Ocean, particularly in 7oN–18oS. Despite low abundance, S. fenestrata is widespread in the central Indian Ocean and warrants further investigation into its role in ecosystem functioning.
Acknowledgements
The authors are grateful to the Director, of CSIR-National Institute of Oceanography (Goa, India), for his support and encouragement. The authors thank Dr Samir Damare, Mr Pranoy Paul, Mr Sathish K., Mr Milind, Mr Anthony, Mr Kallaithan, Mr Kuldeep and the RV Sindhu Sadhana crew (SSD079 and SSD086) for their help in sampling. We thank the three anonymous reviewers for their valuable suggestions for improving the manuscript. This paper is NIO contribution number 7182.
FUNDING
CSIR-National Institute of Oceanography—TraceBioMe (MLP2018); Ministry of Earth Sciences, New Delhi under Poly metallic Nodule Program (GAP2128).
DATA AVAILABILITY
All data are incorporated into the article and its online supplementary material.


