
Volume 31, Issue 4
April 2018
Cover image
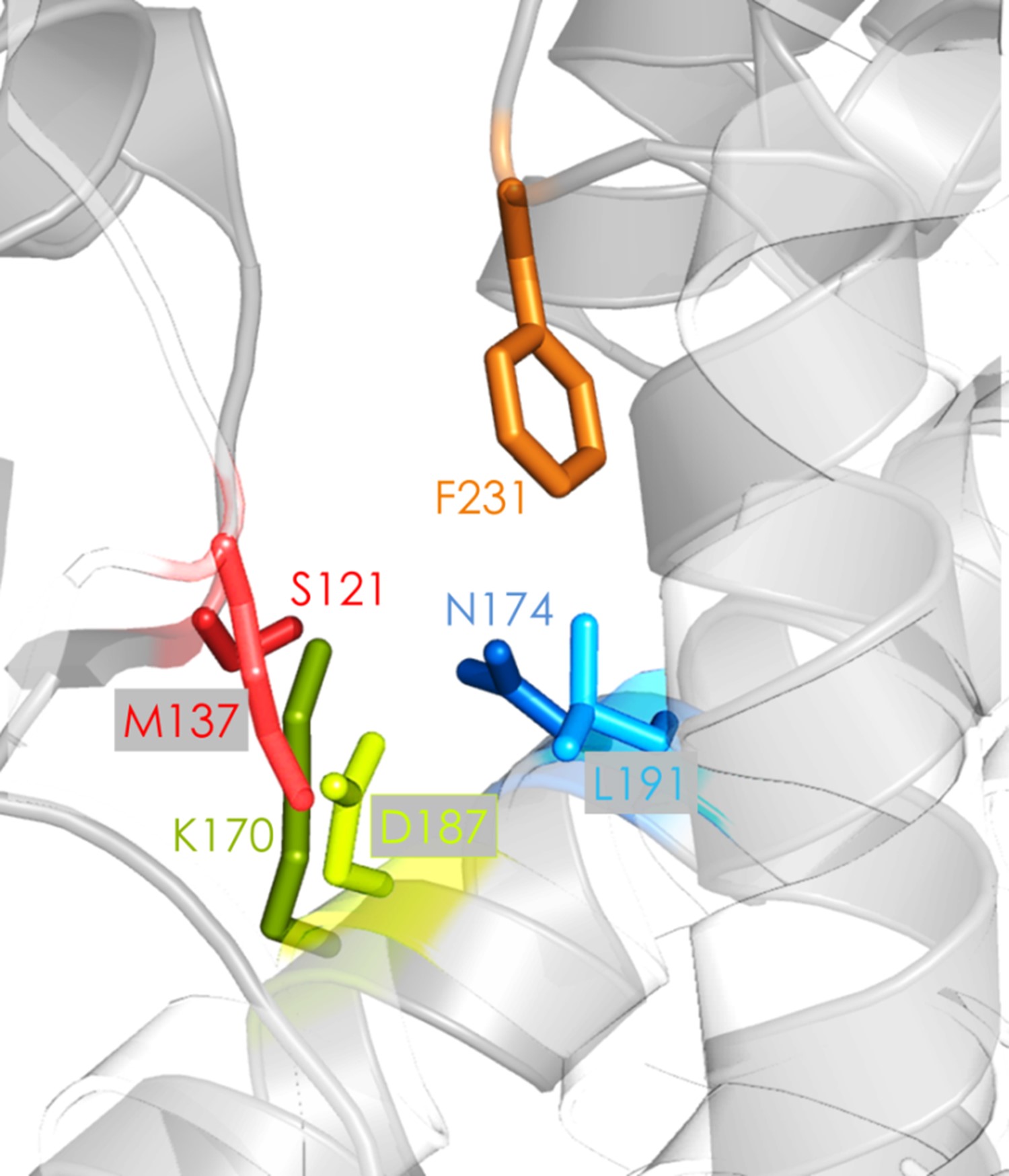
Cover image

cover illustration Homologous imine reductases (IRED) and b-hydroxyacid dehydrogenases(bHADs) with similarly oriented active site residues were engineered for generating novel imine-reducing enzymes. Active site amino acid residues of bHADs were substituted with the
corresponding residues from a R-selective IRED and then screened for promiscuous imine reduction activity. Analysis of the selected variants demonstrated how active site mutations
alter the chemistry of IREDs and bHADs.
ISSN 1741-0126
EISSN 1741-0134
Issue navigation
Volume 31, Issue 4, April 2018
Short Communication
Creation of a formate: malate oxidoreductase by fusion of dehydrogenase enzymes with PEGylated cofactor swing arms
Harun F Ozbakir and others
Protein Engineering, Design and Selection, Volume 31, Issue 4, April 2018, Pages 103–108, https://doi.org/10.1093/protein/gzy005
Original Articles
New imine-reducing enzymes from β-hydroxyacid dehydrogenases by single amino acid substitutions
Maike Lenz and others
Protein Engineering, Design and Selection, Volume 31, Issue 4, April 2018, Pages 109–120, https://doi.org/10.1093/protein/gzy006
Towards conformational fidelity of a quaternary HIV-1 epitope: computational design and directed evolution of a minimal V1V2 antigen
Jennifer I Lai and others
Protein Engineering, Design and Selection, Volume 31, Issue 4, April 2018, Pages 121–133, https://doi.org/10.1093/protein/gzy010
A platform for chemical modification of mandelate racemase: characterization of the C92S/C264S and γ-thialysine 166 variants
Mitesh Nagar and others
Protein Engineering, Design and Selection, Volume 31, Issue 4, April 2018, Pages 135–145, https://doi.org/10.1093/protein/gzy011
Advertisement
Advertisement

