-
PDF
- Split View
-
Views
-
Cite
Cite
Mei Fong Liew, Hui Fang Lim, Mui Cheng Liang, Ives Lim, Zhaohong Tan, Rachel Ying Min Tan, Qi Hui Sam, Win Mar Soe, Sen Hee Tay, Shengli Xu, Matthew Wook Chang, Roger Foo, Tuck Wah Soong, Sharada Ravikumar, Louis Yi Ann Chai, Dominant Negative TRAF3 Variant With Recurrent Mycobacterium abscessus Infection and Bronchiectasis, Open Forum Infectious Diseases, Volume 9, Issue 8, August 2022, ofac379, https://doi.org/10.1093/ofid/ofac379
Close - Share Icon Share
Abstract
Host factors leading to pulmonary nontuberculous mycobacteria (PNTM) disease are poorly understood compared with disseminated NTM disease, which is linked to the interleukin 12–interferon gamma signaling pathway. We investigated the tumor necrosis factor receptor associated factor 3 (TRAF3) R338W variant in a patient with recurrent PNTM infection, demonstrating TRAF3- and TNF-α-deficient phenotypes via ex vivo immune and cloning-transfection cellular studies.
Host factors predisposing to nontuberculous mycobacteria (NTM) infection are progressively being recognized. The vast majority of these are immune defects linked to the interleukin 12 (IL-12)–interferon gamma (IFN-γ) pathway, which orchestrates the innate, myeloid, and type 1 T helper cell (Th1) effector arms of the host response against Mycobacteria [1]. Here, mutations of at least 12 genes—IL-12B, IL-12RB1, IL-12RB2, ISG-15, TYK-2, IRF-8, SPPL-2A, CYBB, IFNGR-1, IFNGR-2, STAT-1, and NEMO—have been reported to cause the spectrum of Mendelian susceptibility to mycobacterial disease (MSMD) [2]. The clinical manifestations previously described, however, have invariably been disseminated or extrapulmonary NTM disease in young patients. Factors leading to isolated pulmonary NTM (PNTM) disease are less understood and have been attributed to (i) underlying structural parenchymal lung abnormalities including chronic obstructive pulmonary disease, post-tuberculosis cavitatory lung disease, or (ii) primary ciliary disease and cystic fibrosis [1, 3]. We describe here a case of pulmonary Mycobacterium abscessus disease with cavitatory bronchiectasis linked to a novel TRAF3 dominant negative variant with reduced TRAF3 expression and TNF-α secretion.
METHODS
Peripheral blood mononuclear cells (PBMCs) were isolated by density centrifugation on Ficoll-Paque, washed, resuspended in RPMI, and stimulated with lipopolysaccharide (LPS), Pam3Cys (P3C), Poly I:C, or Mycobacterium abscessus extract for 24 hours. The culture supernatants were assayed for cytokine production by enzyme-linked immunosorbent assay (ELISA). To preparethe TRAF3 Western blot, the cells were pelleted after 2 hours of stimulation and lysed using RIPA buffer. Equal amounts of protein from cell lysate were run on 10% SDS-polyacrylamide gel, transferred to PVDF membrane, and probed for TRAF3 (∼65 kDa) using sc-1828 antibody (Santa Cruz Biotechnology [SCBT]). β-actin (∼42 kDa) was the loading control. R338W and WT TRAF3 were amplified from patient cDNA and cloned into pGEM-T Easy (Promega). The polymerase chain reaction primers for the TRAF3 region of interest were forward–CTGAACACTTGCCACCTGTC, reverse–AACGTTTGTCTTTGCTGGGG. The amplified 684 base-pair fragment was sequenced and analyzed with Unipro UGENE software. Subcloning was performed in pIRES-EGFP vector (Clontech/TaKaRa Bio) via EcoRI/SalI sites. Sanger sequencing was repeated to confirm the subcloned plasmids. Transfection was carried out in HEK293FT cells and RAW264.7 cells using the Lipofectamine 3000 Reagent protocol in 6-well or 24-well plates with DNA-lipid complex added to the cells, with high transfection efficiency demonstrated (Supplementary Figure 1). The transfected cells were stimulated with LPS or Poly I:C, and Western blot analysis was performed as described. The transfected RAW264.7 cells were stimulated with LPS or NTM, and ELISA was performed on the supernatants. Full details of the experimental methodology are described in the Supplementary Data.
RESULTS
The patient was a 51-year-old female nonsmoker of Asian origin with recurrent Mycobacterium abscessus pulmonary infections over 6 years with progressive severe cavitary bronchiectasis. A timeline of the patient’s clinical course is summarized in Supplementary Figure 2. First diagnosed in 2014, the patient was started on multiple lines of antibiotics consisting of amikacin, clarithromycin, cefoxitin, clofazimine, and linezolid, with relapses whenever her regimen was simplified to 2 drugs. In 2015, she underwent a right lower lobectomy for refractory M. abscessus infection. Pathological examination revealed bronchiectasis, consolidation, and cavitation with multiple foci of necrotizing granulomatous inflammation. An operative specimen culture grew M. abscessus, and she continued on treatment for 9 more months. Seventeen months after stopping treatment, she had a relapse of pulmonary M. abscessus infection in 2019 with worsening chronic productive cough, hemoptysis, and weight loss, but she declined treatment. Clinical assessment did not reveal evidence of primary ciliary dyskinesia, chronic rhinosinusitis, or situs inversus. She had background hepatitis B chronic infection, for which she had received entecavir treatment, and there was a paternal history of pulmonary tuberculosis. Laboratory studies revealed no lymphopenia. She had normal levels of the immunoglobulin (Ig)A, IgM, IgE, IgG subclasses and tested negative for antinuclear antibodies, extractable nuclear antigen antibodies, and anti-IFN-γ autoantibodies [4].
Functional immune studies using the patient’s PBMCs showed diminished TNF-α and IL-6 response. This was mostly clearly seen with Poly I:C (via TLR3), partly seen through LPS (through TLR4) (Figure 1A), and less with Pam3Cys (TLR2). Cytokine response to M. abscessus was markedly reduced. Overall, IL-1β production was least affected. Whole-exome sequencing (WES) of the patient did not reveal rare nonsynonymous coding variants of the genes linked to MSMD, CFTR, and primary ciliary dyskinesia. However, WES identified the presence of a hemizygous missense variant in TRAF3, at exon 11 c.1012C > T, confirmed by Sanger sequencing (Figure 1B). This substitution resulted in amino acid change from arginine to tryptophan at position 338 of the mature protein (R338W) at a conserved position, involving the coiled-coil domain of the protein. The alternate allele frequency was 2.78e-05 in gnomAD. In silico analyses predicted this R338W variant to be deleterious (SIFT score 0.00, PolyPhene2 score 0.972, CADD score 29.6). Other than TRAF3, no other rare variants of genes linked to the TLR3 pathway (TLR3, TICAM1, UNC93B1, TBK1, and IRF-3) [5] were picked up.
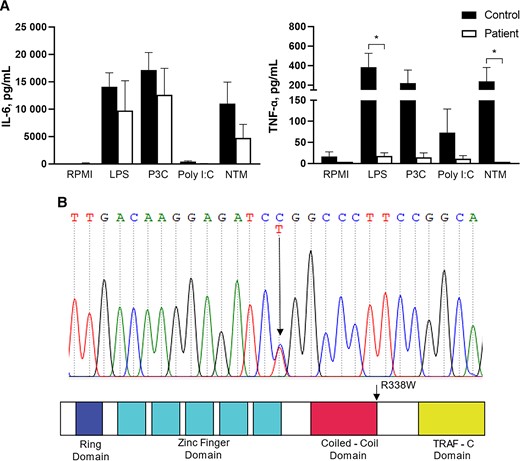
A, Peripheral blood mononuclear cells from the patient (white bar, patient) and healthy volunteers (black bar, control, n = 7) were stimulated with LPS, P3C, Poly I:C and Mycobacterium abscessus (NTM), or RPMI. Culture supernatants were harvested, and concentrations of IL-6 and TNF-α were measured. Data were pooled from 3 experiments. *P < .05 based on Mann Whitney U test. B, Sanger sequence demonstrating the heterozygous cytosine C to thymine T switch at the c.1012 exon 11 of TRAF3 in the proband and location of R338W in the predicted structure by domains of TRAF3. Abbreviations: IL-6, interleukin 6; LPS, lipopolysaccharide; NTM, nontuberculous mycobacteria; P3C, Pam3Cys; TNF-α, tumor necrosis factor alpha.
To verify this finding, Western Blot was performed, demonstrating consistently diminished TRAF3 expression in the patient’s PBMCs when stimulated with Poly I:C and LPS (Figure 2A). TRAF3 R338W was cloned, transfected along with TRAF3 WT and empty vector in HEK 293FT cells, and stimulated with LPS and Poly I:C. The TRAF3 R338W–transfected cells showed markedly reduced expression of TRAF3 compared with WT-transfected cells (Figure 2B). To attempt to elucidate the dominant negative effect of the variant, RAW 264.7 cells transfected with equal amounts of TRAF3 WT and TRAF3 R338W plasmids demonstrated diminished TRAF3 expression (Figure 2C). Furthermore, TRAF3 R338W–transfected RAW 264.7 cells showed significantly attenuated TNF-α production in response to LPS and M. abscessus compared with TRAF3 WT (Figure 2D), mirroring the patient’s PBMC response (Figure 1A).
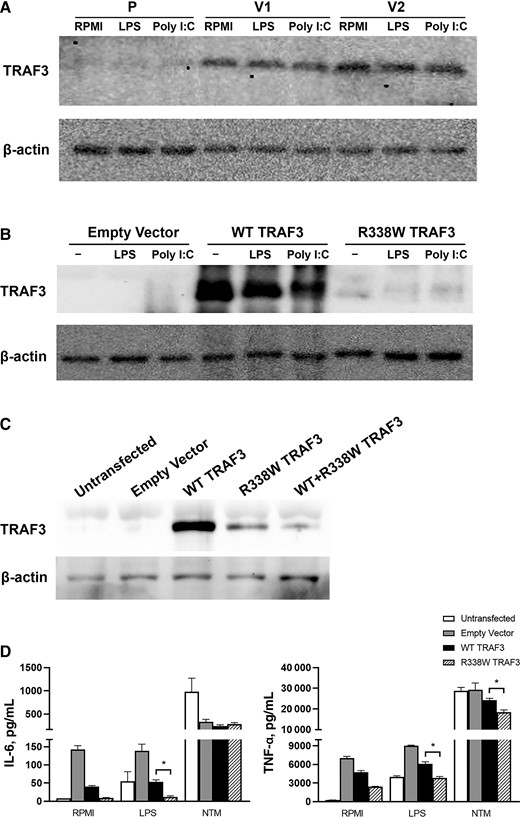
A, Western blot for TRAF3 in PBMCs from the patient (P) and healthy volunteers (V1 and V2). PBMCs were isolated from the patient and healthy volunteers and were unstimulated (RPMI) or stimulated with LPS or Poly I:C and showed diminished TRAF3 expression in patient cells. Data representative of 2 independent experiments are shown. B, Empty vector, wild-type (WT TRAF3), and mutant (R338W TRAF3) plasmids were transfected in HEK 293FT cells. Twenty-four hours after transfection, cells were stimulated with LPS or Poly I:C for 24 hours. Western blot analysis demonstrated diminished TRAF3 expression in transfected R338W cells compared with WT. Data are representative of 2 independent experiments. C, Empty vector, wild-type (WT TRAF3), mutant (R338W TRAF3) plasmids were transfected in RAW264.7 cells. The cells were also transfected with equal amounts of both plasmids (WT + R338W TRAF3). Western blot was performed on the cells 48 hours after transfection. Diminished TRAF3 expression was observed in R338W and WT + R338W TRAF3–transfected cells compared with WT. Data representative of 2 independent experiments are shown. D, Empty vector, wild-type (WT TRAF3), and mutant (R338W TRAF3) plasmids were transfected in RAW264.7 cells. The transfected cells were stimulated after 24 hours with LPS or NTM. ELISA for mouse IL-6 and TNF-α was performed on the cell supernatants 24 hours after stimulation. Data were pooled from 2 experiments. *P < .05 based on Mann-Whitney U test. Abbreviations: ELISA, enzyme-linked immunosorbent assay; IL-6, interleukin 6; LPS, lipopolysaccharide; NTM, nontuberculous mycobacteria; PBMC, peripheral blood mononuclear cell; TNF-α, tumor necrosis factor alpha; WT, wild-type.
The patient was lost to follow up and presented in 2020 with progression of disease. Alongside symptoms of dyspnea, hemoptysis, and marked weight loss, the lungs showed diffuse consolidation and bronchiectasis with persistent elucidation of M. abscessus. She was recommenced on a treatment regimen of intravenous amikacin, cefoxitin, and oral clofazimine. Her hospitalization was complicated by hydropneumothorax with superimposed Pseudomonas aeruginosa and Aspergillus fumigatus pneumonia. The patient eventually succumbed to the disease, with a request to not escalate treatment.
DISCUSSION
TRAF3 deficiency has been ascribed to immunodeficiency through the TLR3 pathway, resulting in herpes simplex encephalitis (HSE) [6]. We demonstrate here a novel heterozygous TRAF3 mutation with a dominant negative effect in an adult patient with recurrent PNTM infection, showing that the clinical sequelae of TRAF3 deficiency may well extend beyond viruses to susceptibility to bacterial pathogens. This is not unanticipated given that recently TRAF3 has been implicated in host defense against nontuberculous Mycobacteria [7]. TRAF3 is a central mediator of signal transduction downstream of not just TLR3, which recognizes viral RNA, but also TLR4, through TRIF as well as MyD88, resulting in both MAPK and NF-κB activation [8]. In line with this, we were able to show reduction of TNF-α through TLR3 (Poly I:C) and TLR4 (LPS) ligands over reduction of TLR2 (Pam3Cys, which signals solely through MyD88). The clear attenuation of TNF-α production was seen both in the patient and in TRAF3 R338W–transfected cells, as well as in response to stimulation by M. abscessus. This sequelae of diminished TNF-α from TRAF3 R338W in conjunction with localized PNTM disease may be relatable, taking reference from a Food and Drug Administration (FDA) MedWatch database of reportable NTM infection cases in patients receiving anti-TNF treatment, in which the majority had a pulmonary site of disease [9].
The previously described TRAF3 R118W defect causing HSE, located in the first zinc finger domain of the protein, was shown to result in loss of function through a dominant negative effect [6]. The finding of the TRAF3 R338W heterozygous mutation in the current case, located at end of the coiled-coil domain of the protein, is notable for 2 reasons. Mutations in the TRAF3 coiled-coil region have been shown to affect cellular signaling through a dominant-negative effect, which we in turn have also attempted to demonstrate with co-transfection studies [10]. Furthermore, the TRAF3 coiled-chain domain has been recognized to serve as an adaptor molecule for NF-κB activation [11].
Susceptibility to intracellular pathogens like Candida infection is known to differ between disseminated and organ-specific disease [12]. Likewise, this experiment in the nature of an immunodeficient patient points us to looking beyond traditional IL-12–IFN-γ signaling to involvement of alternative pathways like TLR3–TRAF3, either directly in host pathogen recognition or indirectly through their influence on ciliary function [13] in PNTM disease.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. L.Y.A.C. was supported by the Clinician Scientist Awards (CSA Senior Investigator and Investigator), Individual Research Grant (IRG), Bedside and Bench (B&B) Grants, Centre Grant, and the Training Fellowship Award from the National Medical Research Council (NMRC), Singapore. L.Y.A.C. also acknowledges the Aspiration Grant and Summit Research Program, iHealthtech Grant, Bench to Bedside Grant, and Seed Fund from the National University Health System, Infectious Diseases and Synthetic Biology Translational Research Program of the National University of Singapore, and the Synthetic Biology Research and Development Program of the National Research Foundation, Singapore. H.F.L. was supported by the Clinician Scientist-Individual Research Grant-New Investigator Grant (CS-IRG-NIG) from NMRC, Singapore. The authors acknowledge funding from Alexandra Hospital for this manuscript.
Author contributions. M.F.L., H.F.L., S.H.T., T.W.S., S.X., S.R., and L.Y.A.C. conceived the study and participated in its design and coordination. M.C.L., I.L., Z.T., R.Y.M.T., Q.H.S., W.M.S., and S.R. performed the experiments. M.F.L., H.F.L., S.H.T., M.W.C., R.F., T.W.S., S.R., and L.Y.A.C. participated in the interpretation of data and revised the paper critically. All authors read and approved the final manuscript.
Patient consent. Informed consent was obtained from the patient and healthy volunteers to participate in the study, which was approved by the National Healthcare Group Domain Specific Research Board (2007/00517).
References
Author notes
Mei Fong Liew, Hui Fang Lim, Sharada Ravikumar and Louis Yi Ann Chai shares equal authorship.
Potential conflicts of interest. The authors declare that they have no conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
- phenotype
- tumor necrosis factors
- lung
- bronchiectasis
- nontuberculous mycobacteria
- cloning
- immunocompromised host
- interferon type ii
- recombinant interferon-gamma
- interleukin-12
- tumor necrosis factor receptor
- transfection
- infections
- lung infections
- host factor
- signal pathway
- signal transduction pathways
- dominant-negative mutation
- mycobacterium abscessus infections





Comments