-
PDF
- Split View
-
Views
-
Cite
Cite
Guillaume Vial, Laure Barthod, Sophie Schneider, Patrick Mercié, Pierre Duffau, Agathe Vermorel, Emmanuel Ribeiro, Multisystem T-cell Chronic Active Epstein-Barr Virus Infection: From the Eye to the Kidney, Open Forum Infectious Diseases, Volume 9, Issue 8, August 2022, ofac351, https://doi.org/10.1093/ofid/ofac351
Close - Share Icon Share
Abstract
Chronic active Epstein-Barr virus (CAEBV) infection is usually a fatal disease associated with clonal proliferation of EBV-infected T or NK cells. We present the case of a 33-year-old Peruvian patient who developed a multisystem CAEBV, notably responsible for exceptional ophthalmological and renal damage. We describe the clinicopathological features of EBV-induced lymphoproliferative disorder.
Primary Epstein-Barr virus (EBV) infection is usually asymptomatic or sometimes causes infectious mononucleosis, which resolves spontaneously after an adapted specific immune response. Chronic active EBV (CAEBV) infection is a rare and heterogenous syndrome characterized by recurrent or prolonged infectious mononucleosis-like symptoms associated with elevated peripheral blood EBV levels in patients without known immunodeficiency, malignancy, or autoimmune disorder [1]. In fact, patients probably have minor defects in cellular immunity against EBV-infected cells [2, 3]. The pathogenesis is uncertain; Asian and Latin American cases suggest a genetic predisposition. CAEBV was originally considered a pediatric disease, but recent studies have identified an increasing number of adult-onset cases. CAEBV is characterized by a systemic clonal proliferation of EBV-infected NK or T cells [3]. Proposed guidelines for the diagnosis of CAEBV require persistent or recurrent mononucleosis-like symptoms, an unusual pattern of anti-EBV antibodies and/or detection of increased EBV genomes in affected tissues (including peripheral blood), and the absence at diagnosis of a differential diagnosis. A case of CAEBV must fulfill each criterion [4]. Although no single genetic defect has been identified, a positive association with human leukocyte antigen (HLA) A26 and a negative association with B52 have been observed. The most common signs are fever, hepatosplenomegaly, hepatitis, pancytopenia, lymphadenopathy, and skin involvement. Less common signs have been described as interstitial pneumonia, central nervous system involvement, periphery neuropathy, and myocarditis [5].
Most patients show an indolent course with cutaneous forms (hydroa vacciniforme–like, severe mosquito bite allergy), and patients with systemic forms show an aggressive course and fatal outcomes due to serious complications: multi-organ failure, hemophagocytic lymphohistiocytis, and malignant transformation (extranodal NK/T-cell lymphoma, NK-cell leukemia) [2, 3]. The quantification of circulating EBV DNA loads is useful for diagnosis of CAEBV [4] and has prognostic value as survival is poorer when the viral load is high at diagnosis [6]. Monitoring for EBV DNA is useful to assess response to treatment. Unfortunately, there is no current consensus regarding the optimal component to measure in peripheral blood: EBV loads in peripheral blood mononuclear cells, in plasma, and in serum are generally correlated with each other, but discordant results have been observed in some patients [6, 7].
A 33-year-old Peruvian woman, without history of immunodeficiency or lymphoma, was admitted for intermittent fever, oral aphthous ulcer, periorbital edema, chronic rhinosinusitis, and recent abnormal liver function tests. The viral panel was positive for EBV nuclear antigen immunoglobulin G and EBV viral capside antigen immunoglobulin G (immunoglobulin M was negative). The EBV DNA polymerase chain reaction (PCR) in the blood was 836 000 UI/mL (5.92 log10). An immune-flowFISH confirmed that the EBV infection was in the CD3+ CD8+ cells. Blood tests showed activation of T cells (HLA- DR+ CD8+ T cells >50%). There was no evidence of T-cell clone in the blood immunophenotyping. Hemophagocytic lymphohistiocytosis (HLH) was considered unlikely due the absence of hemophagocytosis in the bone marrow and the absence of typical abnormalities in blood tests. The H-score [8] was only 19, indicating a very low probability of HLH. Bone marrow biopsy showed a T-cell infiltrate estimated at 10%, without lymphoma. The patient underwent several biopsies of oral aphthous (Figure 1A), liver (Figure 1B), and skin (Figure 1D). Pathology and immunohistochemistry revealed CD3+ CD8+ CD30+ granzyme B+ perforin+ cytotoxic T-cell infiltrate with EBV-encoded RNA (EBER) positivity and monoclonal rearrangement of the TCRG gene of gamma chains of TCR. Due to the occurrence of respiratory failure, a computed tomography (CT) scan was performed (Figure 1F); it showed pleural, pericardial, and peritoneal effusion and mucosal thickening of the maxillary sinus. A fibroscopy showed bronchial secretion without endobronchial lesions, and bronchoalveolar lavage fluid (BLF) showed an alveolitis >1 000 000 cells/mL (lymphocytes 74%), EBER+ cytotoxic T cells were the predominant cells, and EBV DNA in the BLF was >5 000 000 UI/mL (>6.7 log10).
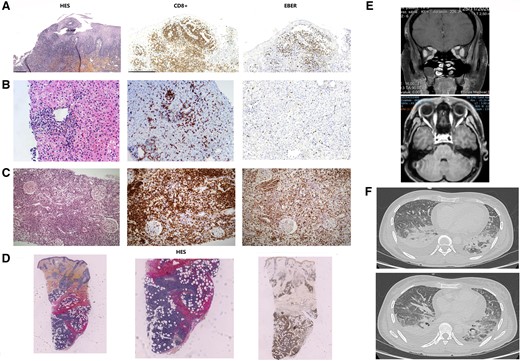
Pathological and radiological illustrations of EBV-induced lymphoproliferation. Multiorgan dysfunction due to CD3+ CD8+ CD30+ EBER+ cytotoxic T-cell infiltrate. For each biopsy, the CD8+ and EBER+ character is confirmed (A–D). In detail, oral aphthous biopsy showed atypical lymphoproliferation with epitheliotropism corresponding to hydroa vacciniforme-like (A). Liver biopsy revealed sinusoidal and portal infiltrate (B). Renal biopsy showed a massive tubulointerstitial infiltrate without glomerulopathy or vasculopathy (C). Skin biopsy showed a diffuse nodular infiltrate of the dermis and hypodermis (D). Axial (low) and coronal (top) contrast-enhanced T1 magnetic resonance imaging of the orbits showed multilocal edema and enlargement of the various ocular muscles (E). Chest CT showed pleural and pericardial effusion (F). Abbreviations: CT, computed tomography; EBV, Epstein-Barr virus.
The clinical picture was then supplemented by diplopia and increased creatinine. Radiological evaluation was preferred to surgical orbital biopsy and revealed bilateral ocular myositis and masticatory muscle myositis on magnetic resonance imaging (MRI) of the orbits (Figure 1E). Kidney biopsy when creatininemia was 140 µmol/L showed a diffuse tubulointerstitial EBER+ T-cell infiltrate; 19 glomeruli were considered normal (Figure 1C).
Many treatments were tried: corticosteroids, rituximab, cyclosporine, PEGylated interferon α-2a, CHOP chemotherapy (cyclophosphamide, doxorubicin, vincristine, prednisone), vinblastine. They transiently reduced systemic symptoms, laboratory abnormalities, and EBV DNA PCR levels. The patient developed several opportunistic infections during treatment (pneumocystis pneumonia, pulmonary aspergillosis, SARS-CoV-2 infection, Staphylococcus aureus pneumonia). Finally, a flare-up of CAEBV required a transfer to an intensive care unit. The medical evolution was unfavorable, with multi-organ failure and gastrointestinal bleeding leading to the death of the patient.
This is a rare case of CAEBV in a South American patient presenting with mucocutaneous, sinus, pulmonary, hepatic, ocular, and renal involvement. Clinicopathological features are illustrated by multiple biopsies showing EBV+ lymphocytic infiltrate. Our case is uncommon because the ocular and renal locations are exceptional.
Ocular myositis is a rare inflammatory disorder of 1 or more extraocular eye muscles. The diagnosis is based on the MRI; eye muscle biopsy is usually not indicated. Usually considered an idiopathic orbital inflammatory syndrome, some cases are associated with systemic diseases (sarcoidosis, lupus, vasculitis) or infections (Lyme disease, cysticercosis, herpes zoster). Corticosteroid therapy typically results in prompt improvement and remission [9]. CAEBV is not a well-known etiology of ocular myositis. A 71-year-old woman was described with chronic generalized myositis associated with CAEBV; she had orbital myositis as the initial symptom. Creatine kinase was elevated. CT showed edematous changes in the pterygoid, masseter, and temporal muscles, MRI showed extraocular muscle and tongue swelling, and muscle biopsy from the deltoid showed necrosis and infiltrate of EBER+ T cells. Treatment with corticosteroid and cyclosporin was insufficient, and the patient died [10]. Another 3-year-old boy was reported to have ocular involvement and intracranial infiltration by extranodal nasal-type NK/T-cell lymphoma combined with CAEBV. He achieved complete remission through chemotherapy. The parents refused allogeneic hematopoietic stem cell transplantation (HSCT) [11]. To the best of our knowledge, we report the third case of CAEBV with ocular myositis. More studies are needed to assess the prognosis impact of ocular myositis in CAEBV patients, especially in adults.
Acute kidney injury and nephrotic syndrome are uncommon complications of acute infection with EBV (infectious mononucleosis). Renal biopsy can show acute tubular necrosis and tubulointerstitial nephritis, or rarely minimal-change disease with focal or diffuse podocyte foot process effacement [12]. In CAEBV, there is a lack of data on renal involvement, but this complication seems rare. In Japan, a 70-year-old patient with CAEBV developed acute tubulointerstitial nephritis and minimal-change nephrotic syndrome. A renal biopsy revealed papillary infoldings of atypical tubular epithelium and adjacent dense infiltration of EBV+ lymphocytes. Surprisingly, renal function improved spontaneously after this patient decided to stay at home. The author did not report outpatient treatment [13]. A recent Chinese study summarized the clinicopathological characteristics of CAEBV in 19 adults. Of the 19 patients, 12 died, half due to multiple organ failure. No specific renal impairment was declared, and no renal biopsy or autopsy was performed to document a renal infiltrate [14].
This case shows that CAEBV is an aggressive lymphoproliferative disorder capable of infiltrating all types of organs as targets. The functional and vital prognoses are uncertain until the disease is brought under control. Our data suggest an urgent need to perform allogenic HSCT as soon as possible in young patients.
Acknowledgments
We are indebted to Dr. Lionel Galicier and Dr. Edouard Forcade for their assistance in the management of the patient.
Financial support. No source of funding to declare.
Patient consent. The patient’s written consent was obtained. Ethical committees were not required for this study.
References
Author notes
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.





Comments