-
PDF
- Split View
-
Views
-
Cite
Cite
Chen-Hua Liu, Hsin-Yun Sun, Cheng-Yuan Peng, Szu-Min Hsieh, Sheng-Shun Yang, Wei-Yu Kao, Yu-Lueng Shih, Chih-Lin Lin, Chun-Jen Liu, Wang-Hui Sheng, Yi-Chun Lo, Wen-Chun Liu, Jo-Hsuan Wu, Tung-Hung Su, Tai-Chung Tseng, Pei-Jer Chen, Chien-Ching Hung, Jia-Horng Kao, Hepatitis C Virus Reinfection in People With HIV in Taiwan After Achieving Sustained Virologic Response With Antiviral Treatment: The RECUR Study, Open Forum Infectious Diseases, Volume 9, Issue 8, August 2022, ofac348, https://doi.org/10.1093/ofid/ofac348
Close - Share Icon Share
Abstract
Data on hepatitis C virus (HCV) reinfection in East Asian people with HIV after treatment-induced sustained virologic response (SVR) are limited.
HIV/HCV-coinfected patients in Taiwan who achieved SVR12 with interferon (IFN) or direct-acting antivirals (DAAs) between 2005 and 2021 underwent HCV RNA measurements at SVR24 and then biannually. HCV reinfection was defined as the detection of different HCV strains beyond SVR12. HIV-negative, low-risk individuals with SVR12 served as reference patients. Crude reinfection rates and secular trends were assessed. Multivariate Cox regression analysis was performed to identify baseline factors associated with HCV reinfection.
A total of 216 HIV-positive and 1589 reference patients were recruited, with median follow-up durations of 3.0 and 6.0 years, respectively. During a total of 772 person-years of follow-up (PYFU), the HCV reinfection rate in HIV-positive patients was 4.02 per 100 PYFU (95% CI, 2.85–5.65), while the HCV reinfection rate in reference patients was 0.14 per 100 PYFU (95% CI, 0.09–0.23) during 10 862 PYFU. HIV-positive patients had a higher risk of HCV reinfection than reference patients (hazard ratio [HR], 17.63; 95% CI, 7.10–43.80; P < .001). No baseline factors were predictive of HCV reinfection in HIV-positive patients. The incidence of HCV reinfection in HIV-positive patients increased after 2015, when DAAs were made available in Taiwan.
The risk of HCV reinfection remains high in HIV/HCV-coinfected patients with treatment-induced SVR12. In addition to mass screening and treatment scale-up, strategies to reduce reinfection are needed for HCV microelimination in HIV-positive patients in Taiwan.
Hepatitis C virus (HCV) infection remains a major public health problem in people with HIV. While the global prevalence of HCV infection is estimated to be 1.0%, the prevalence of HCV infection is 2.4% in HIV-positive patients, which confirms ∼22 million people with HIV/HCV coinfection worldwide [1, 2]. Among HIV-positive subjects, the prevalence rates of HCV infection in heterosexual individuals, men who have sex with men (MSM), and people who inject drugs (PWID) are 4.0%, 6.4%, and 82.4%, respectively. In addition to a consistently higher prevalence rate of HCV infection in HIV-positive individuals than in HIV-negative individuals, HIV/HCV-coinfected patients have faster progression of hepatic fibrosis and a higher risk of liver-related morbidity and mortality if HCV is left untreated [3–5]. In contrast, health-related outcomes are significantly improved once HCV is eradicated with antiviral treatment [5–7].
The introduction of interferon (IFN)-free direct-acting antivirals (DAAs) has affected a paradigmatic shift in HCV care because most patients can successfully eradicate HCV with a short course of highly tolerated DAAs [8]. Based on the epoch-making advances, the World Health Organization (WHO) has targeted HCV elimination before 2030 by achieving a goal of 90% diagnosis, 80% treatment uptake, and 65% reduction in mortality among HCV-infected individuals [9]. While many countries have made efforts toward HCV elimination through multidisciplinary policies in screening, treatment, and post-treatment surveillance, only a limited proportion of them are on track to meet the target [10].
In addition to HCV macroelimination in the general population, HCV microelimination in high-risk populations, including HIV-positive patients, prisoners, PWID, migrants, and psychiatric patients, is also challenging. Studies have indicated the potential success of HCV microelimination in HIV/HCV-coinfected patients by screening and treatment scale-up [11–16]. The reported risks of HCV reinfection in HIV/HCV-coinfected patients with treatment-induced SVR12 varied significantly among Western studies, ranging from 0% to 17.0% per 100 person-years of follow-up (PYFU) [14–22]. Moreover, several studies have shown a decrease in the incidence of HCV reinfection following the widespread use of DAAs [15, 16, 18, 21]. Data regarding HCV reinfection in East Asians with HIV/HCV coinfection who achieved SVR12 with anti-HCV treatment were limited. To date, 2 studies indicated that the HCV reinfection rates among HIV-positive patients in Taiwan who attained spontaneous or treatment-induced HCV clearance were 8.2% and 6.7% per 100 PYFU, respectively [23, 24]. However, both studies were conducted retrospectively on a single-center basis and did not assess the secular trend of HCV reinfection in HIV-positive patients in the era of IFN and DAA. To this end, we conducted a multicenter prospective study to compare (1) the incidence rates of HCV reinfection in HIV/HCV-coinfected patients who achieved SVR12 by IFN or DAAs, using an HIV-negative, low-risk population as the reference group; (2) the baseline factors associated with HCV reinfection; and (3) the secular trend of HCV reinfection in the era of IFN and DAA in Taiwan.
METHODS
Patients
Between January 2005 and December 2021, patients with chronic HCV monoinfection or HIV/HCV coinfection aged ≥20 years who achieved treatment-induced SVR12, defined as those with undetectable serum HCV RNA levels (Cobas TaqMan HCV Test, version 2.0, Roche Diagnostics GmbH, Mannheim, Germany; lower limit of detection [LLOD], 15 IU/mL) 12 weeks after stopping IFN or DAAs, were invited to participate in the Reinfection of hEpatitis C in patients with immUnodeficiency viRus (RECUR) study, which prospectively assessed the risk of HCV reinfection at 7 academic centers in Taiwan. Chronic HCV infection was defined as seropositivity for HCV antibody (anti-HCV; Abbott HCV EIA 2.0, Abbott Laboratories, Abbott Park, IL, USA) and HCV RNA for ≥6 months. HIV infection was defined as patients who were seropositive for HIV antibody (anti-HIV; Abbott Architect HIV Ag/Ab Combo, Abbott Laboratories, Abbott Park, IL, USA) and HIV RNA (Cobas AmpliPrep TaqMan HIV-1 Test, version 2.0, Roche Diagnostics GmbH, Mannheim, Germany; LLOD, 20 copies/mL). Patients who were seropositive for HBV surface antigen (HBsAg; Abbott Architect HBsAg qualitative assay, Abbott Laboratories, Abbott Park, IL, USA) underwent organ transplantation, received post-SVR12 follow-up of <3 months, or refused to provide written informed consent were excluded from the study. HIV-negative, low-risk reference patients were defined as HIV-seronegative individuals who were reported not to be MSM, PWID, incarcerated, or on hemodialysis [25].
Patient Consent
The study was approved by the Research Ethics Committee or Institutional Review Board of each center and was conducted in accordance with the principles of the Declaration of Helsinki in 1975. All participants provided written informed consent before participating in the study.
Study Design
Baseline patient demographics, hemogram, serum albumin, total bilirubin (upper limit of normal [ULN], 1.0 mg/dL), alanine aminotransferase (ALT; ULN, 30 U/L for males and 19 U/L for females), and estimated glomerular filtration rate (eGFR) were assessed at SVR12 [26]. Hepatic fibrosis was staged at SVR12 by transient elastography (FibroScan, Echosens, Paris, France) with a cutoff of liver stiffness ≤7.0 kPa, 7.1–9.4 kPa, 9.5–12.3 kPa, and ≥12.4 kPa representing F0–1, F2, F3, and F4, respectively. HCV RNA level, HCV genotype/subtype (Abbott RealTime HCV Genotyping II assay, Abbott Laboratories, Abbott Park, IL, USA; or Roche Cobas HCV genotyping assay, Roche Diagnostics GmbH, Mannheim, Germany), anti-HIV, and HIV RNA level were assessed before antiviral treatment [27, 28].
Eligible patients underwent HCV RNA testing at SVR24 and then biannually until the last follow-up. Additional unscheduled HCV RNA tests were performed at the physician’s discretion. If patients had a detectable HCV RNA level during post-SVR12 surveillance, HCV genotype/subtype testing was performed again using the Abbott RealTime HCV Genotyping II assay or Roche Cobas HCV genotyping assay. We compared the HCV genotype/subtype results at the time of recurrent HCV viremia with those before antiviral treatment. HCV reinfection was defined as the detection of different HCV strains beyond the SVR12. HCV reinfection was confirmed if the paired samples yielded different genotype/subtype results. In samples yielding the same genotype/subtype results, Sanger sequencing of the archived paired samples was performed in the HCV core and nonstructural 5B (NS5B) regions, where the generated sequences were submitted to GenBank, followed by phylogenetic analysis to compare the similarity of HCV strains [28]. For patients with documented HCV reinfection, the treating physicians inquired about the potential route of viral transmission, including sexual contact, injection drug use (IDU), blood transfusion, surgical or dental treatments, needlestick, tattoo, ear piercing, etc.
The main outcome was HCV reinfection in patients who achieved SVR12 with antiviral treatment. We assessed the following baseline factors potentially associated with HCV reinfection: HIV status, cluster of differentiation 4 (CD4) count, age, sex, body mass index (BMI), pretreatment HCV RNA level and genotype, hepatic fibrosis, ALT level, and type of antiviral treatment (IFN or DAA).
Statistical Analysis
All statistical analyses were performed using the Statistical Program for Social Sciences (SPSS Statistics, version 23.0, IBM Corp., Armonk, NY, USA). Baseline characteristics were shown as median (range) and number (percentage) and compared using the Mann-Whitney U test, or χ2 with Fisher exact test when appropriate. The incidence rates were shown as the number of HCV reinfections in HIV-positive and reference patients per 100 PYFU with 95% CI. In patients with HCV reinfection, the date of recurrent HCV viremia was taken as the outcome date. Patients without HCV reinfection were censored at the date of last follow-up. The cumulative incidence rates of HCV reinfection between HIV-positive and reference patients and between HIV-positive patients with IFN and DAAs were calculated using the Kaplan-Meier method and compared using the log-rank test. We used a multivariate Cox regression model, which was shown as hazard ratio (HR) with 95% CI, to assess the association between baseline factors and HCV reinfection. The trends in the incidence of HCV reinfection from 2005 to 2021 between HIV-positive and reference patients were compared by generalized estimating equation (GEE). All statistical tests were 2-tailed, and results were statistically significant when a P value was <.05.
RESULTS
Patient Characteristics
Of the 2433 patients who achieved SVR12, 246 (10.1%) and 2187 (89.9%) were HIV positive and HIV negative, respectively. After excluding 30 patients who had HBV coinfection (n = 7), who received a post-SVR12 follow-up at <3 months (n = 15) and refused to provide written informed consent (n = 8), 216 HIV-positive patients were eligible for the study. Among the 2187 HIV-negative patients, 1589 were included as reference patients after excluding 598 ineligible patients (Figure 1).

Table 1 shows the baseline patient characteristics. The median duration of post-SVR12 follow-up in HIV-positive patients was shorter than that in reference patients (3.0 years vs 6.0 years; P < .001). HIV-positive patients were younger (P < .001), had higher eGFR (P < .001), lower BMI (P < .001), and ALT levels (P = .001), and had higher proportions of males and individuals undergoing treatment with DAAs (P < .001) than reference patients. Among HIV-positive patients, all received antiretroviral therapy (ART), 200 (92.6%) were MSM, 210 (97.2%) had an HIV RNA <20 copies/mL, and 82 (38.0%) had a CD4 count <500 × 106 cells/L. During the post-SVR12 follow-up, 14 and 19 unscheduled HCV RNA tests were performed in HIV-positive and reference patients, respectively. The mean number of HCV RNA tests among HIV-positive and reference patients was 2.02 and 2.00 times per year, respectively.
| Characteristicsa . | HIV-Positive Patients (n = 216) . | Reference Patients (n = 1589) . |
|---|---|---|
| Age, median (range), y | 36 (22–79) | 57 (20–94) |
| Age >40 y | 78 (36.1) | 1420 (89.4) |
| Male | 210 (97.2) | 857 (53.9) |
| BMI, median (range), kg/m2 | 22.8 (16.9–36.7) | 24.7 (15.0–46.6) |
| BMI ≥23 kg/m2 | 100 (46.3) | 1105 (69.5) |
| HIV risk group | ||
| MSM | 200 (92.6) | … |
| Heterosexual | 4 (1.9) | … |
| PWID | 11 (5.1) | … |
| Hemophilia | 1 (0.5) | … |
| ART treatment | 216 (100) | … |
| HIV RNA <20 copies/mL | 210 (97.2) | … |
| Duration of post-SVR12 follow-up, median (range), y | 3.0 (0.25–10.5) | 6.0 (0.5–17) |
| Antiviral treatment | ||
| IFN | 109 (50.5) | 1185 (74.6) |
| DAA | 107 (49.5) | 404 (25.4) |
| HCV RNA, median (range), log10 IU/mL | 6.56 (1.41–7.75) | 6.01 (1.34–7.70) |
| HCV RNA >6 000 000 IU/mL | 90 (41.7) | 232 (14.6) |
| HCV genotype/subtype | ||
| 1 | 81 (37.5) | 856 (53.9) |
| 2 | 86 (39.8) | 616 (38.8) |
| 3 | 5 (2.3) | 8 (0.5) |
| 4 | 0 (0) | 1 (0.1) |
| 6 | 32 (14.8) | 27 (1.7) |
| Mixed | 9 (4.2) | 76 (4.8) |
| Invalid | 3 (1.4) | 5 (0.3) |
| Stage of hepatic fibrosisb | ||
| 0–1 | 145 (67.2) | 624 (39.2) |
| 2 | 38 (17.6) | 435 (27.4) |
| 3 | 17 (7.9) | 210 (13.2) |
| 4 | 15 (6.9) | 311 (19.6) |
| Failed | 1 (0.5) | 9 (0.6) |
| Hemoglobin, median (range), g/dL | 15.3 (11.3–19.2) | 14.1 (8.2–18.2) |
| White blood cell count, median (range), 106 cells/L | 5825 (2600–15 470) | 5210 (1210–17 640) |
| CD4 cell count, median (range), 106 cells/L | 552 (228–1243) | … |
| CD4 cell count <500 × 106 cells/L | 82 (38.0) | … |
| Platelet count, median (range), 109 cells/L | 241 (91–465) | 180 (29–461) |
| Albumin, median (range), g/dL | 4.7 (4.0–5.5) | 4.5 (2.6–5.5) |
| Total bilirubin, median (range),c ULN | 0.7 (0.3–3.8) | 0.8 (0.2–4.8) |
| ALT, median (range),c ULN | 0.6 (0.2–13.5) | 0.8 (0.2–19.1) |
| ALT > ULN | 42 (19.4) | 474 (29.8) |
| eGFR, median (range), mL/min/1.73m2 | 96 (44–133) | 83 (7–144) |
| CKD staged | ||
| 1 (eGFR ≥90 mL/min/1.73m2) | 130 (60.2) | 664 (41.8) |
| 2 (eGFR 60–89 mL/min/1.73m2) | 76 (35.2) | 627 (39.5) |
| 3 (eGFR 30–59 mL/min/1.73m2) | 10 (4.7) | 285 (17.9) |
| 4 (eGFR 15–29 mL/min/1.73m2) | 0 (0) | 10 (0.6) |
| 5 (eGFR <15 mL/min/1.73m2) | 0 (0) | 3 (0.2) |
| Characteristicsa . | HIV-Positive Patients (n = 216) . | Reference Patients (n = 1589) . |
|---|---|---|
| Age, median (range), y | 36 (22–79) | 57 (20–94) |
| Age >40 y | 78 (36.1) | 1420 (89.4) |
| Male | 210 (97.2) | 857 (53.9) |
| BMI, median (range), kg/m2 | 22.8 (16.9–36.7) | 24.7 (15.0–46.6) |
| BMI ≥23 kg/m2 | 100 (46.3) | 1105 (69.5) |
| HIV risk group | ||
| MSM | 200 (92.6) | … |
| Heterosexual | 4 (1.9) | … |
| PWID | 11 (5.1) | … |
| Hemophilia | 1 (0.5) | … |
| ART treatment | 216 (100) | … |
| HIV RNA <20 copies/mL | 210 (97.2) | … |
| Duration of post-SVR12 follow-up, median (range), y | 3.0 (0.25–10.5) | 6.0 (0.5–17) |
| Antiviral treatment | ||
| IFN | 109 (50.5) | 1185 (74.6) |
| DAA | 107 (49.5) | 404 (25.4) |
| HCV RNA, median (range), log10 IU/mL | 6.56 (1.41–7.75) | 6.01 (1.34–7.70) |
| HCV RNA >6 000 000 IU/mL | 90 (41.7) | 232 (14.6) |
| HCV genotype/subtype | ||
| 1 | 81 (37.5) | 856 (53.9) |
| 2 | 86 (39.8) | 616 (38.8) |
| 3 | 5 (2.3) | 8 (0.5) |
| 4 | 0 (0) | 1 (0.1) |
| 6 | 32 (14.8) | 27 (1.7) |
| Mixed | 9 (4.2) | 76 (4.8) |
| Invalid | 3 (1.4) | 5 (0.3) |
| Stage of hepatic fibrosisb | ||
| 0–1 | 145 (67.2) | 624 (39.2) |
| 2 | 38 (17.6) | 435 (27.4) |
| 3 | 17 (7.9) | 210 (13.2) |
| 4 | 15 (6.9) | 311 (19.6) |
| Failed | 1 (0.5) | 9 (0.6) |
| Hemoglobin, median (range), g/dL | 15.3 (11.3–19.2) | 14.1 (8.2–18.2) |
| White blood cell count, median (range), 106 cells/L | 5825 (2600–15 470) | 5210 (1210–17 640) |
| CD4 cell count, median (range), 106 cells/L | 552 (228–1243) | … |
| CD4 cell count <500 × 106 cells/L | 82 (38.0) | … |
| Platelet count, median (range), 109 cells/L | 241 (91–465) | 180 (29–461) |
| Albumin, median (range), g/dL | 4.7 (4.0–5.5) | 4.5 (2.6–5.5) |
| Total bilirubin, median (range),c ULN | 0.7 (0.3–3.8) | 0.8 (0.2–4.8) |
| ALT, median (range),c ULN | 0.6 (0.2–13.5) | 0.8 (0.2–19.1) |
| ALT > ULN | 42 (19.4) | 474 (29.8) |
| eGFR, median (range), mL/min/1.73m2 | 96 (44–133) | 83 (7–144) |
| CKD staged | ||
| 1 (eGFR ≥90 mL/min/1.73m2) | 130 (60.2) | 664 (41.8) |
| 2 (eGFR 60–89 mL/min/1.73m2) | 76 (35.2) | 627 (39.5) |
| 3 (eGFR 30–59 mL/min/1.73m2) | 10 (4.7) | 285 (17.9) |
| 4 (eGFR 15–29 mL/min/1.73m2) | 0 (0) | 10 (0.6) |
| 5 (eGFR <15 mL/min/1.73m2) | 0 (0) | 3 (0.2) |
Abbreviations: ALT, alanine aminotransferase; ART, antiretroviral therapy; BMI, body mass index; CD, cluster of differentiation; CKD, chronic kidney disease; DAA, direct-acting antiviral; eGFR, estimated glomerular filtration rate; HCV, hepatitis C virus; IFN, interferon; IU, international unit; MSM, men who have sex with men; PWID, people with injection drugs; SVR, sustained virologic response; ULN, upper limit of normal.
Values are shown as numbers (percentages) unless otherwise indicated. All patient characteristics were collected at SVR12, except for data regarding HCV RNA, HCV GT, type of antiviral treatment, HIV risk group, and HIV RNA, which were collected before antiviral treatment. The P values were all <.001 between the HIV-positive and reference groups, except for ALT > ULN, where the P value was .001. The P values were not available for the HIV risk group and CD4 count.
Assessed by transient elastography (FibroScan, Echosens, Paris, France) with a cutoff of liver stiffness of ≤7.0 kPa, 7.1–9.4 kPa, 9.5–12.3 kPa, and ≥12.4 kPa to be F0–1, F2, F3, and F4, respectively. The assessment was considered to have failed if the subjects had primary failure or unreliable measurements with transient elastography.
The ULN for total bilirubin was 1.0 mg/dL. The ULN for ALT was 30 U/L in men and 19 U/L in women.
Assessed using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.
| Characteristicsa . | HIV-Positive Patients (n = 216) . | Reference Patients (n = 1589) . |
|---|---|---|
| Age, median (range), y | 36 (22–79) | 57 (20–94) |
| Age >40 y | 78 (36.1) | 1420 (89.4) |
| Male | 210 (97.2) | 857 (53.9) |
| BMI, median (range), kg/m2 | 22.8 (16.9–36.7) | 24.7 (15.0–46.6) |
| BMI ≥23 kg/m2 | 100 (46.3) | 1105 (69.5) |
| HIV risk group | ||
| MSM | 200 (92.6) | … |
| Heterosexual | 4 (1.9) | … |
| PWID | 11 (5.1) | … |
| Hemophilia | 1 (0.5) | … |
| ART treatment | 216 (100) | … |
| HIV RNA <20 copies/mL | 210 (97.2) | … |
| Duration of post-SVR12 follow-up, median (range), y | 3.0 (0.25–10.5) | 6.0 (0.5–17) |
| Antiviral treatment | ||
| IFN | 109 (50.5) | 1185 (74.6) |
| DAA | 107 (49.5) | 404 (25.4) |
| HCV RNA, median (range), log10 IU/mL | 6.56 (1.41–7.75) | 6.01 (1.34–7.70) |
| HCV RNA >6 000 000 IU/mL | 90 (41.7) | 232 (14.6) |
| HCV genotype/subtype | ||
| 1 | 81 (37.5) | 856 (53.9) |
| 2 | 86 (39.8) | 616 (38.8) |
| 3 | 5 (2.3) | 8 (0.5) |
| 4 | 0 (0) | 1 (0.1) |
| 6 | 32 (14.8) | 27 (1.7) |
| Mixed | 9 (4.2) | 76 (4.8) |
| Invalid | 3 (1.4) | 5 (0.3) |
| Stage of hepatic fibrosisb | ||
| 0–1 | 145 (67.2) | 624 (39.2) |
| 2 | 38 (17.6) | 435 (27.4) |
| 3 | 17 (7.9) | 210 (13.2) |
| 4 | 15 (6.9) | 311 (19.6) |
| Failed | 1 (0.5) | 9 (0.6) |
| Hemoglobin, median (range), g/dL | 15.3 (11.3–19.2) | 14.1 (8.2–18.2) |
| White blood cell count, median (range), 106 cells/L | 5825 (2600–15 470) | 5210 (1210–17 640) |
| CD4 cell count, median (range), 106 cells/L | 552 (228–1243) | … |
| CD4 cell count <500 × 106 cells/L | 82 (38.0) | … |
| Platelet count, median (range), 109 cells/L | 241 (91–465) | 180 (29–461) |
| Albumin, median (range), g/dL | 4.7 (4.0–5.5) | 4.5 (2.6–5.5) |
| Total bilirubin, median (range),c ULN | 0.7 (0.3–3.8) | 0.8 (0.2–4.8) |
| ALT, median (range),c ULN | 0.6 (0.2–13.5) | 0.8 (0.2–19.1) |
| ALT > ULN | 42 (19.4) | 474 (29.8) |
| eGFR, median (range), mL/min/1.73m2 | 96 (44–133) | 83 (7–144) |
| CKD staged | ||
| 1 (eGFR ≥90 mL/min/1.73m2) | 130 (60.2) | 664 (41.8) |
| 2 (eGFR 60–89 mL/min/1.73m2) | 76 (35.2) | 627 (39.5) |
| 3 (eGFR 30–59 mL/min/1.73m2) | 10 (4.7) | 285 (17.9) |
| 4 (eGFR 15–29 mL/min/1.73m2) | 0 (0) | 10 (0.6) |
| 5 (eGFR <15 mL/min/1.73m2) | 0 (0) | 3 (0.2) |
| Characteristicsa . | HIV-Positive Patients (n = 216) . | Reference Patients (n = 1589) . |
|---|---|---|
| Age, median (range), y | 36 (22–79) | 57 (20–94) |
| Age >40 y | 78 (36.1) | 1420 (89.4) |
| Male | 210 (97.2) | 857 (53.9) |
| BMI, median (range), kg/m2 | 22.8 (16.9–36.7) | 24.7 (15.0–46.6) |
| BMI ≥23 kg/m2 | 100 (46.3) | 1105 (69.5) |
| HIV risk group | ||
| MSM | 200 (92.6) | … |
| Heterosexual | 4 (1.9) | … |
| PWID | 11 (5.1) | … |
| Hemophilia | 1 (0.5) | … |
| ART treatment | 216 (100) | … |
| HIV RNA <20 copies/mL | 210 (97.2) | … |
| Duration of post-SVR12 follow-up, median (range), y | 3.0 (0.25–10.5) | 6.0 (0.5–17) |
| Antiviral treatment | ||
| IFN | 109 (50.5) | 1185 (74.6) |
| DAA | 107 (49.5) | 404 (25.4) |
| HCV RNA, median (range), log10 IU/mL | 6.56 (1.41–7.75) | 6.01 (1.34–7.70) |
| HCV RNA >6 000 000 IU/mL | 90 (41.7) | 232 (14.6) |
| HCV genotype/subtype | ||
| 1 | 81 (37.5) | 856 (53.9) |
| 2 | 86 (39.8) | 616 (38.8) |
| 3 | 5 (2.3) | 8 (0.5) |
| 4 | 0 (0) | 1 (0.1) |
| 6 | 32 (14.8) | 27 (1.7) |
| Mixed | 9 (4.2) | 76 (4.8) |
| Invalid | 3 (1.4) | 5 (0.3) |
| Stage of hepatic fibrosisb | ||
| 0–1 | 145 (67.2) | 624 (39.2) |
| 2 | 38 (17.6) | 435 (27.4) |
| 3 | 17 (7.9) | 210 (13.2) |
| 4 | 15 (6.9) | 311 (19.6) |
| Failed | 1 (0.5) | 9 (0.6) |
| Hemoglobin, median (range), g/dL | 15.3 (11.3–19.2) | 14.1 (8.2–18.2) |
| White blood cell count, median (range), 106 cells/L | 5825 (2600–15 470) | 5210 (1210–17 640) |
| CD4 cell count, median (range), 106 cells/L | 552 (228–1243) | … |
| CD4 cell count <500 × 106 cells/L | 82 (38.0) | … |
| Platelet count, median (range), 109 cells/L | 241 (91–465) | 180 (29–461) |
| Albumin, median (range), g/dL | 4.7 (4.0–5.5) | 4.5 (2.6–5.5) |
| Total bilirubin, median (range),c ULN | 0.7 (0.3–3.8) | 0.8 (0.2–4.8) |
| ALT, median (range),c ULN | 0.6 (0.2–13.5) | 0.8 (0.2–19.1) |
| ALT > ULN | 42 (19.4) | 474 (29.8) |
| eGFR, median (range), mL/min/1.73m2 | 96 (44–133) | 83 (7–144) |
| CKD staged | ||
| 1 (eGFR ≥90 mL/min/1.73m2) | 130 (60.2) | 664 (41.8) |
| 2 (eGFR 60–89 mL/min/1.73m2) | 76 (35.2) | 627 (39.5) |
| 3 (eGFR 30–59 mL/min/1.73m2) | 10 (4.7) | 285 (17.9) |
| 4 (eGFR 15–29 mL/min/1.73m2) | 0 (0) | 10 (0.6) |
| 5 (eGFR <15 mL/min/1.73m2) | 0 (0) | 3 (0.2) |
Abbreviations: ALT, alanine aminotransferase; ART, antiretroviral therapy; BMI, body mass index; CD, cluster of differentiation; CKD, chronic kidney disease; DAA, direct-acting antiviral; eGFR, estimated glomerular filtration rate; HCV, hepatitis C virus; IFN, interferon; IU, international unit; MSM, men who have sex with men; PWID, people with injection drugs; SVR, sustained virologic response; ULN, upper limit of normal.
Values are shown as numbers (percentages) unless otherwise indicated. All patient characteristics were collected at SVR12, except for data regarding HCV RNA, HCV GT, type of antiviral treatment, HIV risk group, and HIV RNA, which were collected before antiviral treatment. The P values were all <.001 between the HIV-positive and reference groups, except for ALT > ULN, where the P value was .001. The P values were not available for the HIV risk group and CD4 count.
Assessed by transient elastography (FibroScan, Echosens, Paris, France) with a cutoff of liver stiffness of ≤7.0 kPa, 7.1–9.4 kPa, 9.5–12.3 kPa, and ≥12.4 kPa to be F0–1, F2, F3, and F4, respectively. The assessment was considered to have failed if the subjects had primary failure or unreliable measurements with transient elastography.
The ULN for total bilirubin was 1.0 mg/dL. The ULN for ALT was 30 U/L in men and 19 U/L in women.
Assessed using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.
Comparison of HCV Reinfection in HIV-Positive and Reference Patients
Among HIV-positive patients who achieved SVR12, 31 (14.4%) had confirmed HCV reinfection (Figure 1). During a total of 772 PYFU, the incidence rate of HCV reinfection was 4.02 per 100 PYFU (95% CI, 2.85–5.65). Furthermore, the incidence rate of HCV reinfection in HIV-positive MSM was 4.12 per 100 PYFU (94% CI, 2.90–5.82). Twenty-seven (87.1%) HIV-positive patients had HCV genotype/subtype switching at the time of recurrent HCV viremia, and 30 (96.8%) were MSM. All reinfections were detected at biannual HCV RNA surveillance, except for 1 (patient No. 31) detected at unscheduled testing (Supplementary Table 1). Viral sequencing and phylogenetic analysis of 4 patients with identical commercial HCV genotype/subtype results revealed different viral strains that indicated HCV reinfection (Supplementary Figure 1). Thirty (96.8%) HCV reinfections were attributed to high-risk sexual behavior, and 1 (3.2%) was attributed to injection drug use.
Fifteen (0.9%) reference patients had HCV reinfection (Figure 1). The incidence rate of HCV reinfection was 0.14 per 100 PYFU (95% CI, 0.09–0.23) during a total of 10 862 PYFU. Thirteen (86.7%) reference patients had HCV genotype/subtype switching at the time of recurrent HCV viremia (Supplementary Table 1). Two reference patients had identical commercial HCV genotype/subtype results, and viral sequencing and phylogenetic analysis yielded different viral strains (Supplementary Figure 2). The most common route was unsafe medical injection, which accounted for 53.3% of reinfections in reference patients.
The cumulative incidence rate of HCV reinfection was higher in HIV-positive patients than in reference patients (log-rank test, P < .001) (Figure 2A). The cumulative risk of HCV reinfection at 3 years post-SVR12 in HIV-positive and reference patients was 14.6% and 0.5%, respectively. Multivariate analysis revealed that HIV seropositivity was significantly associated with HCV reinfection (HR, 17.63; 95% CI, 7.10–43.80; P < .001), while age >40 years, male sex, BMI ≥23 kg/m2, HCV RNA >6 000 000 IU/mL, HCV genotype 1 infection, hepatic fibrosis ≥F3, ALT > ULN, and treatment with DAAs were not associated with HCV reinfection (Table 2).
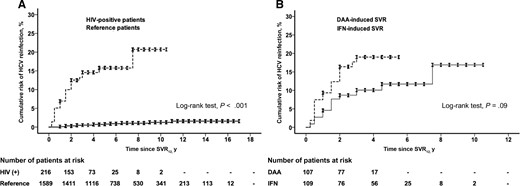
Cumulative incidences of HCV reinfection in (A) HIV-positive and reference patients with treatment-induced SVR12 and (B) HIV-positive patients with DAA- and IFN-induced SVR12. Abbreviations: DAA, direct-acting antiviral; HCV, hepatitis C virus; IFN, interferon; SVR, sustained virologic response.
Baseline Factors Associated With HCV Reinfection in All Patients Using the Multivariate Cox Regression Model
| . | Multivariate Analysis . | |||
|---|---|---|---|---|
| No Reinfection (n = 1759) . | Reinfection (n = 46) . | HR (95% CI) . | P Value . | |
| Patient group | ||||
| HIV-positive | 185 (10.5) | 31 (67.4) | 17.63 (7.10–43.80) | <.001 |
| Reference | 1574 (89.5) | 15 (32.6) | Ref | |
| Age, y | ||||
| >40 | 1476 (83.9) | 22 (47.8) | 0.82 (0.40–1.69) | .59 |
| ≤40 | 283 (16.1) | 24 (52.2) | Ref | |
| Sex | ||||
| Male | 1028 (58.4) | 39 (84.8) | 1.19 (0.46–3.11) | .72 |
| Female | 731 (41.6) | 7 (15.2) | Ref | |
| BMI, kg/m2 | ||||
| ≥23 | 1184 (67.3) | 21 (45.7) | 0.78 (0.42–1.45) | .44 |
| <23 | 575 (32.7) | 25 (54.3) | Ref | |
| HCV RNA, IU/mL | ||||
| >6 000 000 | 311 (17.7) | 11 (23.9) | 0.64 (0.31–1.31) | .22 |
| ≤6 000 000 | 1448 (82.3) | 35 (76.1) | Ref | |
| HCV genotypea | ||||
| 1 | 915 (52.0) | 22 (47.8) | 1.31 (0.71–2.39) | .39 |
| Non-1 | 844 (48.0) | 24 (52.2) | Ref | |
| Hepatic fibrosisb | ||||
| F3–F4 | 545 (31.2) | 8 (17.4) | 0.95 (0.42–2.14) | .90 |
| F0–F2 | 1204 (68.8) | 38 (82.6) | Ref | |
| ALT | ||||
| >ULN | 510 (29.0) | 6 (13.0) | 0.50 (0.21–1.21) | .13 |
| ≤ULN | 1249 (71.0) | 40 (87.0) | Ref | |
| Antiviral treatment | ||||
| DAA | 489 (27.8) | 22 (47.8) | 1.70 (0.91–3.21) | .10 |
| IFN | 1270 (72.2) | 24 (52.2) | Ref | |
| . | Multivariate Analysis . | |||
|---|---|---|---|---|
| No Reinfection (n = 1759) . | Reinfection (n = 46) . | HR (95% CI) . | P Value . | |
| Patient group | ||||
| HIV-positive | 185 (10.5) | 31 (67.4) | 17.63 (7.10–43.80) | <.001 |
| Reference | 1574 (89.5) | 15 (32.6) | Ref | |
| Age, y | ||||
| >40 | 1476 (83.9) | 22 (47.8) | 0.82 (0.40–1.69) | .59 |
| ≤40 | 283 (16.1) | 24 (52.2) | Ref | |
| Sex | ||||
| Male | 1028 (58.4) | 39 (84.8) | 1.19 (0.46–3.11) | .72 |
| Female | 731 (41.6) | 7 (15.2) | Ref | |
| BMI, kg/m2 | ||||
| ≥23 | 1184 (67.3) | 21 (45.7) | 0.78 (0.42–1.45) | .44 |
| <23 | 575 (32.7) | 25 (54.3) | Ref | |
| HCV RNA, IU/mL | ||||
| >6 000 000 | 311 (17.7) | 11 (23.9) | 0.64 (0.31–1.31) | .22 |
| ≤6 000 000 | 1448 (82.3) | 35 (76.1) | Ref | |
| HCV genotypea | ||||
| 1 | 915 (52.0) | 22 (47.8) | 1.31 (0.71–2.39) | .39 |
| Non-1 | 844 (48.0) | 24 (52.2) | Ref | |
| Hepatic fibrosisb | ||||
| F3–F4 | 545 (31.2) | 8 (17.4) | 0.95 (0.42–2.14) | .90 |
| F0–F2 | 1204 (68.8) | 38 (82.6) | Ref | |
| ALT | ||||
| >ULN | 510 (29.0) | 6 (13.0) | 0.50 (0.21–1.21) | .13 |
| ≤ULN | 1249 (71.0) | 40 (87.0) | Ref | |
| Antiviral treatment | ||||
| DAA | 489 (27.8) | 22 (47.8) | 1.70 (0.91–3.21) | .10 |
| IFN | 1270 (72.2) | 24 (52.2) | Ref | |
Abbreviations: ALT, alanine aminotransferase; BMI, body mass index; DAA, direct-acting antiviral; HCV, hepatitis C virus; HR, hazard ratio; IFN, interferon; ULN, upper limit of normal.
Patients with mixed HCV infection and those with invalid HCV genotypes were categorized as having HCV GT non-1 infection.
Ten patients without HCV reinfection were excluded from the multivariate analysis because of measurement failures with transient elastography.
Baseline Factors Associated With HCV Reinfection in All Patients Using the Multivariate Cox Regression Model
| . | Multivariate Analysis . | |||
|---|---|---|---|---|
| No Reinfection (n = 1759) . | Reinfection (n = 46) . | HR (95% CI) . | P Value . | |
| Patient group | ||||
| HIV-positive | 185 (10.5) | 31 (67.4) | 17.63 (7.10–43.80) | <.001 |
| Reference | 1574 (89.5) | 15 (32.6) | Ref | |
| Age, y | ||||
| >40 | 1476 (83.9) | 22 (47.8) | 0.82 (0.40–1.69) | .59 |
| ≤40 | 283 (16.1) | 24 (52.2) | Ref | |
| Sex | ||||
| Male | 1028 (58.4) | 39 (84.8) | 1.19 (0.46–3.11) | .72 |
| Female | 731 (41.6) | 7 (15.2) | Ref | |
| BMI, kg/m2 | ||||
| ≥23 | 1184 (67.3) | 21 (45.7) | 0.78 (0.42–1.45) | .44 |
| <23 | 575 (32.7) | 25 (54.3) | Ref | |
| HCV RNA, IU/mL | ||||
| >6 000 000 | 311 (17.7) | 11 (23.9) | 0.64 (0.31–1.31) | .22 |
| ≤6 000 000 | 1448 (82.3) | 35 (76.1) | Ref | |
| HCV genotypea | ||||
| 1 | 915 (52.0) | 22 (47.8) | 1.31 (0.71–2.39) | .39 |
| Non-1 | 844 (48.0) | 24 (52.2) | Ref | |
| Hepatic fibrosisb | ||||
| F3–F4 | 545 (31.2) | 8 (17.4) | 0.95 (0.42–2.14) | .90 |
| F0–F2 | 1204 (68.8) | 38 (82.6) | Ref | |
| ALT | ||||
| >ULN | 510 (29.0) | 6 (13.0) | 0.50 (0.21–1.21) | .13 |
| ≤ULN | 1249 (71.0) | 40 (87.0) | Ref | |
| Antiviral treatment | ||||
| DAA | 489 (27.8) | 22 (47.8) | 1.70 (0.91–3.21) | .10 |
| IFN | 1270 (72.2) | 24 (52.2) | Ref | |
| . | Multivariate Analysis . | |||
|---|---|---|---|---|
| No Reinfection (n = 1759) . | Reinfection (n = 46) . | HR (95% CI) . | P Value . | |
| Patient group | ||||
| HIV-positive | 185 (10.5) | 31 (67.4) | 17.63 (7.10–43.80) | <.001 |
| Reference | 1574 (89.5) | 15 (32.6) | Ref | |
| Age, y | ||||
| >40 | 1476 (83.9) | 22 (47.8) | 0.82 (0.40–1.69) | .59 |
| ≤40 | 283 (16.1) | 24 (52.2) | Ref | |
| Sex | ||||
| Male | 1028 (58.4) | 39 (84.8) | 1.19 (0.46–3.11) | .72 |
| Female | 731 (41.6) | 7 (15.2) | Ref | |
| BMI, kg/m2 | ||||
| ≥23 | 1184 (67.3) | 21 (45.7) | 0.78 (0.42–1.45) | .44 |
| <23 | 575 (32.7) | 25 (54.3) | Ref | |
| HCV RNA, IU/mL | ||||
| >6 000 000 | 311 (17.7) | 11 (23.9) | 0.64 (0.31–1.31) | .22 |
| ≤6 000 000 | 1448 (82.3) | 35 (76.1) | Ref | |
| HCV genotypea | ||||
| 1 | 915 (52.0) | 22 (47.8) | 1.31 (0.71–2.39) | .39 |
| Non-1 | 844 (48.0) | 24 (52.2) | Ref | |
| Hepatic fibrosisb | ||||
| F3–F4 | 545 (31.2) | 8 (17.4) | 0.95 (0.42–2.14) | .90 |
| F0–F2 | 1204 (68.8) | 38 (82.6) | Ref | |
| ALT | ||||
| >ULN | 510 (29.0) | 6 (13.0) | 0.50 (0.21–1.21) | .13 |
| ≤ULN | 1249 (71.0) | 40 (87.0) | Ref | |
| Antiviral treatment | ||||
| DAA | 489 (27.8) | 22 (47.8) | 1.70 (0.91–3.21) | .10 |
| IFN | 1270 (72.2) | 24 (52.2) | Ref | |
Abbreviations: ALT, alanine aminotransferase; BMI, body mass index; DAA, direct-acting antiviral; HCV, hepatitis C virus; HR, hazard ratio; IFN, interferon; ULN, upper limit of normal.
Patients with mixed HCV infection and those with invalid HCV genotypes were categorized as having HCV GT non-1 infection.
Ten patients without HCV reinfection were excluded from the multivariate analysis because of measurement failures with transient elastography.
Baseline Risk Factors for HCV Reinfection in HIV-Positive Patients
The median duration of post-SVR12 follow-up in HIV-positive patients receiving IFN and DAAs (range) was 4.5 (0.25–10.5) years and 3.0 (0.25–5.5) years, respectively (P < .001). The cumulative incidence rates of HCV reinfection were comparable between HIV-positive patients who received DAAs and those who received IFN (log-rank test, P = .09) (Figure 2B). The cumulative risk of HCV reinfection at 4 years post-SVR12 in HIV-positive patients with DAA- and IFN-induced SVR12 was 19.0% and 10.1%, respectively. Multivariate analysis revealed that MSM, CD4 count >500 × 106 cells/L, age >40 years, male sex, BMI ≥23 kg/m2, HCV RNA >6 000 000 IU/mL, HCV genotype 1 infection, hepatic fibrosis ≥F3, ALT > ULN, and treatment with DAAs were not associated with HCV reinfection (Table 3).
Baseline Factors Associated With HCV Reinfection in HIV-Positive Patients Using the Multivariate Cox Regression Model
| . | Multivariate Analysis . | |||
|---|---|---|---|---|
| No Reinfection (n = 185) . | Reinfection (n = 31) . | HR (95% CI) . | P Value . | |
| HIV risk group | ||||
| MSM | 170 (91.9) | 30 (96.8) | 1.41 (0.16–12.32) | .76 |
| Non-MSM | 15 (8.1) | 1 (3.2) | Ref | |
| CD4 count, 106 cells/L | ||||
| <500 | 73 (39.5) | 9 (29.0) | 0.63 (0.28–1.39) | .25 |
| ≥500 | 112 (60.5) | 22 (71.0) | Ref | |
| Age, y | ||||
| >40 | 69 (37.3) | 9 (29.0) | 0.84 (0.36–1.96) | .68 |
| ≤40 | 116 (62.7) | 22 (71.0) | Ref | |
| Sex | ||||
| Male | 179 (96.8) | 31 (100) | 112 264.20 (0.00–∞) | .98 |
| Female | 6 (3.2) | 0 (0) | Ref | |
| BMI, kg/m2 | ||||
| ≥23 | 91 (49.2) | 9 (29.0) | 0.53 (0.24–1.16) | .11 |
| <23 | 94 (50.8) | 22 (71.0) | Ref | |
| HCV RNA, IU/mL | ||||
| >6 000 000 | 79 (42.7) | 11 (35.5) | 0.79 (0.37–1.72) | .56 |
| ≤6 000 000 | 106 (57.3) | 20 (64.5) | Ref | |
| HCV genotypea | ||||
| 1 | 67 (36.2) | 14 (45.2) | 1.77 (0.85–3.70) | .13 |
| Non-1 | 118 (63.8) | 17 (54.8) | Ref | |
| Hepatic fibrosisb | ||||
| F3–F4 | 29 (15.8) | 3 (9.7) | 0.84 (0.23–3.07) | .80 |
| F0–F2 | 155 (84.2) | 28 (90.3) | Ref | |
| ALT | ||||
| >ULN | 40 (21.6) | 2 (6.5) | 0.29 (0.07–1.23) | .09 |
| ≤ULN | 145 (78.4) | 29 (93.5) | Ref | |
| Antiviral treatment | ||||
| DAA | 88 (47.6) | 19 (61.3) | 1.84 (0.87–3.91) | .11 |
| IFN | 97 (52.4) | 12 (39.7) | Ref | |
| . | Multivariate Analysis . | |||
|---|---|---|---|---|
| No Reinfection (n = 185) . | Reinfection (n = 31) . | HR (95% CI) . | P Value . | |
| HIV risk group | ||||
| MSM | 170 (91.9) | 30 (96.8) | 1.41 (0.16–12.32) | .76 |
| Non-MSM | 15 (8.1) | 1 (3.2) | Ref | |
| CD4 count, 106 cells/L | ||||
| <500 | 73 (39.5) | 9 (29.0) | 0.63 (0.28–1.39) | .25 |
| ≥500 | 112 (60.5) | 22 (71.0) | Ref | |
| Age, y | ||||
| >40 | 69 (37.3) | 9 (29.0) | 0.84 (0.36–1.96) | .68 |
| ≤40 | 116 (62.7) | 22 (71.0) | Ref | |
| Sex | ||||
| Male | 179 (96.8) | 31 (100) | 112 264.20 (0.00–∞) | .98 |
| Female | 6 (3.2) | 0 (0) | Ref | |
| BMI, kg/m2 | ||||
| ≥23 | 91 (49.2) | 9 (29.0) | 0.53 (0.24–1.16) | .11 |
| <23 | 94 (50.8) | 22 (71.0) | Ref | |
| HCV RNA, IU/mL | ||||
| >6 000 000 | 79 (42.7) | 11 (35.5) | 0.79 (0.37–1.72) | .56 |
| ≤6 000 000 | 106 (57.3) | 20 (64.5) | Ref | |
| HCV genotypea | ||||
| 1 | 67 (36.2) | 14 (45.2) | 1.77 (0.85–3.70) | .13 |
| Non-1 | 118 (63.8) | 17 (54.8) | Ref | |
| Hepatic fibrosisb | ||||
| F3–F4 | 29 (15.8) | 3 (9.7) | 0.84 (0.23–3.07) | .80 |
| F0–F2 | 155 (84.2) | 28 (90.3) | Ref | |
| ALT | ||||
| >ULN | 40 (21.6) | 2 (6.5) | 0.29 (0.07–1.23) | .09 |
| ≤ULN | 145 (78.4) | 29 (93.5) | Ref | |
| Antiviral treatment | ||||
| DAA | 88 (47.6) | 19 (61.3) | 1.84 (0.87–3.91) | .11 |
| IFN | 97 (52.4) | 12 (39.7) | Ref | |
Abbreviations: ALT, alanine aminotransferase; BMI, body mass index; CD, cluster of differentiation; DAA, direct-acting antiviral; HCV, hepatitis C virus; HR, hazard ratio; IFN, interferon; MSM, men who have sex with men; ULN, upper limit of normal.
Patients with mixed HCV infection and those with invalid HCV genotypes were categorized as having HCV GT non-1 infection.
One patient without HCV reinfection was excluded from the analysis because of measurement failure with transient elastography.
Baseline Factors Associated With HCV Reinfection in HIV-Positive Patients Using the Multivariate Cox Regression Model
| . | Multivariate Analysis . | |||
|---|---|---|---|---|
| No Reinfection (n = 185) . | Reinfection (n = 31) . | HR (95% CI) . | P Value . | |
| HIV risk group | ||||
| MSM | 170 (91.9) | 30 (96.8) | 1.41 (0.16–12.32) | .76 |
| Non-MSM | 15 (8.1) | 1 (3.2) | Ref | |
| CD4 count, 106 cells/L | ||||
| <500 | 73 (39.5) | 9 (29.0) | 0.63 (0.28–1.39) | .25 |
| ≥500 | 112 (60.5) | 22 (71.0) | Ref | |
| Age, y | ||||
| >40 | 69 (37.3) | 9 (29.0) | 0.84 (0.36–1.96) | .68 |
| ≤40 | 116 (62.7) | 22 (71.0) | Ref | |
| Sex | ||||
| Male | 179 (96.8) | 31 (100) | 112 264.20 (0.00–∞) | .98 |
| Female | 6 (3.2) | 0 (0) | Ref | |
| BMI, kg/m2 | ||||
| ≥23 | 91 (49.2) | 9 (29.0) | 0.53 (0.24–1.16) | .11 |
| <23 | 94 (50.8) | 22 (71.0) | Ref | |
| HCV RNA, IU/mL | ||||
| >6 000 000 | 79 (42.7) | 11 (35.5) | 0.79 (0.37–1.72) | .56 |
| ≤6 000 000 | 106 (57.3) | 20 (64.5) | Ref | |
| HCV genotypea | ||||
| 1 | 67 (36.2) | 14 (45.2) | 1.77 (0.85–3.70) | .13 |
| Non-1 | 118 (63.8) | 17 (54.8) | Ref | |
| Hepatic fibrosisb | ||||
| F3–F4 | 29 (15.8) | 3 (9.7) | 0.84 (0.23–3.07) | .80 |
| F0–F2 | 155 (84.2) | 28 (90.3) | Ref | |
| ALT | ||||
| >ULN | 40 (21.6) | 2 (6.5) | 0.29 (0.07–1.23) | .09 |
| ≤ULN | 145 (78.4) | 29 (93.5) | Ref | |
| Antiviral treatment | ||||
| DAA | 88 (47.6) | 19 (61.3) | 1.84 (0.87–3.91) | .11 |
| IFN | 97 (52.4) | 12 (39.7) | Ref | |
| . | Multivariate Analysis . | |||
|---|---|---|---|---|
| No Reinfection (n = 185) . | Reinfection (n = 31) . | HR (95% CI) . | P Value . | |
| HIV risk group | ||||
| MSM | 170 (91.9) | 30 (96.8) | 1.41 (0.16–12.32) | .76 |
| Non-MSM | 15 (8.1) | 1 (3.2) | Ref | |
| CD4 count, 106 cells/L | ||||
| <500 | 73 (39.5) | 9 (29.0) | 0.63 (0.28–1.39) | .25 |
| ≥500 | 112 (60.5) | 22 (71.0) | Ref | |
| Age, y | ||||
| >40 | 69 (37.3) | 9 (29.0) | 0.84 (0.36–1.96) | .68 |
| ≤40 | 116 (62.7) | 22 (71.0) | Ref | |
| Sex | ||||
| Male | 179 (96.8) | 31 (100) | 112 264.20 (0.00–∞) | .98 |
| Female | 6 (3.2) | 0 (0) | Ref | |
| BMI, kg/m2 | ||||
| ≥23 | 91 (49.2) | 9 (29.0) | 0.53 (0.24–1.16) | .11 |
| <23 | 94 (50.8) | 22 (71.0) | Ref | |
| HCV RNA, IU/mL | ||||
| >6 000 000 | 79 (42.7) | 11 (35.5) | 0.79 (0.37–1.72) | .56 |
| ≤6 000 000 | 106 (57.3) | 20 (64.5) | Ref | |
| HCV genotypea | ||||
| 1 | 67 (36.2) | 14 (45.2) | 1.77 (0.85–3.70) | .13 |
| Non-1 | 118 (63.8) | 17 (54.8) | Ref | |
| Hepatic fibrosisb | ||||
| F3–F4 | 29 (15.8) | 3 (9.7) | 0.84 (0.23–3.07) | .80 |
| F0–F2 | 155 (84.2) | 28 (90.3) | Ref | |
| ALT | ||||
| >ULN | 40 (21.6) | 2 (6.5) | 0.29 (0.07–1.23) | .09 |
| ≤ULN | 145 (78.4) | 29 (93.5) | Ref | |
| Antiviral treatment | ||||
| DAA | 88 (47.6) | 19 (61.3) | 1.84 (0.87–3.91) | .11 |
| IFN | 97 (52.4) | 12 (39.7) | Ref | |
Abbreviations: ALT, alanine aminotransferase; BMI, body mass index; CD, cluster of differentiation; DAA, direct-acting antiviral; HCV, hepatitis C virus; HR, hazard ratio; IFN, interferon; MSM, men who have sex with men; ULN, upper limit of normal.
Patients with mixed HCV infection and those with invalid HCV genotypes were categorized as having HCV GT non-1 infection.
One patient without HCV reinfection was excluded from the analysis because of measurement failure with transient elastography.
Secular Trends of HCV Reinfection by Calendar Year
The trends in the incidence of HCV reinfection in HIV-positive and reference patients according to calendar year showed a significant difference (P < .001) (Figure 3). While the incidence rates of HCV reinfection between 2005 and 2021 in reference patients ranged from 0 to 0.26 per 100 PYFU, the incidence rates of HCV reinfection in HIV-positive patients remained 0 per 100 PYFU between 2005 and 2014 when IFN was the only available treatment option and increased to 2.82 per 100 PYFU in 2015 when patients in Taiwan could get access to the out-of-pocket brand-name and generic DAAs. The incidence rate of HCV reinfection in HIV-positive patients progressively increased from 3.85 to 4.30 per 100 PYFU in 2017 and 2018, when limited brand-name DAA reimbursement was carried out. Following the unlimited brand-name DAA reimbursement in 2019, the incidence rates of HCV reinfection in HIV-positive patients were 4.39, 5.74, and 6.42 per 100 PYFU in 2019, 2020 and 2021, respectively (Figure 3).
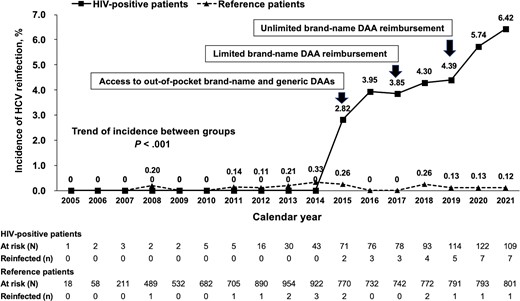
Secular trends of HCV reinfection in HIV-positive and reference patients with treatment-induced SVR12 by calendar year. Abbreviations: DAA, direct-acting antiviral; HCV, hepatitis C virus; SVR12, sustained virologic response at 12 months.
DISCUSSION
In line with meta-analyses and population-based cohort studies, our prospective study confirmed that the crude and cumulative incidence rates of HCV reinfection in HIV-positive patients were much higher than those in reference patients who achieved SVR12 with HCV treatment in Taiwan [22, 29]. No specific baseline factors, such as HIV risk group, CD4 count, or type of antiviral treatment received, predicted HCV reinfection in HIV-positive patients. Moreover, the increasing trend of HCV reinfection in HIV-positive patients with treatment-induced SVR12 after 2015 indicated a significant public health alert in Taiwan.
Compared with the highly varied reported incidence of HCV reinfection from 0% to 17% in HIV-positive patients, the incidence of HCV reinfection in our patients with treatment-induced SVR12 was 4.02 per 100 PYFU, which was in line with a recent large-scale pan-Europe survey that demonstrated an overall incidence of HCV reinfection of 4.65 per 100 PYFU in HIV-positive patients who achieved SVR with IFN or DAA [21]. Because most patients were MSM, we further demonstrated the risk of HCV reinfection in our HIV-positive MSM to be 4.12 per 100 PYFU, which was also similar to a long-term cohort that reported an incidence of 3.85 per 100 PYFU in the Netherlands [18]. Different patient HIV risk groups and risk behaviors, duration of post-SVR follow-up, definition of HCV reinfection, type of HCV RNA surveillance, and HCV therapeutic policies by health authorities may contribute to the risk estimate discrepancies across various studies [30].
Although the risk of HCV reinfection of our HIV-positive patients was much higher than that of the reference patients, we found that no baseline factors predicted HCV reinfection [21]. Multiple sex partners, unprotected anal intercourse, group sex, sharing sex toys, and amphetamine abuse in HIV-positive MSM and ongoing use of cocaine, methamphetamine, or heroin injection in HIV-positive PWID after achieving SVR were highly associated with HCV reinfection; these factors were evident in our HIV-positive patients with HCV reinfection [17, 20, 24, 30]. Furthermore, studies also showed that a new diagnosis of sexually transmitted infection or syphilis during post-SVR12 follow-up was highly associated with risk of HCV infection among HIV-positive patients [23, 31].
While most Western studies have demonstrated decreasing incidence rates of HCV reinfection in HIV-positive patients following the implementation of HCV screening and DAA treatment scale-up after 2014, the secular trend of HCV reinfection tended to increase in our HIV-positive patients after 2015, when DAAs were made available in Taiwan [15, 16, 18, 21, 32]. Several factors may contribute to the divergent trends of HCV reinfection. First, prior studies have suggested that the frequency of HCV RNA surveillance may affect the reported incidence [18, 24]. However, our study had a protocol-defined frequency for HCV RNA testing, and only 14 (0.9%) unscheduled tests were performed during the post-SVR12 surveillance in HIV-positive patients, making overestimation of the incidence after 2015 unlikely. Our reasoning is supported by an independent study reporting the risk of HCV reinfection to be 5.66 per 100 PYFU between 2019 and 2021 in HIV-positive patients in Taiwan by quarterly HCV RNA testing, which was similar to our biannual surveillance showing incidence rates of 4.39, 5.74, and 6.42 per 100 PYFU in 2019, 2020, and 2021, respectively [31]. Second, although the introduction of DAAs in Taiwan after 2015 increased HCV treatment uptake in HIV-positive patients, delayed full DAA reimbursement and unsatisfactory HCV screening programs may contribute to the persistence of the viremic pool. Third, the increasing use of recreational drugs and pre-exposure prophylaxis (PrEP) for HIV among MSM may potentiate viral transmission through high-risk sexual behaviors [33–35]. Fourth, compared with the suboptimal efficacy and tolerance of IFN, the awareness of potent and safe DAAs to manage HCV reinfection in HIV-positive patients may reduce vigilance for the unsafe sex and shared injections that potentiate reinfection [36–39]. As observed in our cohort, studies from Germany, the Netherlands, and Spain indicated that the risk of HCV reinfection tended to increase in the era of DAAs among MSM with high-risk sexual behaviors regardless of HIV status [17, 20, 40].
With the availability of DAAs, the WHO has targeted HCV elimination by 2030. While HCV microelimination among HIV-positive patients may be achievable in the foreseeable future in most European countries, Australia, and the United States, health care providers should speed up HCV microelimination among HIV-positive patients in Taiwan [10, 12–16, 18]. To fulfill the WHO’s target of HCV elimination by attaining 90% diagnosis and 80% treatment uptake, 72% of HIV-positive patients with HCV are assumed to be treated, and at least 70% should achieve HCV cure based on an SVR12 rate of 95% with DAAs. Currently, data are not available concerning the screening and treatment rates for HCV among HIV-positive MSM patients in Taiwan. Studies have shown that the SVR12 rates in HIV-positive patients in Taiwan with pangenotypic sofosbuvir/velpatasvir (SOF/VEL) and glecaprevir/pibrentasvir (GLE/PIB) range from 97.1% to 98.2% and are comparable to the response rates in the usual population [39, 41, 42]. By increasing patient awareness, mass screening, point-of-care testing, and link-to-care programs, the HCV reservoir in at-risk populations could be efficiently controlled. Furthermore, education on harm reduction and prudential post-treatment surveillance are also needed to lessen HCV transmission and facilitate timely intervention [43].
The strengths of our study included (1) a sizable number of HIV-positive and reference patients with 3.0–6.0 years of post-SVR12 follow-up to compare the risks of HCV reinfection; (2) a multicenter prospective cohort with protocol-defined post-SVR12 surveillance for HCV RNA to avoid over- or underestimating HCV reinfection; (3) robust evidence to confirm HCV reinfection by commercial genotyping assays and sequencing analysis. However, our study had several limitations. First, we were unable to assess the association of patterns or frequencies of ongoing high-risk sexual behaviors or injection drug use with HCV reinfection beyond SVR12 in HIV-positive patients because we did not systemically inquire about these risk factors at each visit. Second, comparing the risk of HCV reinfection in high-risk patients with or without HIV infection was not possible because we had only 6 high-risk participants without HIV infection (1 MSM, 3 PWID, and 2 incarcerated individuals) for risk comparison. Third, the duration of post-SVR12 follow-up in HIV-positive patients was relatively short, and a longer duration of surveillance is needed to delineate the risk and trend of HCV reinfection in Taiwan.
In conclusion, the risk of HCV reinfection in HIV-positive patients with treatment-induced SVR12 was much higher than that in reference patients in Taiwan. In addition to HCV screening and treatment scale-up, programs for safe sex counseling, interventions to reduce recreational drug use, and vigilant post-SVR12 surveillance should be implemented to eliminate HCV infection in HIV-positive patients as early as possible.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors thank Hui-Ju Lin and Pin-Chin Huang for clinical data management and the 7th Core Lab of National Taiwan University Hospital and the 1st Common Laboratory of National Taiwan University Hospital, Yun-Lin Branch, for instrumental and technical support.
Financial support. This work was funded by the Ministry of Science and Technology, Taiwan (MOST; 110-2314-B-002-172) and National Taiwan University Hospital (111-IF0011).
Potential conflicts of interest. CH Liu: advisory board for AbbVie, Gilead Sciences, Merck Sharp & Dohme; speaker’s bureau for Abbott, AbbVie, Gilead Sciences, Merck Sharp & Dohme; research grant from AbbVie, Gilead Science, Merck Sharp & Dohme. CC Hung: advisory board for Gilead Sciences; speaker’s bureau for Gilead Sciences; research grant from Gilead Sciences. JH Kao: advisory board for Abbott, AbbVie, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, Novartis, Roche; speaker’s bureau for Abbott, AbbVie, Bayer, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, Novartis, Roche. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. Conception and design: CH Liu; analysis and interpretation of data: CH Liu; drafting of the article: CH Liu, CC Hung, JH Kao; critical revision of the article for important intellectual content: CH Liu, HY Sun, CY Peng, SM Hsieh, SS Yang, WY Kao, YL Shih, CL Lin, CJ Liu, WH Sheng, YC Lo, WC Liu, JH Wu, TH Su, TC Tseng, PJ Chen, JH Kao; final approval of the article: CH Liu, HY Sun, CY Peng, SM Hsieh, SS Yang, WY Kao, YL Shih, CL Lin, CJ Liu, WH Sheng, YC Lo, WC Liu, JH Wu, TH Su, TC Tseng, PJ Chen, JH Kao; provision of study materials or patients: CH Liu, HY Sun, CY Peng, SM Hsieh, SS Yang, WY Kao, YL Shih, CL Lin, CJ Liu, WH Sheng, YC Lo, WC Liu, JH Wu, TH Su, TC Tseng, PJ Chen, JH Kao; statistical expertise: CH Liu; administrative, technical, or logistic support: CC Hung, JH Kao; collection and assembly of data: CH Liu.





Comments