-
PDF
- Split View
-
Views
-
Cite
Cite
F Julian Lantry, Nusrat J Epsi, Simon D Pollett, Mark P Simons, David A Lindholm, Rhonda E Colombo, Anthony C Fries, Ryan C Maves, Anuradha Ganesan, Gregory C Utz, Tahaniyat Lalani, Alfred G Smith, Rupal M Mody, Christopher J Colombo, Sharon W Chi, Cristian Madar, Nikhil Huprikar, Derek T Larson, Samantha Bazan, Christopher C Broder, Eric D Laing, Caroline English, Charlotte Lanteri, Katrin Mende, David R Tribble, Brian K Agan, Timothy H Burgess, Stephanie A Richard, EPICC COVID-19 Cohort Study Group , Anatomical Site, Viral Ribonucleic Acid Abundance, and Time of Sampling Correlate With Molecular Detection of Severe Acute Respiratory Syndrome Coronavirus 2 During Infection, Open Forum Infectious Diseases, Volume 9, Issue 3, March 2022, ofab623, https://doi.org/10.1093/ofid/ofab623
Close - Share Icon Share
Abstract
Nasopharyngeal (NP) swabs are the standard for SARS-CoV-2 diagnosis. If less invasive alternatives to NP swabs (eg, oropharyngeal [OP] or nasal swabs [NS]) are comparably sensitive, the use of these techniques may be preferable in terms of comfort, convenience, and safety.
This study compared the detection of SARS-CoV-2 in swab samples collected on the same day among participants with at least one positive PCR test.
Overall, 755 participants had at least one set of paired swabs. Concordance between NP and other swab types was 75% (NS), 72% (OP), 54% (rectal swabs [RS]), and 78% (NS/OP combined). Kappa values were moderate for the NS, OP, and NS/OP comparisons (0.50, 0.45, and 0.54, respectively). Highest sensitivity relative to NP (0.87) was observed with a combination of NS/OP tests (positive if either NS or OP was positive). Sensitivity of the non-NP swab types was highest in the first week postsymptom onset and decreased thereafter. Similarly, virus RNA quantity was highest in the NP swabs as compared with NS, OP, and RS within two weeks postsymptom onset. OP and NS performance decreased as virus RNA quantity decreased. No differences were noted between NS specimens collected at home or in clinic.
NP swabs detected more SARS-CoV-2 cases than non-NP swabs, and the sensitivity of the non-NP swabs decreased with time postsymptom onset. While other swabs may be simpler to collect, NP swabs present the best chance of detecting SARS-CoV-2 RNA, which is essential for clinical care as well as genomic surveillance.
The rapid and accurate detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a fundamental aspect of clinical care and population surveillance to limit its spread. The Centers for Disease Control and Prevention (CDC) has recommended the use of swabs to collect upper respiratory specimens for the detection of SARS-CoV-2 [1]. Nasopharyngeal (NP) swabs have been regarded as the preferred collection device for SARS-CoV-2 detection [2, 3]. However, non-NP collection routes, such as anterior nasal swabs (NS), may be preferred based on patient comfort and potential decreased infection risk to the healthcare worker [2, 4]. The existing literature is mixed regarding the comparability of different sample collection methods for SARS-CoV-2 detection [2, 5], including whether such differences change with time postsymptom onset or may simply be explained by differences in collection adequacy. Moreover, there are limited data on the performance of rectal swabs (RS) compared with upper respiratory specimens for detection of SARS-CoV-2 virus [6].
The primary goal of this analysis was to compare NP swabs for the detection of SARS-CoV-2 with alternative collection types (NS, oropharyngeal swabs [OP], and RS) using paired samples collected on the same day from participants enrolled in the Epidemiology, Immunology, and Clinical Characteristics of Emerging Infectious Diseases with Pandemic Potential (EPICC) cohort study. Second, the study compared time-varying viral ribonucleic acid (RNA) load estimates determined from quantitative polymerase chain reaction (qPCR) cycle threshold (Ct) values among the different sample types. In addition to examining how anatomical site, RNA abundance, and illness time changed PCR sensitivity, we further investigated whether self-collection of NS specimens changed PCR assay sensitivity.
METHODS
The EPICC study is a longitudinal cohort study investigating the risk factors associated with and the short- and long-term effects of SARS-CoV-2 infection in 10 military treatment facilities, 9 of which provided data and samples for this analysis (Brooke Army Medical Center, Carl R. Darnall Army Medical Center, Fort Belvoir Community Hospital, Madigan Army Medical Center, Naval Medical Center Portsmouth, Naval Medical Center San Diego, Tripler Army Medical Center, Walter Reed National Military Medical Center, and William Beaumont Army Medical Center). The EPICC study is enrolling participants who are military healthcare beneficiaries (active duty, dependents, or retirees) who have confirmed SARS-CoV-2 infection, a coronavirus disease 2019 (COVID-19)-like illness, a high-risk exposure to SARS-CoV-2, or who have been tested for or vaccinated against SARS-CoV-2 [7]. Participants included in this analysis were required to have tested positive for SARS-CoV-2 infection (confirmed case of COVID-19 based on any PCR-positive test within 7 days presymptom onset to 21 days postsymptom onset) and have at least 1 pair of swabs from different sites collected on the same day within 30 days of symptom onset.
The EPICC study personnel collected swab specimens using synthetic flocked swabs from outpatients at day 0 and 14 postenrollment from March 2020 onward. Beginning in July 2020, participants were offered the option of at-home NS collection if they were unable to present for in-person collection. Inpatients had swabs collected on day 0, 3, and 7 and then weekly thereafter, if they continued to be hospitalized. Nasal swabs were self-collected, whereas OP and NP were collected by clinic staff, and RS were either self-collected or clinic staff-collected. Because concurrent, standardized swab collections were required for comparison, we excluded results from clinical specimens collected before enrollment into this study.
The qPCR was performed on study samples using the SARS-CoV-2 (2019-nCoV) CDC qPCR Probe Assay research use only kit (catalog no. 10006770; Integrated DNA Technologies, Inc., Coralville, IA). Two regions of the nucleocapsid (N) gene are targeted with the assay (N1 and N2), and an additional primer/probe is included to detect the human Ribonuclease P gene (RP) in the samples as a quality control measure. Samples with Ct values of <40 for both N1 and N2 were considered to be SARS-CoV-2 positive consistent with the instructions for use. Viral RNA load (genome equivalents [GE]/reaction) were determined using standard curves specific to each assay plate. Each curve was calculated based on at least 3 dilutions of known SARS-CoV-2 gene copy numbers. These plate-specific curves represent the relationship between Ct and viral copy number for each plate and then allowed for conversion of Ct values to GE/reaction for each swab specimen [8].
To compare SARS-CoV-2 detection among NP, OP, NS, and RS samples collected on the same day from the same participant, we calculated positive result concordance, discordance, and associated kappa (SE) values for NP/OP, NP/NS, NP/RS, and NP/OP + NS paired sets. For the OP + NS paired set, participants had NP, OP, and NS samples, and results from the OP and NS swab were combined; if either the OP or the NS sample was positive, the OP + NS category was positive. We also determined the sensitivity of OP, NS, RS, and OP + NS samples compared with the NP samples as the reference. For this initial analysis, we tested only the first paired sample set collected for each person for the analysis. We also compared the sensitivity in different categories of time since onset of symptoms (<7 days, 7–13 days, and 14–30 days) and age groups (18–44, 45–64, and 65+); for the time since onset analysis, we retained the first paired sample collected in each time period for each person. Viral load quantity (GE/reaction) and RP values (to estimate collection adequacy [9]) were compared among swab sample types using paired t tests for paired samples and standard t tests for nonpaired samples. Viral load quantity (GE/reaction) for the N1 and N2 nucleocapsid gene targets was log10 transformed for the comparisons.
Patient Consent Statement
The EPICC was conducted in accordance with the Declaration of Helsinki and good clinical practice guidelines. Informed consent was obtained from all individual participants in the study and this study was approved by the Uniformed Services University Institutional Review Board (IDCRP-085).
RESULTS
In total, 755 SARS-CoV-2-positive participants were included in this analysis (Table 1), among whom 63.6% had both a positive clinical and research sample PCR test. Six hundred thirty-four participants had paired NP/NS samples, 560 had paired NP/OP samples, 92 had paired NP/RS samples, and 427 had paired NP/OP + NS samples. Among the samples collected in the first week postsymptom onset, NP detected the greatest percentage of cases (75%), followed by NS (66%), OP (62%), and RS (29%). Overall concordance values ranged from 54% for the NP/RS pairs to 72% for the NP/OP pairs and 75% for the NP/NS pairs (Table 2). Likewise, kappa values were moderate for the OP and NS comparisons (0.45 and 0.50), whereas the kappa value was quite low for RS (0.16).
Demographic Characteristics of EPICC Participants With at Least One Set of Paired Swab Samples
| Characteristics . | N = 755 . |
|---|---|
| Age Group | |
| <18 | 14 (1.9%) |
| 18–44 | 357 (47.3%) |
| 45–64 | 269 (35.6%) |
| 65+ | 115 (15.2%) |
| Male | 476 (63.0%) |
| Race | |
| Asian | 36 (4.8%) |
| Black | 121 (16.0%) |
| Hispanic or Latino | 189 (25.0%) |
| Other | 34 (4.5%) |
| White | 375 (49.7%) |
| Military Status | |
| Active duty | 321 (42.5%) |
| Dependent | 188 (24.9%) |
| Retired military | 246 (32.6%) |
| Days Postsymptom Onset Category | |
| <7 DPSO | 110 (14.6%) |
| 7–13 DPSO | 225 (29.8%) |
| 14–30 DPSO | 420 (55.6%) |
| Research Swab Positive | 480 (63.6%) |
| Positive Test Reported in MDR/Survey | 641 (84.9%) |
| Characteristics . | N = 755 . |
|---|---|
| Age Group | |
| <18 | 14 (1.9%) |
| 18–44 | 357 (47.3%) |
| 45–64 | 269 (35.6%) |
| 65+ | 115 (15.2%) |
| Male | 476 (63.0%) |
| Race | |
| Asian | 36 (4.8%) |
| Black | 121 (16.0%) |
| Hispanic or Latino | 189 (25.0%) |
| Other | 34 (4.5%) |
| White | 375 (49.7%) |
| Military Status | |
| Active duty | 321 (42.5%) |
| Dependent | 188 (24.9%) |
| Retired military | 246 (32.6%) |
| Days Postsymptom Onset Category | |
| <7 DPSO | 110 (14.6%) |
| 7–13 DPSO | 225 (29.8%) |
| 14–30 DPSO | 420 (55.6%) |
| Research Swab Positive | 480 (63.6%) |
| Positive Test Reported in MDR/Survey | 641 (84.9%) |
Abbreviations: DPSO, days postsymptom onset; EPICC, Epidemiology, Immunology, and Clinical Characteristics of Emerging Infectious Diseases with Pandemic Potential; MDR, Military Health System Data Repository.
Demographic Characteristics of EPICC Participants With at Least One Set of Paired Swab Samples
| Characteristics . | N = 755 . |
|---|---|
| Age Group | |
| <18 | 14 (1.9%) |
| 18–44 | 357 (47.3%) |
| 45–64 | 269 (35.6%) |
| 65+ | 115 (15.2%) |
| Male | 476 (63.0%) |
| Race | |
| Asian | 36 (4.8%) |
| Black | 121 (16.0%) |
| Hispanic or Latino | 189 (25.0%) |
| Other | 34 (4.5%) |
| White | 375 (49.7%) |
| Military Status | |
| Active duty | 321 (42.5%) |
| Dependent | 188 (24.9%) |
| Retired military | 246 (32.6%) |
| Days Postsymptom Onset Category | |
| <7 DPSO | 110 (14.6%) |
| 7–13 DPSO | 225 (29.8%) |
| 14–30 DPSO | 420 (55.6%) |
| Research Swab Positive | 480 (63.6%) |
| Positive Test Reported in MDR/Survey | 641 (84.9%) |
| Characteristics . | N = 755 . |
|---|---|
| Age Group | |
| <18 | 14 (1.9%) |
| 18–44 | 357 (47.3%) |
| 45–64 | 269 (35.6%) |
| 65+ | 115 (15.2%) |
| Male | 476 (63.0%) |
| Race | |
| Asian | 36 (4.8%) |
| Black | 121 (16.0%) |
| Hispanic or Latino | 189 (25.0%) |
| Other | 34 (4.5%) |
| White | 375 (49.7%) |
| Military Status | |
| Active duty | 321 (42.5%) |
| Dependent | 188 (24.9%) |
| Retired military | 246 (32.6%) |
| Days Postsymptom Onset Category | |
| <7 DPSO | 110 (14.6%) |
| 7–13 DPSO | 225 (29.8%) |
| 14–30 DPSO | 420 (55.6%) |
| Research Swab Positive | 480 (63.6%) |
| Positive Test Reported in MDR/Survey | 641 (84.9%) |
Abbreviations: DPSO, days postsymptom onset; EPICC, Epidemiology, Immunology, and Clinical Characteristics of Emerging Infectious Diseases with Pandemic Potential; MDR, Military Health System Data Repository.
| Swab Pairs . | Number . | Both Positive . | Both Negative . | Discordant, NP+ . | Discordant, NP− . | Overall Concordance . | Overall Discordance . | Kappa (SE) . | Sensitivity (95% CI) . |
|---|---|---|---|---|---|---|---|---|---|
| NP/NS | 634 | 188 (30%) | 287 (45%) | 124 (20%) | 35 (5%) | 475 (75%) | 159 (25%) | 0.50 (0.03) | 0.60 (0.55–0.66) |
| NP/OP | 560 | 211 (38%) | 190 (34%) | 129 (23%) | 30 (5%) | 401 (72%) | 159 (28%) | 0.45 (0.04) | 0.62 (0.57–0.67) |
| NP/RS | 92 | 10 (11%) | 40 (43%) | 41 (45%) | 1 (1%) | 50 (54%) | 42 (46%) | 0.16 (0.06) | 0.20 (0.10–0.33) |
| NP/OP + NS | 427 | 212 (50%) | 120 (28%) | 33 (8%) | 62 (14%) | 332 (78%) | 95 (22%) | 0.54 (0.04) | 0.87 (0.82–0.91) |
| Swab Pairs . | Number . | Both Positive . | Both Negative . | Discordant, NP+ . | Discordant, NP− . | Overall Concordance . | Overall Discordance . | Kappa (SE) . | Sensitivity (95% CI) . |
|---|---|---|---|---|---|---|---|---|---|
| NP/NS | 634 | 188 (30%) | 287 (45%) | 124 (20%) | 35 (5%) | 475 (75%) | 159 (25%) | 0.50 (0.03) | 0.60 (0.55–0.66) |
| NP/OP | 560 | 211 (38%) | 190 (34%) | 129 (23%) | 30 (5%) | 401 (72%) | 159 (28%) | 0.45 (0.04) | 0.62 (0.57–0.67) |
| NP/RS | 92 | 10 (11%) | 40 (43%) | 41 (45%) | 1 (1%) | 50 (54%) | 42 (46%) | 0.16 (0.06) | 0.20 (0.10–0.33) |
| NP/OP + NS | 427 | 212 (50%) | 120 (28%) | 33 (8%) | 62 (14%) | 332 (78%) | 95 (22%) | 0.54 (0.04) | 0.87 (0.82–0.91) |
Abbreviations: CI, confidence interval; NP, nasopharyngeal swab; NS, nasal swab; OP, oropharyngeal swab; RS, rectal swab; OP + NS, OP or NS positive; SE, standard error.
| Swab Pairs . | Number . | Both Positive . | Both Negative . | Discordant, NP+ . | Discordant, NP− . | Overall Concordance . | Overall Discordance . | Kappa (SE) . | Sensitivity (95% CI) . |
|---|---|---|---|---|---|---|---|---|---|
| NP/NS | 634 | 188 (30%) | 287 (45%) | 124 (20%) | 35 (5%) | 475 (75%) | 159 (25%) | 0.50 (0.03) | 0.60 (0.55–0.66) |
| NP/OP | 560 | 211 (38%) | 190 (34%) | 129 (23%) | 30 (5%) | 401 (72%) | 159 (28%) | 0.45 (0.04) | 0.62 (0.57–0.67) |
| NP/RS | 92 | 10 (11%) | 40 (43%) | 41 (45%) | 1 (1%) | 50 (54%) | 42 (46%) | 0.16 (0.06) | 0.20 (0.10–0.33) |
| NP/OP + NS | 427 | 212 (50%) | 120 (28%) | 33 (8%) | 62 (14%) | 332 (78%) | 95 (22%) | 0.54 (0.04) | 0.87 (0.82–0.91) |
| Swab Pairs . | Number . | Both Positive . | Both Negative . | Discordant, NP+ . | Discordant, NP− . | Overall Concordance . | Overall Discordance . | Kappa (SE) . | Sensitivity (95% CI) . |
|---|---|---|---|---|---|---|---|---|---|
| NP/NS | 634 | 188 (30%) | 287 (45%) | 124 (20%) | 35 (5%) | 475 (75%) | 159 (25%) | 0.50 (0.03) | 0.60 (0.55–0.66) |
| NP/OP | 560 | 211 (38%) | 190 (34%) | 129 (23%) | 30 (5%) | 401 (72%) | 159 (28%) | 0.45 (0.04) | 0.62 (0.57–0.67) |
| NP/RS | 92 | 10 (11%) | 40 (43%) | 41 (45%) | 1 (1%) | 50 (54%) | 42 (46%) | 0.16 (0.06) | 0.20 (0.10–0.33) |
| NP/OP + NS | 427 | 212 (50%) | 120 (28%) | 33 (8%) | 62 (14%) | 332 (78%) | 95 (22%) | 0.54 (0.04) | 0.87 (0.82–0.91) |
Abbreviations: CI, confidence interval; NP, nasopharyngeal swab; NS, nasal swab; OP, oropharyngeal swab; RS, rectal swab; OP + NS, OP or NS positive; SE, standard error.
When compared with results from NP swabs, NS and OP swab results had similar sensitivity (NS, 0.60; OP, 0.62) (Table 2). Rectal swabs had very low sensitivity (0.20). Sensitivity was higher for all non-NP (alternative) types in the first week after symptom onset and decreased thereafter (Figure 1). A subanalysis considering performance of the alternative sample types by age category identified a trend toward lower sensitivity in all alternative sample types in the youngest age group (18–44) (Supplementary Figure 1), but the differences were not statistically significant.
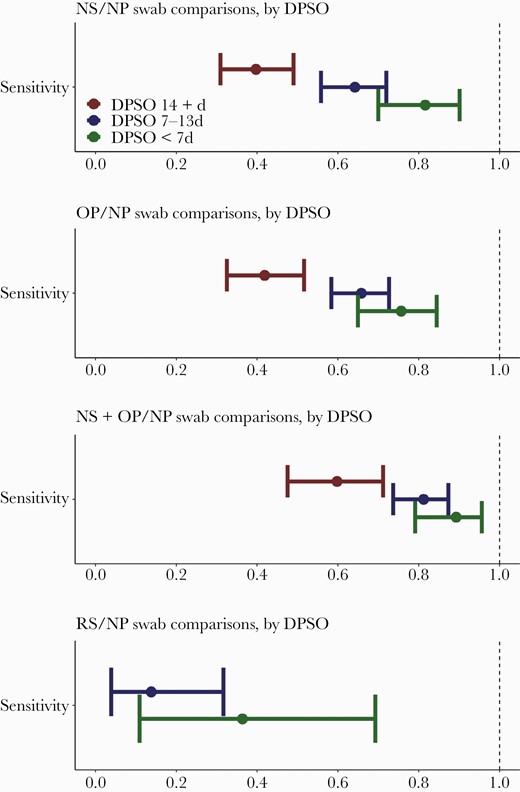
Sensitivity of alternative swab types compared with nasopharyngeal (NP) swabs collected on the same day in the Epidemiology, Immunology, and Clinical Characteristics of Emerging Infectious Diseases with Pandemic Potential (EPICC) study, categorized by days postsymptom onset (DPSO). d, days; NS, nasal swab; OP, oropharyngeal swab; RS, rectal swab.
When we compared N1 and N2 RNA quantity (GE/reaction) between NP/OP, NP/NS, and NP/RS sample pairs, we found that the viral copy number was higher in the NP sample than it was in the OP samples (N1, mean log10 difference = 0.95; 95% confidence interval [CI], 0.69–1.21 and N2, 0.80; 95% CI, 0.55–1.05), the NS samples (N1, 0.54; 95% CI, 0.35–0.72 and N2, 0.48; 95% CI, 0.28–0.68), and the RS samples (N1, 2.58; 95% CI, 1.02–4.15 and N2, 2.51; 95% CI, 0.51–4.50) (Figure 2). The RP gene Ct was higher in the alternative swab types. The majority of paired samples (N = 420; 55.6%) were collected between 14 and 30 days postsymptom onset, whereas 29.8% (N = 225) were collected within 7 to 13 days, and 14.6% (N = 110) were collected within 7 days (Table 1). The median days postsymptom onset for the collections in the first week postsymptom onset was 4 days. When analyzed separately by days postsymptom onset category, N1 and N2 quantities were consistently higher in NP samples compared with OP and NS samples in the first 2 weeks postsymptom onset, but they were not statistically significantly different in the 14 + day period (Supplementary Figure 2). When compared by agreement category (concordant NP+/NS+ and discordant NP+/NS−), concordant samples were found to have higher virus quantity than discordant samples (Supplementary Figure 3). The results were similar for those samples that were positive for the alternative types and negative for the NP swabs (data not shown). The EPICC study introduced the option of self-collection of nasal swabs at home in July 2020, thus allowing for comparison of the different settings (clinic vs home collection of nasal swabs), and few differences were observed in the amount of virus detected in the different settings (Supplementary Figure 4).
![Severe acute respiratory syndrome coronavirus 2 log10 N1 quantity (genome equivalents [GE]/reaction), log10 N2 quantity (GE/reaction), and RNAse P gene (RP) cycle threshold (Ct) values, by swab type. Paired t tests were used to compare sample types. NP, nasopharyngeal swab; NS, nasal swab; OP, oropharyngeal swab; RS, rectal swab.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ofid/9/3/10.1093_ofid_ofab623/1/m_ofab623_fig2.jpeg?Expires=1750236219&Signature=zQ3~DfNtbj-MUTe8bZzKFLwNgzlKUl3brwP2vi8t2bjaOFBFfx7ZzQVVGUvHKCLrpWrz9TgNF4r3rLchPHIN1FK58GsEm6uN1~UNzG3hbfA4ZldQyK61-M4xIHuxmOQeCRjrf0S3lzDcA4nCUMiRJVPTUdZIWqbj9J8Sx~dgaFCAYS73QXZzeOaX75gaZtF0ohAEGDVsEXPkwe9s8uN4no0j~h5Tl8T5C94pX9L0zhPAZrGfbGudrBoWt2LSSe6rGn~SmvGiPT2p4ShjercoU4pZ1QyS6bt1yfZgqfFLqm9VzTzFusemtM5TYZ~u3O6xozO-LJSpgHwlp~dBTmF3hA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Severe acute respiratory syndrome coronavirus 2 log10 N1 quantity (genome equivalents [GE]/reaction), log10 N2 quantity (GE/reaction), and RNAse P gene (RP) cycle threshold (Ct) values, by swab type. Paired t tests were used to compare sample types. NP, nasopharyngeal swab; NS, nasal swab; OP, oropharyngeal swab; RS, rectal swab.
DISCUSSION
This study was a subanalysis within EPICC, enabled by the robust longitudinal collection of multiple sample types. Our findings demonstrate that NP swabs routinely yielded better detection of SARS-CoV-2 nucleic acid in SARS-CoV-2-positive participants than alternative swab types (OP, NS, RS) collected concurrently. Nasal swabs and OP had relatively low sensitivity compared with NP swabs, whereas kappa scores ranged from fair to moderate. Rectal swabs had very low sensitivity and kappa, compared with NP swabs, which is consistent with other studies [10, 11]. In addition, sampling time, but not age, was a predictor of diagnostic performance of the test with different sampling techniques in our study, as has been noted elsewhere [12].
Our findings highlight the importance of early collection, because the sensitivity of OP and NS swabs compared with NP swabs was highest when the samples were collected within 7 days postsymptom onset and decreased in later timepoints. These results are similar to those from a systematic review comparing alternative swab types to NP swabs [2], which found that the alternative swabs identified a lower percentage of positives than the NP swabs; however, the review noted some degree of publication bias, ie, the published studies appeared to be biased toward those that demonstrate that alternative sample types perform well. Rectal swab samples did not perform well relative to NP samples, although the numbers were limited.
The quantity of virus detected in the swab samples was significantly higher for NP swabs than for NS and OP swabs, despite higher RP values in the alternative sample types (RP values suggest adequate collection technique) [9]. These results would support a hypothesis that differences in detection for the NP swabs (compared with other swabs) are not explained by poor collection technique for NS and OP swabs. Our findings are consistent with prior studies showing a higher RNA abundance in NP swabs (compared with OP swabs) [3], although our study also demonstrated this in comparison with nasal and rectal swabs. All of the EPICC participants included in this analysis were symptomatic; given that NP swabs are more sensitive than the alternative sample types, one might expect that NP would perform better in asymptomatic individuals with potentially lower viral loads, but further research is needed.
In some cases, NS samples may be preferred based on the possibility for at-home collection of samples [9], which has the potential to increase enrollment in studies and facilitate expanded genomic surveillance. More importantly, no difference in virus quantity was observed between NS collected at home or in the clinic for these EPICC participants, indicating that self-collected NS specimens are a reasonable alternative when clinic visits are not possible, which is similar to previously published studies [4]. A previous study has demonstrated that combined oropharyngeal/nares swabs may be comparable to NP swabs [13]; when we compared a combined OP + NS value with NP, sensitivity was much higher, indicating that a combined OP + NS strategy may be a good alternative when self-collection at home is preferred.
EPICC is a large SARS-CoV-2 longitudinal cohort that has been recruiting since March 2020, and multiple swab types were collected in EPICC that allow for the exploration of different SARS-CoV-2 diagnostic testing methods. However, this analysis has some limitations. First, individuals were identified for recruitment into the cohort after an interaction with a healthcare system, sometimes weeks after developing symptoms, which resulted in relatively low sensitivity of all research sample types, particularly those collected beyond 1 week postsymptom onset. However, this limitation allowed for the examination of swab performance across a wide time range relative to symptom onset. Second, in the absence of a gold standard, the performance of alternative swab types was compared with results from NP swabs, which is an imperfect reference standard. In EPICC, NP swabs were found to perform best in detecting SARS-CoV-2 in the subset of SARS-CoV-2-positive participants with paired samples; therefore, NP swabs were used as the reference in this analysis. The viability of virus that was detected in the swabs was not determined for this analysis; it is possible that some of the positive tests detected dead virus particles, and a positive test in this case does not identify infectivity.
CONCLUSIONS
The results of this study confirm NP swabs as the preferred collection route for detecting SARS-CoV-2, indicating that the convenience and comfort of OP and NS samples are offset by lower sensitivity unless a combined OP + NS strategy is used. The study also confirms that NS collected at home are of equivalent quality to clinic-based NS in diagnosing infection. Nasopharyngeal swabs present the best chance of detecting SARS-CoV-2 virus for clinical and public health indications, the latter of which is critical as variants of concern continue to emerge.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We appreciate the Epidemiology, Immunology, and Clinical Characteristics of Emerging Infectious Diseases with Pandemic Potential (EPICC) participants for their central role in this study. Many thanks to the Infectious Disease Clinical Research Program (IDCRP) team at the clinical research sites—physician/clinical investigators, site managers, regulatory staff, clinical research coordinators, and laboratory personnel—for their support of this study and contributions to its success under very challenging circumstances. We thank Marietta Grother for her editorial assistance. We thank the members of the EPICC COVID-19 Cohort Study Group for their many contributions in conducting the study and ensuring effective protocol operations. The following members were all closely involved with the design, implementation, and/or oversight of the study and have met group authorship criteria for this manuscript: Brooke Army Medical Center, Fort Sam Houston, TX - Col. J. Cowden, LTC M. Darling, T. Merritt, and CPT T. Wellington; Fort Belvoir Community Hospital, Fort Belvoir, VA - A. Rutt; Madigan Army Medical Center, Joint Base Lewis McChord, WA - S. Chambers and W. Robb-McGrath; Naval Medical Center San Diego, San Diego, CA - CDR C. Berjohn and N. Kirkland; Uniformed Services University of the Health Sciences, Bethesda, MD - C. Broder, C. Byrne, M. Fritschlanski, COL P. Hickey, E. Laing, LTC J. Livezey, E. Parmelee, J. Rusiecki, and A. Scher; Womack Army Medical Center, Fort Bragg, NC - B. Barton, LTC D. Hostler, LTC (Ret) J. Hostler, MAJ K. Lago, and C. Maldonado; William Beaumont Army Medical Center, El Paso, TX - M. Wayman. We also acknowledge all who have contributed to the EPICC COVID-19 study: Brooke Army Medical Center, Fort Sam Houston, TX - Col J. Cowden, LTC M. Darling, S. DeLeon, Maj D. Lindholm, LTC A. Markelz, K. Mende, S. Merritt, T. Merritt, LTC N. Turner, and CPT T. Wellington; Carl R. Darnall Army Medical Center, Fort Hood, TX - LTC S. Bazan and P. K. Love; Fort Belvoir Community Hospital, Fort Belvoir, VA - N. Dimascio-Johnson, MAJ E. Ewers, LCDR K. Gallagher, LCDR D. Larson, and A. Rutt; Henry M. Jackson Foundation, Inc., Bethesda, MD - P. Blair, J. Chenoweth, and D. Clark; Madigan Army Medical Center, Joint Base Lewis McChord, WA - S. Chambers, LTC C. J. Colombo, R. Colombo, CAPT C. Conlon, CAPT K. Everson, COL P. Faestel, COL T. Ferguson, MAJ L. Gordon, LTC S. Grogan, CAPT S. Lis, COL C. Mount, LTC D. Musfeldt, CPT D. Odineal, LTC M. Perreault, W. Robb-McGrath, MAJ R. Sainato, C. Schofield, COL C. Skinner, M. Stein, MAJ M. Switzer, MAJ M. Timlin, MAJ S. Wood; Naval Medical Center Portsmouth, Portsmouth, VA - S. Banks, R. Carpenter, L. Kim, CAPT K. Kronmann, T. Lalani, LCDR T. Lee, LCDR A. Smith, R. Smith, R. Tant, and T. Warkentien; Naval Medical Center San Diego, San Diego, CA - CDR C. Berjohn, S. Cammarata, N. Kirkland, CAPT (Ret) R. Maves, and CAPT (Ret) G. Utz; Tripler Army Medical Center, Honolulu, HI - S. Chi, LTC R. Flanagan, MAJ M. Jones, C. Lucas, LTC C. Madar, K. Miyasato, and C. Uyehara; Uniformed Services University of the Health Sciences, Bethesda, MD - B. Agan, L. Andronescu, A. Austin, C. Broder, CAPT T. Burgess, C. Byrne, COL K. Chung, J. Davies, C. English, N. Epsi, C. Fox, M. Fritschlanski, M. Grother, A. Hadley, COL P. Hickey, E. Laing, LTC C. Lanteri, LTC J. Livezey, A. Malloy, R. Mohammed, C. Morales, P. Nwachukwu, C. Olsen, E. Parmelee, S. Pollett, S. Richard, J. Rozman, J. Rusiecki, E. Samuels, M. Sanchez, A. Scher, CDR M. Simons, A. Snow, K. Telu, D. Tribble, and L. Ulomi; United States Air Force School of Aerospace Medicine, Dayton, OH - Sgt T. Chao, R. Chapleau, A. Fries, C. Harrington, S. Huntsberger, S. Purves, K. Reynolds, J. Rodriguez, and C. Starr; Womack Army Medical Center, Fort Bragg, NC - B. Barton, LTC D. Hostler, LTC (Ret) J. Hostler, MAJ K. Lago, C. Maldonado, and J. Mehrer; William Beaumont Army Medical Center, El Paso, TX - MAJ T. Hunter, J. Mejia, R. Mody, R. Resendez, P. Sandoval, and M. Wayman; Walter Reed National Military Medical Center, Bethesda, MD - I. Barahona, A. Baya, A. Ganesan, MAJ N. Huprikar, and B. Johnson; Walter Reed Army Institute of Research, Silver Spring, MD - S. Peel.
Disclaimer. The contents of this publication are the sole responsibility of the author(s) and do not necessarily reflect the views, opinions, or policies of Uniformed Services University of the Health Sciences (USUHS); the Department of Defense (DoD); the Departments of the Army, Navy, or Air Force; Brooke Army Medical Center; Walter Reed National Military Medical Center; Naval Medical Center San Diego; Madigan Army Medical Center; Naval Medical Center Portsmouth; Carl R. Darnall Army Medical Center; William Beaumont Army Medical Center; Tripler Army Medical Center; United States Air Force School of Aerospace Medicine; Fort Belvoir Community Hospital; the Defense Health Agency; the U.S. government; or the Henry M. Jackson Foundation for the Advancement of Military Medicine. Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. government. The investigators have adhered to the policies for protection of human subjects as prescribed in 45 CFR 46. Some of the authors are service members or employees of the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
Financial support. This work was funded by awards from the Defense Health Program (HU00012020067) and the National Institute of Allergy and Infectious Disease (HU00011920111). The protocol was executed by the IDCRP, a DoD program executed by the USUHS through a cooperative agreement by the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. (HJF). This project has been funded in part by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health, under an interagency agreement (Y1-AI-5072).
Potential conflicts of interest. S. D. P., M. P. S., T. H. B., and D. R. T. (The Uniformed Services University [USU] IDCRP, a US Department of Defense institution, and the Henry M. Jackson Foundation [HJF]) were funded under a Cooperative Research and Development Agreement to conduct an unrelated Phase III coronavirus disease 2019 (COVID-19) monoclonal antibody immune-prophylaxis trial sponsored by AstraZeneca. The HJF, in support of the USU IDCRP, was funded by the Department of Defense Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense to augment the conduct of an unrelated Phase III vaccine trial sponsored by AstraZeneca. Both of these trials were part of the US Government COVID-19 response. Neither is related to the work presented here. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.





Comments