-
PDF
- Split View
-
Views
-
Cite
Cite
Akiko Sayama, Michiko Okamoto, Raita Tamaki, Mariko Saito-Obata, Mayuko Saito, Taro Kamigaki, Yusuke Sayama, Irene Lirio, Joanna Ina G Manalo, Veronica L Tallo, Socorro P Lupisan, Hitoshi Oshitani, Comparison of Rhinovirus A–, B–, and C–Associated Respiratory Tract Illness Severity Based on the 5′-Untranslated Region Among Children Younger Than 5 Years, Open Forum Infectious Diseases, Volume 9, Issue 10, October 2022, ofac387, https://doi.org/10.1093/ofid/ofac387
Close - Share Icon Share
Abstract
Rhinoviruses (RVs) are among the most frequently detected viruses from hospitalized children with severe acute respiratory infections, being classified into RV-A, RV-B, and RV-C (4 clades: C, GAC1, GAC2, and A2). This study aimed to compare the clinical characteristics and respiratory tract illness severity between the RV species and RV-C clades in children in primary care and hospital settings in rural communities in the Philippines.
Clinical samples and information of children <5 years old in the Philippines were collected from 2014 to 2016. The samples were tested by reverse-transcription polymerase chain reaction (RT-PCR) targeting the 5′-untranslated region. PCR-positive samples were sequenced, and RV species were identified by phylogenetic analysis.
Overall, 3680 respiratory tract illness episodes in 1688 cohort children were documented; 713 of those were RV positive and identified as RV-A (n = 271), RV-B (n = 47), and RV-C (n = 395: C [n = 76], GAG1 [n = 172], GAG2 [n = 8], A2 [n = 138], and unidentified [n = 1]). Severe illnesses, low oxygen saturation, cough, and wheezing were more common in patients with RV-C, especially with GAC1, than in those with RV-A or RV-B. Furthermore, severe illness was significantly more common in RV-C (GAC1)–positive cases than in RV-A–positive cases (odds ratio, 2.61 [95% CI, 1.17–4.13]).
Children infected with RV-C had more severe illnesses than children infected with RV-A and RV-B. Moreover, emerging clades of RV-C were associated with increased severity.
Rhinoviruses (RVs) are members of the Enterovirus genus in the Picornaviridae family. RVs were previously classified into 2 species: RV-A and RV-B, in which >100 serotypes have been identified using cross-neutralization assays [1]. With the development of molecular techniques, the novel RV species RV-C was discovered in patients with acute respiratory illness [2, 3]. Due to the difficulty to culture RV-C using standard cell culture, polymerase chain reaction (PCR) and sequencing have typically been used to classify RV-C [4]. To date, >50 genotypes considered equivalent to serotypes have been identified in RV-C [1]. Based on the phylogenetic analysis of the partial sequencing of the 5′-untranslated region (UTR), RV-C is classified into 3 novel clades, A2 [5, 6], GAC1, and GAC2 [7]. Sequence analysis of VP4/VP2 and 5′-UTR revealed possible recombination between RV-C and RV-A for these emerging clades [7, 8].
Recently, PCR combined with sequencing has been used to detect and differentiate RV species in clinical samples. Capsid genes analysis, including VP2 and VP4 [1, 9], is required to determine species and serotypes/genotypes. However, PCR targeting the 5′-UTR is more sensitive for detecting RV because the 5′-UTR has greater homology [9].
Rhinoviruses represent the most common cause of mild respiratory illnesses [10]. However, in recent etiological studies, RV was frequently detected in children with lower respiratory tract illness (LRTI) [11]. Conversely, a recent case-control study has shown high RV positivity in healthy controls and a small attributable fraction to LRTI [12]. Some studies have indicated that RV-C was associated with more severe illness [13–15], and our cohort study also demonstrated an association between RV-C and severe clinical signs in children with acute respiratory tract illnesses (RTIs) [16]. However, clinical manifestation or severity differences among RV species still need clarification. Most previous etiological studies investigated hospitalized severe cases.
Furthermore, to our knowledge, no study has examined the clinical impact of RV-C emerging clades (C, GAC1, GAC2, and A2) on RV infection severity. Therefore, this study aimed to compare the symptoms and severity of RTIs between different RV species and RV-C clades by analyzing data from a community-based cohort study of children aged <5 years in 2 primary healthcare facilities and a hospital on a remote island in the Philippines. We used 5′-UTR sequencing results to identify species and RV to include more RV-associated cases in the analysis.
METHODS
Patient Consent Statement
Gurdians of all participants gave written informed consent. This study was approved by the Institutional Review Board of Research Institute for Tropical Medicine (RITM) (number 2013-002) in the Philippines and the Ethics Committee of Tohoku University Graduate School of Medicine (numbers 2012-1-63, 2014-1-790, 2016-1-258, and 2018-1-70).
Study Design
This case-comparison study analyzed data from a cohort study conducted in Biliran, the Philippines, as previously described [17]. In brief, the inclusion criteria of the baseline cohort children were children <5 years old, living in 2 selected municipalities (Caibiran and Kawayan) at least 1 month before the recruitment, and children whose parental consent was obtained. A child was excluded if parental consent was unavailable or did not meet the above criteria. The cohort children were enrolled and followed from February 2014 to June 2016. A nasopharyngeal swab sample was collected when a child had cough, nasal discharge or nasal obstruction, or respiratory distress within 14 days and visited or was admitted to the primary care health unit (Rural Health Unit) or Biliran Provincial Hospital.
Rhinovirus Detection and Phylogenetic Analysis
RNA was extracted and synthesized to complementary DNA (cDNA) from nasopharyngeal swabs. Reverse-transcription PCR (RT-PCR) targeting the partial 5′-UTR of rhinoviruses was performed using DK001 and DK004 as primers [7, 18]. Amplified cDNAs whose bands were observed using gel electrophoresis were subjected to Sanger sequencing for molecular classification, as previously described [19]. Enteroviruses (EVs) were also detected by RT-PCR using DK001 and DK004 as primers as well as RV. Adenovirus (AdV) and parainfluenza (PIV, types 1–4) were detected by conventional PCR; influenza virus A(H1N1)pdm, A(H3N3), and B by real-time PCR; and respiratory syncytial virus (RSV) and human metapneumovirus (HMPV) by multiplex real-time PCR. The primers and probes used are shown in Supplementary Table 1.
The reference sequences were obtained from the list of International Committee on Taxomony of Viruses Picornaviridae Study Group [1]. We selected sequences only if the whole-genome sequences were available in GenBank (Supplementary Table 2). We performed multiple alignments using the Clustal Omega algorithm and included 345 nucleotides (nt 166–510) to analyze the clinical samples. Sequences that included >20 undetermined nucleotides were excluded from the analysis. A phylogenetic tree was constructed using the neighbor-joining method with 100 bootstrap replicates in MEGA7 software (https://www.megasoftware.net/). Using a phylogenetic tree, RV-C clades, including RV-C (GAC1), RV-C (A2), and RV-C (GAC2), were determined using previously described methods [7]. RV-C sequences that were not categorized into these clades were grouped into RV-C (C). When some RV-A reference sequences were clustered with RV-C (GAG1) or RV-C (A2) clade, RV-As belonging to these clades were categorized as RV-C in this study. The sequences generated in this study have been assigned DNA Data Bank of Japan/European Molecular Biology Laboratory/GenBank accession numbers LC591112–LC591835.
Case Definition of RTI With RV and Severity
An RTI case was defined as an episode with cough or difficulty breathing. When symptoms newly appeared ≥7 days after the resolution of the symptoms of the last episode, it was considered a new case. However, if RV was detected within the last 15 days or if the same RV species were detected within a month, these cases were not considered as new RV-associated cases.
We classified RTI severity based on the criteria proposed by the expert group (RTI, LRTI, severe LRTI, and very severe LRTI) [20] with some modifications [21]. Because the criteria do not include children with only nasal discharge or congestion, we categorized such episodes as “coryza” and included it as a nonsevere category. If percutaneous arterial oxygen saturation (SpO2) was measured after oxygen treatment or bronchodilator therapy, SpO2 was considered to be <95%. We could not determine the LRTI category without the SpO2 measurement for some episodes, and these episodes were categorized as undefined LRTI. LRTI severity was compared between severe cases (severe LRTI and very severe LRTI) and nonsevere cases (coryza, RTI, and LRTI).
Statistical Analysis
Age in months was compared using Kruskal-Wallis and Bonferroni-Dunn tests. The χ2 and Fisher exact tests were used to compare the categorical variables. We compared clinical symptoms between RV species (RV-A, RV-B, and RV-C), then compared between RV-C clades (GAC1, A2, C, and GAC2). When P values were < .05, P values were adjusted using Bonferroni corrections for multiple comparisons.
Adjusted odds ratios (aORs) and 95% confidence intervals (CIs) were calculated using multiple logistic regression adjusted for age, sex, and place of residence (municipality). P values were also adjusted for multiple comparisons using the Tukey and Hochberg corrections. All tests were 2-tailed, and P values < .05 were considered significant. All statistical analyses were performed using R version 3.6.0 software [22].
RESULTS
Study Population
In total, 3680 RTI episodes in 1688 cohort children were included in the analysis. Among those, RVs were detected in 972 episodes. Of those, 134 episodes were excluded because of low sequence data quality to determine the species. In addition, 259 episodes were excluded due to the codetection of other viruses (n = 111: RSV, 31; PIV, 24; AdV, 18; influenza, 15; HMPV, 12; cytomegalovirus, 8; EV, 2; AdV and PIV, 1), detection of multiple RV species in 1 episode (n = 6), and missing clinical data (n = 8). Finally, 713 RV-associated episodes were included in the analysis.
Phylogenetic Characteristics of RVs and Clades
Based on the phylogenetic analysis of 5′-UTR sequences, RVs were categorized as RV-A (n = 271 [38.0%]), RV-B (n = 47 [6.6%]), or RV-C (n = 395 [55.4%]) (Figure 1 and Table 1). RV-C clades were further divided into RV-C (C) clade (n = 76), GAG1 clade (n = 172), GAG2 clade (n = 8), A2 clade (n = 138), and unidentified (n = 1) (Table 1). One unidentified RV-C was excluded because of the codetection of different RV-C clades within the same episode. Although some of RV-A reference sequences were clustered with GAG1 (5 serotypes/genotypes) or A2 clade (9 serotypes/genotypes), RVs belonging to these clades were categorized as RV-C.
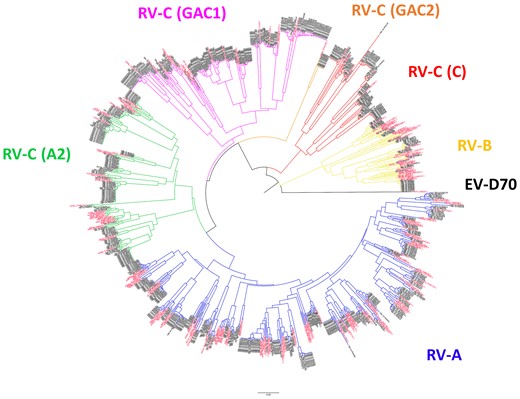
Phylogenetic analysis of the 5′-UTR sequence of rhinovirus. Phylogenetic analysis was performed by using MEGA software, which features a bootstrap test with the neighbor-joining method with pairwise deletion option. The 345 nucleotide in the 5′-UTR of the available rhinovirus sequences in GenBank was used to create the phylogenetic tree. Black indicates sequences of collected samples, and red indicates reference sequences. Abbreviations: EV, enterovirus; RV, rhinovirus.
Patient Background, Clinical Signs, Symptoms, and Severity by Rhinovirus (RV)-A, RV-B, and RV-C Species and RV-C Clades
| Characteristic . | Species (n = 713) . | RV-C Clades (n = 394)a . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| RV-A . | RV-B . | RV-C . | P Valueb . | C . | GAC1 . | A2 . | GAC2 . | P Valuec . | |
| Background, clinical symptoms, and signs | n = 271 | n = 47 | n = 395 | n = 76 | n = 172 | n = 138 | n = 8 | ||
| Age, mo, median (IQR) | 16 (8–30) | 11 (6–30) | 18 (9–31) | .243 | 21 (10–33) | 18 (9–31) | 16 (8–30) | 20 (11–30) | .363 |
| Municipality | |||||||||
| Caibiran | 172 (63.5) | 26 (55.3) | 220 (55.7) | 48 (63.2) | 85 (49.4) | 82 (59.4) | 5 (62.5) | ||
| Kawayan | 99 (36.5) | 21 (44.7) | 175 (44.3) | .121 | 28 (36.8) | 87 (50.6) | 56 (40.6) | 3 (37.5) | .088 |
| Sex | |||||||||
| Male | 147 (54.2) | 22 (46.8) | 217 (54.9) | 41 (53.9) | 96 (55.8) | 77 (55.8) | 3 (37.5) | ||
| Female | 124 (45.8) | 25 (53.2) | 178 (45.1) | .571 | 35 (46.1) | 76 (44.2) | 61 (44.2) | 5 (62.5) | .812 |
| Fever (≥37.5°C) | 38 (14.0) | 3 (6.4) | 50 (12.7) | .349 | 9 (11.8) | 21 (12.2) | 17 (12.3) | 3 (37.5) | .250 |
| SpO2 <95% | 35 (12.9) | 5 (10.6) | 74 (18.7) | .077 | 14 (18.4) | 38 (22.1) | 18 (13.0) | 4 (50.0) | .008 |
| Cough | 239 (88.2) | 44 (93.6) | 363 (91.9) | .209 | 70 (92.1) | 163 (94.8) | 122 (88.4) | 7 (87.5) | .227 |
| Nasal discharge or Congestion | 262 (96.7) | 41 (87.2) | 376 (95.2) | .020d | 73 (96.1) | 164 (95.3) | 131 (94.9) | 7 (87.5) | .107 |
| Fast breathing | 115 (42.4) | 14 (29.8) | 161 (40.8) | .265 | 34 (44.7) | 70 (40.7) | 52 (37.7) | 5 (62.5) | .384 |
| Difficulty breathing | 33 (12.2) | 4 (8.5) | 63 (15.9) | .205 | 11 (14.5) | 29 (16.9) | 19 (13.8) | 4 (50.0) | .039 |
| Alar flaring | 14 (5.2) | 1 (2.1) | 21 (5.3) | .637 | 2 (2.6) | 11 (6.4) | 6 (4.3) | 2 (25.0) | .102 |
| Chest indrawing | 14 (5.2) | 2 (4.3) | 41 (10.4) | .032 | 9 (11.8) | 17 (9.9) | 10 (7.2) | 5 (62.5) | <.001d |
| Decreased breath Sounds | 3 (1.1) | 1 (2.1) | 6 (1.5) | .823 | 2 (2.6) | 3 (1.7) | 1 (0.7) | 0 (0.0) | .863 |
| Rales | 98 (36.2) | 16 (34.0) | 166 (42.0) | .235 | 26 (34.2) | 69 (40.1) | 64 (46.4) | 6 (75.0) | .084 |
| Wheezing | 53 (19.6) | 5 (10.6) | 91 (23.0) | .112 | 17 (22.4) | 47 (27.3) | 23 (16.7) | 4 (50.0) | .021 |
| Severity classificatione | n = 260 | n = 47 | n = 369 | n = 70 | n = 161 | n = 132 | n = 5 | ||
| Severe illness | 18 (6.9) | 3 (6.4) | 44 (11.9) | .082 | 7 (10.0) | 23 (14.3) | 12 (9.1) | 2 (40.0) | .033 |
| Characteristic . | Species (n = 713) . | RV-C Clades (n = 394)a . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| RV-A . | RV-B . | RV-C . | P Valueb . | C . | GAC1 . | A2 . | GAC2 . | P Valuec . | |
| Background, clinical symptoms, and signs | n = 271 | n = 47 | n = 395 | n = 76 | n = 172 | n = 138 | n = 8 | ||
| Age, mo, median (IQR) | 16 (8–30) | 11 (6–30) | 18 (9–31) | .243 | 21 (10–33) | 18 (9–31) | 16 (8–30) | 20 (11–30) | .363 |
| Municipality | |||||||||
| Caibiran | 172 (63.5) | 26 (55.3) | 220 (55.7) | 48 (63.2) | 85 (49.4) | 82 (59.4) | 5 (62.5) | ||
| Kawayan | 99 (36.5) | 21 (44.7) | 175 (44.3) | .121 | 28 (36.8) | 87 (50.6) | 56 (40.6) | 3 (37.5) | .088 |
| Sex | |||||||||
| Male | 147 (54.2) | 22 (46.8) | 217 (54.9) | 41 (53.9) | 96 (55.8) | 77 (55.8) | 3 (37.5) | ||
| Female | 124 (45.8) | 25 (53.2) | 178 (45.1) | .571 | 35 (46.1) | 76 (44.2) | 61 (44.2) | 5 (62.5) | .812 |
| Fever (≥37.5°C) | 38 (14.0) | 3 (6.4) | 50 (12.7) | .349 | 9 (11.8) | 21 (12.2) | 17 (12.3) | 3 (37.5) | .250 |
| SpO2 <95% | 35 (12.9) | 5 (10.6) | 74 (18.7) | .077 | 14 (18.4) | 38 (22.1) | 18 (13.0) | 4 (50.0) | .008 |
| Cough | 239 (88.2) | 44 (93.6) | 363 (91.9) | .209 | 70 (92.1) | 163 (94.8) | 122 (88.4) | 7 (87.5) | .227 |
| Nasal discharge or Congestion | 262 (96.7) | 41 (87.2) | 376 (95.2) | .020d | 73 (96.1) | 164 (95.3) | 131 (94.9) | 7 (87.5) | .107 |
| Fast breathing | 115 (42.4) | 14 (29.8) | 161 (40.8) | .265 | 34 (44.7) | 70 (40.7) | 52 (37.7) | 5 (62.5) | .384 |
| Difficulty breathing | 33 (12.2) | 4 (8.5) | 63 (15.9) | .205 | 11 (14.5) | 29 (16.9) | 19 (13.8) | 4 (50.0) | .039 |
| Alar flaring | 14 (5.2) | 1 (2.1) | 21 (5.3) | .637 | 2 (2.6) | 11 (6.4) | 6 (4.3) | 2 (25.0) | .102 |
| Chest indrawing | 14 (5.2) | 2 (4.3) | 41 (10.4) | .032 | 9 (11.8) | 17 (9.9) | 10 (7.2) | 5 (62.5) | <.001d |
| Decreased breath Sounds | 3 (1.1) | 1 (2.1) | 6 (1.5) | .823 | 2 (2.6) | 3 (1.7) | 1 (0.7) | 0 (0.0) | .863 |
| Rales | 98 (36.2) | 16 (34.0) | 166 (42.0) | .235 | 26 (34.2) | 69 (40.1) | 64 (46.4) | 6 (75.0) | .084 |
| Wheezing | 53 (19.6) | 5 (10.6) | 91 (23.0) | .112 | 17 (22.4) | 47 (27.3) | 23 (16.7) | 4 (50.0) | .021 |
| Severity classificatione | n = 260 | n = 47 | n = 369 | n = 70 | n = 161 | n = 132 | n = 5 | ||
| Severe illness | 18 (6.9) | 3 (6.4) | 44 (11.9) | .082 | 7 (10.0) | 23 (14.3) | 12 (9.1) | 2 (40.0) | .033 |
Data are presented as No. (%) unless otherwise indicated. P values in bold means significant by each testing.
Abbreviations: IQR, interquartile range; RV, rhinovirus; SpO2, percutaneous arterial oxygen saturation.
One episode of RV-C infection was excluded because of the codetection of different RV-C clades.
P values were estimated testing among 3 species of RV-A, RV-B, and RV-C (All RV-C clades were included). Age in months was tested using Kruskal-Wallis rank-sum test, and categorical variables were tested using χ2 test or Fisher test (before multiple comparison with Bonferroni correction).
P values were estimated testing among 6 clades of RV-A, RV-B, and RV-C clades (separately). Age in months was tested using Kruskal-Wallis rank-sum test, and categorical variables were tested using χ2 test or Fisher test (before multiple comparison with Bonferroni correction).
P values < .05 when multiple comparison with Bonferroni correction applied.
Severe illness included severe lower respiratory tract illness (LRTI) and very severe LRTI. Thirty-six episodes (5.1%) were not classified and were excluded from the analysis due to incomplete SpO2 measurement for the classification criteria.
Patient Background, Clinical Signs, Symptoms, and Severity by Rhinovirus (RV)-A, RV-B, and RV-C Species and RV-C Clades
| Characteristic . | Species (n = 713) . | RV-C Clades (n = 394)a . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| RV-A . | RV-B . | RV-C . | P Valueb . | C . | GAC1 . | A2 . | GAC2 . | P Valuec . | |
| Background, clinical symptoms, and signs | n = 271 | n = 47 | n = 395 | n = 76 | n = 172 | n = 138 | n = 8 | ||
| Age, mo, median (IQR) | 16 (8–30) | 11 (6–30) | 18 (9–31) | .243 | 21 (10–33) | 18 (9–31) | 16 (8–30) | 20 (11–30) | .363 |
| Municipality | |||||||||
| Caibiran | 172 (63.5) | 26 (55.3) | 220 (55.7) | 48 (63.2) | 85 (49.4) | 82 (59.4) | 5 (62.5) | ||
| Kawayan | 99 (36.5) | 21 (44.7) | 175 (44.3) | .121 | 28 (36.8) | 87 (50.6) | 56 (40.6) | 3 (37.5) | .088 |
| Sex | |||||||||
| Male | 147 (54.2) | 22 (46.8) | 217 (54.9) | 41 (53.9) | 96 (55.8) | 77 (55.8) | 3 (37.5) | ||
| Female | 124 (45.8) | 25 (53.2) | 178 (45.1) | .571 | 35 (46.1) | 76 (44.2) | 61 (44.2) | 5 (62.5) | .812 |
| Fever (≥37.5°C) | 38 (14.0) | 3 (6.4) | 50 (12.7) | .349 | 9 (11.8) | 21 (12.2) | 17 (12.3) | 3 (37.5) | .250 |
| SpO2 <95% | 35 (12.9) | 5 (10.6) | 74 (18.7) | .077 | 14 (18.4) | 38 (22.1) | 18 (13.0) | 4 (50.0) | .008 |
| Cough | 239 (88.2) | 44 (93.6) | 363 (91.9) | .209 | 70 (92.1) | 163 (94.8) | 122 (88.4) | 7 (87.5) | .227 |
| Nasal discharge or Congestion | 262 (96.7) | 41 (87.2) | 376 (95.2) | .020d | 73 (96.1) | 164 (95.3) | 131 (94.9) | 7 (87.5) | .107 |
| Fast breathing | 115 (42.4) | 14 (29.8) | 161 (40.8) | .265 | 34 (44.7) | 70 (40.7) | 52 (37.7) | 5 (62.5) | .384 |
| Difficulty breathing | 33 (12.2) | 4 (8.5) | 63 (15.9) | .205 | 11 (14.5) | 29 (16.9) | 19 (13.8) | 4 (50.0) | .039 |
| Alar flaring | 14 (5.2) | 1 (2.1) | 21 (5.3) | .637 | 2 (2.6) | 11 (6.4) | 6 (4.3) | 2 (25.0) | .102 |
| Chest indrawing | 14 (5.2) | 2 (4.3) | 41 (10.4) | .032 | 9 (11.8) | 17 (9.9) | 10 (7.2) | 5 (62.5) | <.001d |
| Decreased breath Sounds | 3 (1.1) | 1 (2.1) | 6 (1.5) | .823 | 2 (2.6) | 3 (1.7) | 1 (0.7) | 0 (0.0) | .863 |
| Rales | 98 (36.2) | 16 (34.0) | 166 (42.0) | .235 | 26 (34.2) | 69 (40.1) | 64 (46.4) | 6 (75.0) | .084 |
| Wheezing | 53 (19.6) | 5 (10.6) | 91 (23.0) | .112 | 17 (22.4) | 47 (27.3) | 23 (16.7) | 4 (50.0) | .021 |
| Severity classificatione | n = 260 | n = 47 | n = 369 | n = 70 | n = 161 | n = 132 | n = 5 | ||
| Severe illness | 18 (6.9) | 3 (6.4) | 44 (11.9) | .082 | 7 (10.0) | 23 (14.3) | 12 (9.1) | 2 (40.0) | .033 |
| Characteristic . | Species (n = 713) . | RV-C Clades (n = 394)a . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| RV-A . | RV-B . | RV-C . | P Valueb . | C . | GAC1 . | A2 . | GAC2 . | P Valuec . | |
| Background, clinical symptoms, and signs | n = 271 | n = 47 | n = 395 | n = 76 | n = 172 | n = 138 | n = 8 | ||
| Age, mo, median (IQR) | 16 (8–30) | 11 (6–30) | 18 (9–31) | .243 | 21 (10–33) | 18 (9–31) | 16 (8–30) | 20 (11–30) | .363 |
| Municipality | |||||||||
| Caibiran | 172 (63.5) | 26 (55.3) | 220 (55.7) | 48 (63.2) | 85 (49.4) | 82 (59.4) | 5 (62.5) | ||
| Kawayan | 99 (36.5) | 21 (44.7) | 175 (44.3) | .121 | 28 (36.8) | 87 (50.6) | 56 (40.6) | 3 (37.5) | .088 |
| Sex | |||||||||
| Male | 147 (54.2) | 22 (46.8) | 217 (54.9) | 41 (53.9) | 96 (55.8) | 77 (55.8) | 3 (37.5) | ||
| Female | 124 (45.8) | 25 (53.2) | 178 (45.1) | .571 | 35 (46.1) | 76 (44.2) | 61 (44.2) | 5 (62.5) | .812 |
| Fever (≥37.5°C) | 38 (14.0) | 3 (6.4) | 50 (12.7) | .349 | 9 (11.8) | 21 (12.2) | 17 (12.3) | 3 (37.5) | .250 |
| SpO2 <95% | 35 (12.9) | 5 (10.6) | 74 (18.7) | .077 | 14 (18.4) | 38 (22.1) | 18 (13.0) | 4 (50.0) | .008 |
| Cough | 239 (88.2) | 44 (93.6) | 363 (91.9) | .209 | 70 (92.1) | 163 (94.8) | 122 (88.4) | 7 (87.5) | .227 |
| Nasal discharge or Congestion | 262 (96.7) | 41 (87.2) | 376 (95.2) | .020d | 73 (96.1) | 164 (95.3) | 131 (94.9) | 7 (87.5) | .107 |
| Fast breathing | 115 (42.4) | 14 (29.8) | 161 (40.8) | .265 | 34 (44.7) | 70 (40.7) | 52 (37.7) | 5 (62.5) | .384 |
| Difficulty breathing | 33 (12.2) | 4 (8.5) | 63 (15.9) | .205 | 11 (14.5) | 29 (16.9) | 19 (13.8) | 4 (50.0) | .039 |
| Alar flaring | 14 (5.2) | 1 (2.1) | 21 (5.3) | .637 | 2 (2.6) | 11 (6.4) | 6 (4.3) | 2 (25.0) | .102 |
| Chest indrawing | 14 (5.2) | 2 (4.3) | 41 (10.4) | .032 | 9 (11.8) | 17 (9.9) | 10 (7.2) | 5 (62.5) | <.001d |
| Decreased breath Sounds | 3 (1.1) | 1 (2.1) | 6 (1.5) | .823 | 2 (2.6) | 3 (1.7) | 1 (0.7) | 0 (0.0) | .863 |
| Rales | 98 (36.2) | 16 (34.0) | 166 (42.0) | .235 | 26 (34.2) | 69 (40.1) | 64 (46.4) | 6 (75.0) | .084 |
| Wheezing | 53 (19.6) | 5 (10.6) | 91 (23.0) | .112 | 17 (22.4) | 47 (27.3) | 23 (16.7) | 4 (50.0) | .021 |
| Severity classificatione | n = 260 | n = 47 | n = 369 | n = 70 | n = 161 | n = 132 | n = 5 | ||
| Severe illness | 18 (6.9) | 3 (6.4) | 44 (11.9) | .082 | 7 (10.0) | 23 (14.3) | 12 (9.1) | 2 (40.0) | .033 |
Data are presented as No. (%) unless otherwise indicated. P values in bold means significant by each testing.
Abbreviations: IQR, interquartile range; RV, rhinovirus; SpO2, percutaneous arterial oxygen saturation.
One episode of RV-C infection was excluded because of the codetection of different RV-C clades.
P values were estimated testing among 3 species of RV-A, RV-B, and RV-C (All RV-C clades were included). Age in months was tested using Kruskal-Wallis rank-sum test, and categorical variables were tested using χ2 test or Fisher test (before multiple comparison with Bonferroni correction).
P values were estimated testing among 6 clades of RV-A, RV-B, and RV-C clades (separately). Age in months was tested using Kruskal-Wallis rank-sum test, and categorical variables were tested using χ2 test or Fisher test (before multiple comparison with Bonferroni correction).
P values < .05 when multiple comparison with Bonferroni correction applied.
Severe illness included severe lower respiratory tract illness (LRTI) and very severe LRTI. Thirty-six episodes (5.1%) were not classified and were excluded from the analysis due to incomplete SpO2 measurement for the classification criteria.
Patient Characteristics
For 713 RV-associated episodes, the median patient age was 17 months (interquartile range, 8–31 months), and 54% (386/713) were male (Table 1). Severe illness (severe LRTI and very severe LRTI) was found in 9.6% (65/676) of the episodes. Thirty-six episodes (5.1%) were excluded from the analysis of severity due to missing data on SpO2 measurement. The proportion of episodes with nasal discharge or congestion, fast breathing, and rale varied among age groups and places of residence (community) (Supplementary Table 3). In addition, the proportion of episodes with difficulty breathing, wheezing, low SpO2 (<95%), and severe illness were higher in the community of Kawayan compared with Caibiran. Chest indrawing was more likely to be seen in females than males (P = .034).
Comparison of Clinical Symptoms and Signs Among RV-A, RV-B, and RV-C
When clinical symptoms were compared, chest indrawing was more common in RV-C (10.4%)–associated episodes than RV-A (5.2%) (Table 1), and also the difference was significant (aOR, 2.20 [95% CI, 1.17–4.13]; P = .047) (Table 2). Although >95% of children infected with RV-A and RV-C had nasal discharge or congestion (Table 1), those with RV-B infection showed a lower proportion of episodes with these symptoms (87.2%; A vs B: aOR, 0.26 [95% CI, .08–.81], P = .045; B vs C: aOR, 3.07 [95% CI, 1.13–8.32], P = .011) (Table 2). Wheezing was less common in the episodes associated with RV-B than those associated with RV-C (10.6% vs 23.0%; aOR, 2.46 [95% CI, .94–6.44]), and low SpO2 (<95%) and severe illnesses were more common in episodes caused by RV-C species (18.7% and 11.9%, respectively) than in those caused by RV-A (12.9% and 6.9%; aORs, 1.65 [95% CI, 1.06–2.57] and 1.94 [95% CI, 1.09–3.47]) (P = .074 and P = .078, respectively).
Adjusted Odds Ratios for the Comparison of Clinical Outcomes of Episodes by Different Rhinovirus Species and Rhinovirus-C Clades
| Clinical Outcome . | Versus RV-A . | Versus RV-B . | Versus RV-C (C) . | Versus RV-C (GAC1) . | Versus RV-C (A2) . |
|---|---|---|---|---|---|
| aOR (95% CI) . | aOR (95% CI) . | aOR (95% CI) . | aOR (95% CI) . | aOR (95% CI) . | |
| Total (n = 712a) | |||||
| Low SpO2 <95% (yes) | |||||
| RV-A | Ref | … | … | … | … |
| RV-B | 0.87 (.32–2.37) | Ref | … | … | … |
| RV-C | 1.65 (1.06–2.57) | 1.97 (.75–5.19) | … | … | … |
| C | 1.50 (.75–3.00) | 1.81 (.59–5.56) | Ref | … | … |
| GAC1 | 2.16 (1.29–3.64) | 2.54 (.93–7.00) | 1.37 (.68–2.75) | Ref | … |
| A2 | 1.05 (.56–1.93) | 1.28 (.43–3.78) | .66 (.30–1.42) | .49 (.26–.91) | Ref |
| GAC2 | 7.26 (1.65–31.91) | 15.89 (.96–128.83) | 4.71 (1.01–22.04) | 3.46 (.80–14.99) | 7.94 (1.69–37.43) |
| Nasal discharge or congestion (yes) | |||||
| RV-A | Ref. | … | … | … | … |
| RV-B | .26 (.08–.81)b | Ref | … | … | … |
| RV-C | .76 (.34–1.73) | 3.07 (1.13–8.32)b | … | … | … |
| C | 1.05 (.26–4.22) | 3.24 (.73–14.4) | Ref | … | … |
| GAC1 | .86 (.32–2.31) | 3.66 (1.15–11.7) | .97 (.24–3.86) | Ref | … |
| A2 | .67 (.24–1.86) | 2.73 (.84–8.83) | .74 (.18–3.05) | .73 (.25–2.16) | Ref |
| GAC2 | .29 (.03–2.90) | 1.03 (.10–10.87) | .41 (.03–5.20) | 0.17 (.02–2.01) | .30 (.03–3.14) |
| Chest indrawing (yes) | |||||
| RV-A | Ref | … | … | … | … |
| RV-B | .94 (.20–4.38) | Ref | … | … | … |
| RV-C | 2.20 (1.17–4.13)b | 2.54 (.59–10.9) | … | … | … |
| C | 2.46 (1.01–6.00) | 2.99 (.60–14.9) | Ref | … | … |
| GAC1 | 2.20 (1.04–4.68) | 2.32 (.50–10.7) | .85 (.35–2.05) | Ref | … |
| A2 | 1.44 (.62–3.34) | 1.80 (.38–8.56) | .57 (.22–1.49) | .67 (.29–1.52) | Ref |
| GAC2 | 45.53 (8.44–245.73)c | 94.05 (5.73–1544.62)b | 14.05 (2.68–73.55)b | 23.02 (4.22–125.61)c | 20.25 (4.14–99.10)c |
| Wheezing (yes) | |||||
| RV-A | Ref | … | … | … | … |
| RV-B | .52 (.19–1.41) | Ref | … | … | … |
| RV-C | 1.30 (.88–1.91) | 2.46 (.94–6.44) | … | … | … |
| C | 1.19 (.63–2.24) | 2.39 (.80–7.12) | Ref | … | … |
| GAC1 | 1.80 (1.13–2.88) | 3.24 (1.19–8.82) | 1.50 (.78–2.88) | Ref | … |
| A2 | 0.84 (.49–1.45) | 1.65 (.58–4.69) | 0.71 (.35–1.44) | .49 (.28–.87) | Ref |
| GAC2 | 4.86 (1.10–21.50) | 10.52 (1.75–63.28) | 3.43 (.74–15.80) | 2.65 (.61–11.58) | 5.11 (1.18–22.22) |
| Severe illnessd (total n = 676e) | |||||
| RV-A | Ref | … | … | … | … |
| RV-B | 1.05 (.29–3.78) | Ref | … | … | … |
| RV-C | 1.94 (1.09–3.47) | 2.06 (.61–7.00) | … | … | … |
| C | 1.46 (.58–3.70) | 1.57 (.37–6.61) | Ref | … | … |
| GAC1 | 2.61 (1.34–5.09) | 2.58 (.73–9.12) | 1.67 (.67–4.16) | Ref | … |
| A2 | 1.39 (.64–2.99) | 1.44 (.39–5.37) | .86 (.32–2.33) | .53 (.25–1.13) | Ref |
| GAC2 | 14.54 (2.03–104.04) | 11.16 (1.15–108.60) | 5.94 (.76–46.58) | 4.43 (.63–31.12) | 7.12 (.99–51.4) |
| Clinical Outcome . | Versus RV-A . | Versus RV-B . | Versus RV-C (C) . | Versus RV-C (GAC1) . | Versus RV-C (A2) . |
|---|---|---|---|---|---|
| aOR (95% CI) . | aOR (95% CI) . | aOR (95% CI) . | aOR (95% CI) . | aOR (95% CI) . | |
| Total (n = 712a) | |||||
| Low SpO2 <95% (yes) | |||||
| RV-A | Ref | … | … | … | … |
| RV-B | 0.87 (.32–2.37) | Ref | … | … | … |
| RV-C | 1.65 (1.06–2.57) | 1.97 (.75–5.19) | … | … | … |
| C | 1.50 (.75–3.00) | 1.81 (.59–5.56) | Ref | … | … |
| GAC1 | 2.16 (1.29–3.64) | 2.54 (.93–7.00) | 1.37 (.68–2.75) | Ref | … |
| A2 | 1.05 (.56–1.93) | 1.28 (.43–3.78) | .66 (.30–1.42) | .49 (.26–.91) | Ref |
| GAC2 | 7.26 (1.65–31.91) | 15.89 (.96–128.83) | 4.71 (1.01–22.04) | 3.46 (.80–14.99) | 7.94 (1.69–37.43) |
| Nasal discharge or congestion (yes) | |||||
| RV-A | Ref. | … | … | … | … |
| RV-B | .26 (.08–.81)b | Ref | … | … | … |
| RV-C | .76 (.34–1.73) | 3.07 (1.13–8.32)b | … | … | … |
| C | 1.05 (.26–4.22) | 3.24 (.73–14.4) | Ref | … | … |
| GAC1 | .86 (.32–2.31) | 3.66 (1.15–11.7) | .97 (.24–3.86) | Ref | … |
| A2 | .67 (.24–1.86) | 2.73 (.84–8.83) | .74 (.18–3.05) | .73 (.25–2.16) | Ref |
| GAC2 | .29 (.03–2.90) | 1.03 (.10–10.87) | .41 (.03–5.20) | 0.17 (.02–2.01) | .30 (.03–3.14) |
| Chest indrawing (yes) | |||||
| RV-A | Ref | … | … | … | … |
| RV-B | .94 (.20–4.38) | Ref | … | … | … |
| RV-C | 2.20 (1.17–4.13)b | 2.54 (.59–10.9) | … | … | … |
| C | 2.46 (1.01–6.00) | 2.99 (.60–14.9) | Ref | … | … |
| GAC1 | 2.20 (1.04–4.68) | 2.32 (.50–10.7) | .85 (.35–2.05) | Ref | … |
| A2 | 1.44 (.62–3.34) | 1.80 (.38–8.56) | .57 (.22–1.49) | .67 (.29–1.52) | Ref |
| GAC2 | 45.53 (8.44–245.73)c | 94.05 (5.73–1544.62)b | 14.05 (2.68–73.55)b | 23.02 (4.22–125.61)c | 20.25 (4.14–99.10)c |
| Wheezing (yes) | |||||
| RV-A | Ref | … | … | … | … |
| RV-B | .52 (.19–1.41) | Ref | … | … | … |
| RV-C | 1.30 (.88–1.91) | 2.46 (.94–6.44) | … | … | … |
| C | 1.19 (.63–2.24) | 2.39 (.80–7.12) | Ref | … | … |
| GAC1 | 1.80 (1.13–2.88) | 3.24 (1.19–8.82) | 1.50 (.78–2.88) | Ref | … |
| A2 | 0.84 (.49–1.45) | 1.65 (.58–4.69) | 0.71 (.35–1.44) | .49 (.28–.87) | Ref |
| GAC2 | 4.86 (1.10–21.50) | 10.52 (1.75–63.28) | 3.43 (.74–15.80) | 2.65 (.61–11.58) | 5.11 (1.18–22.22) |
| Severe illnessd (total n = 676e) | |||||
| RV-A | Ref | … | … | … | … |
| RV-B | 1.05 (.29–3.78) | Ref | … | … | … |
| RV-C | 1.94 (1.09–3.47) | 2.06 (.61–7.00) | … | … | … |
| C | 1.46 (.58–3.70) | 1.57 (.37–6.61) | Ref | … | … |
| GAC1 | 2.61 (1.34–5.09) | 2.58 (.73–9.12) | 1.67 (.67–4.16) | Ref | … |
| A2 | 1.39 (.64–2.99) | 1.44 (.39–5.37) | .86 (.32–2.33) | .53 (.25–1.13) | Ref |
| GAC2 | 14.54 (2.03–104.04) | 11.16 (1.15–108.60) | 5.94 (.76–46.58) | 4.43 (.63–31.12) | 7.12 (.99–51.4) |
Odds ratios were adjusted for age in years, sex, and place of residence. P values were calculated by multiple comparisons (Tukey method) using logistic regressions and adjusted by Hochberg method, and values in bold means significant.
Abbreviations: aOR, adjusted odds ratios; CI, confidence interval; Ref, reference group of rhinoviruses for calculating adjusted odds ratio; RV, rhinovirus; SpO2, percutaneous arterial oxygen saturation.
One episode of RV-C was excluded because of the codetection of different RV-C clades.
Adjusted P value for multiple comparison < .05.
Adjusted P value for multiple comparison < .01.
aOR was calculated between severe illness (severe lower respiratory tract illness [LRTI] and very severe LRTI) and nonsevere illness (coryza, respiratory tract illness, and LRTI).
Thirty-six episodes (5.1%) were excluded from the analysis due to incomplete SpO2 measurement for the severity classification.
Adjusted Odds Ratios for the Comparison of Clinical Outcomes of Episodes by Different Rhinovirus Species and Rhinovirus-C Clades
| Clinical Outcome . | Versus RV-A . | Versus RV-B . | Versus RV-C (C) . | Versus RV-C (GAC1) . | Versus RV-C (A2) . |
|---|---|---|---|---|---|
| aOR (95% CI) . | aOR (95% CI) . | aOR (95% CI) . | aOR (95% CI) . | aOR (95% CI) . | |
| Total (n = 712a) | |||||
| Low SpO2 <95% (yes) | |||||
| RV-A | Ref | … | … | … | … |
| RV-B | 0.87 (.32–2.37) | Ref | … | … | … |
| RV-C | 1.65 (1.06–2.57) | 1.97 (.75–5.19) | … | … | … |
| C | 1.50 (.75–3.00) | 1.81 (.59–5.56) | Ref | … | … |
| GAC1 | 2.16 (1.29–3.64) | 2.54 (.93–7.00) | 1.37 (.68–2.75) | Ref | … |
| A2 | 1.05 (.56–1.93) | 1.28 (.43–3.78) | .66 (.30–1.42) | .49 (.26–.91) | Ref |
| GAC2 | 7.26 (1.65–31.91) | 15.89 (.96–128.83) | 4.71 (1.01–22.04) | 3.46 (.80–14.99) | 7.94 (1.69–37.43) |
| Nasal discharge or congestion (yes) | |||||
| RV-A | Ref. | … | … | … | … |
| RV-B | .26 (.08–.81)b | Ref | … | … | … |
| RV-C | .76 (.34–1.73) | 3.07 (1.13–8.32)b | … | … | … |
| C | 1.05 (.26–4.22) | 3.24 (.73–14.4) | Ref | … | … |
| GAC1 | .86 (.32–2.31) | 3.66 (1.15–11.7) | .97 (.24–3.86) | Ref | … |
| A2 | .67 (.24–1.86) | 2.73 (.84–8.83) | .74 (.18–3.05) | .73 (.25–2.16) | Ref |
| GAC2 | .29 (.03–2.90) | 1.03 (.10–10.87) | .41 (.03–5.20) | 0.17 (.02–2.01) | .30 (.03–3.14) |
| Chest indrawing (yes) | |||||
| RV-A | Ref | … | … | … | … |
| RV-B | .94 (.20–4.38) | Ref | … | … | … |
| RV-C | 2.20 (1.17–4.13)b | 2.54 (.59–10.9) | … | … | … |
| C | 2.46 (1.01–6.00) | 2.99 (.60–14.9) | Ref | … | … |
| GAC1 | 2.20 (1.04–4.68) | 2.32 (.50–10.7) | .85 (.35–2.05) | Ref | … |
| A2 | 1.44 (.62–3.34) | 1.80 (.38–8.56) | .57 (.22–1.49) | .67 (.29–1.52) | Ref |
| GAC2 | 45.53 (8.44–245.73)c | 94.05 (5.73–1544.62)b | 14.05 (2.68–73.55)b | 23.02 (4.22–125.61)c | 20.25 (4.14–99.10)c |
| Wheezing (yes) | |||||
| RV-A | Ref | … | … | … | … |
| RV-B | .52 (.19–1.41) | Ref | … | … | … |
| RV-C | 1.30 (.88–1.91) | 2.46 (.94–6.44) | … | … | … |
| C | 1.19 (.63–2.24) | 2.39 (.80–7.12) | Ref | … | … |
| GAC1 | 1.80 (1.13–2.88) | 3.24 (1.19–8.82) | 1.50 (.78–2.88) | Ref | … |
| A2 | 0.84 (.49–1.45) | 1.65 (.58–4.69) | 0.71 (.35–1.44) | .49 (.28–.87) | Ref |
| GAC2 | 4.86 (1.10–21.50) | 10.52 (1.75–63.28) | 3.43 (.74–15.80) | 2.65 (.61–11.58) | 5.11 (1.18–22.22) |
| Severe illnessd (total n = 676e) | |||||
| RV-A | Ref | … | … | … | … |
| RV-B | 1.05 (.29–3.78) | Ref | … | … | … |
| RV-C | 1.94 (1.09–3.47) | 2.06 (.61–7.00) | … | … | … |
| C | 1.46 (.58–3.70) | 1.57 (.37–6.61) | Ref | … | … |
| GAC1 | 2.61 (1.34–5.09) | 2.58 (.73–9.12) | 1.67 (.67–4.16) | Ref | … |
| A2 | 1.39 (.64–2.99) | 1.44 (.39–5.37) | .86 (.32–2.33) | .53 (.25–1.13) | Ref |
| GAC2 | 14.54 (2.03–104.04) | 11.16 (1.15–108.60) | 5.94 (.76–46.58) | 4.43 (.63–31.12) | 7.12 (.99–51.4) |
| Clinical Outcome . | Versus RV-A . | Versus RV-B . | Versus RV-C (C) . | Versus RV-C (GAC1) . | Versus RV-C (A2) . |
|---|---|---|---|---|---|
| aOR (95% CI) . | aOR (95% CI) . | aOR (95% CI) . | aOR (95% CI) . | aOR (95% CI) . | |
| Total (n = 712a) | |||||
| Low SpO2 <95% (yes) | |||||
| RV-A | Ref | … | … | … | … |
| RV-B | 0.87 (.32–2.37) | Ref | … | … | … |
| RV-C | 1.65 (1.06–2.57) | 1.97 (.75–5.19) | … | … | … |
| C | 1.50 (.75–3.00) | 1.81 (.59–5.56) | Ref | … | … |
| GAC1 | 2.16 (1.29–3.64) | 2.54 (.93–7.00) | 1.37 (.68–2.75) | Ref | … |
| A2 | 1.05 (.56–1.93) | 1.28 (.43–3.78) | .66 (.30–1.42) | .49 (.26–.91) | Ref |
| GAC2 | 7.26 (1.65–31.91) | 15.89 (.96–128.83) | 4.71 (1.01–22.04) | 3.46 (.80–14.99) | 7.94 (1.69–37.43) |
| Nasal discharge or congestion (yes) | |||||
| RV-A | Ref. | … | … | … | … |
| RV-B | .26 (.08–.81)b | Ref | … | … | … |
| RV-C | .76 (.34–1.73) | 3.07 (1.13–8.32)b | … | … | … |
| C | 1.05 (.26–4.22) | 3.24 (.73–14.4) | Ref | … | … |
| GAC1 | .86 (.32–2.31) | 3.66 (1.15–11.7) | .97 (.24–3.86) | Ref | … |
| A2 | .67 (.24–1.86) | 2.73 (.84–8.83) | .74 (.18–3.05) | .73 (.25–2.16) | Ref |
| GAC2 | .29 (.03–2.90) | 1.03 (.10–10.87) | .41 (.03–5.20) | 0.17 (.02–2.01) | .30 (.03–3.14) |
| Chest indrawing (yes) | |||||
| RV-A | Ref | … | … | … | … |
| RV-B | .94 (.20–4.38) | Ref | … | … | … |
| RV-C | 2.20 (1.17–4.13)b | 2.54 (.59–10.9) | … | … | … |
| C | 2.46 (1.01–6.00) | 2.99 (.60–14.9) | Ref | … | … |
| GAC1 | 2.20 (1.04–4.68) | 2.32 (.50–10.7) | .85 (.35–2.05) | Ref | … |
| A2 | 1.44 (.62–3.34) | 1.80 (.38–8.56) | .57 (.22–1.49) | .67 (.29–1.52) | Ref |
| GAC2 | 45.53 (8.44–245.73)c | 94.05 (5.73–1544.62)b | 14.05 (2.68–73.55)b | 23.02 (4.22–125.61)c | 20.25 (4.14–99.10)c |
| Wheezing (yes) | |||||
| RV-A | Ref | … | … | … | … |
| RV-B | .52 (.19–1.41) | Ref | … | … | … |
| RV-C | 1.30 (.88–1.91) | 2.46 (.94–6.44) | … | … | … |
| C | 1.19 (.63–2.24) | 2.39 (.80–7.12) | Ref | … | … |
| GAC1 | 1.80 (1.13–2.88) | 3.24 (1.19–8.82) | 1.50 (.78–2.88) | Ref | … |
| A2 | 0.84 (.49–1.45) | 1.65 (.58–4.69) | 0.71 (.35–1.44) | .49 (.28–.87) | Ref |
| GAC2 | 4.86 (1.10–21.50) | 10.52 (1.75–63.28) | 3.43 (.74–15.80) | 2.65 (.61–11.58) | 5.11 (1.18–22.22) |
| Severe illnessd (total n = 676e) | |||||
| RV-A | Ref | … | … | … | … |
| RV-B | 1.05 (.29–3.78) | Ref | … | … | … |
| RV-C | 1.94 (1.09–3.47) | 2.06 (.61–7.00) | … | … | … |
| C | 1.46 (.58–3.70) | 1.57 (.37–6.61) | Ref | … | … |
| GAC1 | 2.61 (1.34–5.09) | 2.58 (.73–9.12) | 1.67 (.67–4.16) | Ref | … |
| A2 | 1.39 (.64–2.99) | 1.44 (.39–5.37) | .86 (.32–2.33) | .53 (.25–1.13) | Ref |
| GAC2 | 14.54 (2.03–104.04) | 11.16 (1.15–108.60) | 5.94 (.76–46.58) | 4.43 (.63–31.12) | 7.12 (.99–51.4) |
Odds ratios were adjusted for age in years, sex, and place of residence. P values were calculated by multiple comparisons (Tukey method) using logistic regressions and adjusted by Hochberg method, and values in bold means significant.
Abbreviations: aOR, adjusted odds ratios; CI, confidence interval; Ref, reference group of rhinoviruses for calculating adjusted odds ratio; RV, rhinovirus; SpO2, percutaneous arterial oxygen saturation.
One episode of RV-C was excluded because of the codetection of different RV-C clades.
Adjusted P value for multiple comparison < .05.
Adjusted P value for multiple comparison < .01.
aOR was calculated between severe illness (severe lower respiratory tract illness [LRTI] and very severe LRTI) and nonsevere illness (coryza, respiratory tract illness, and LRTI).
Thirty-six episodes (5.1%) were excluded from the analysis due to incomplete SpO2 measurement for the severity classification.
When the proportion of episodes with each clinical sign or severe illness was compared between RV species and RV-C clades, episodes with chest indrawing (87.5%) were significantly more common in episodes with RV-C (GAC2) than in those with RV-A (P < .01) or RV-B (P < .01) (Table 2). The proportion of episodes with low SpO2 (22.1%), cough (94.8%), wheezing (27.3%), and severe illness (14.3%) were higher in cases associated with GAC1 than in those with RV-A with borderline significance (Table 2).
Comparison of Clinical Symptoms and Signs Between 4 RV-C Clades
Although the number of episodes with GAC2 was limited, the proportion of episodes with chest indrawing (5/8 [62.5%]) was significantly more common than in other RV-C clades. Low SpO2 (4/8 [50%]), wheezing (4/8 [50%]), and severe illness (2/5 [40%]) were relatively high without statistical significance (Tables 1 and 2).
Additionally, within RV-C clades, episodes with A2 were less likely to have low SpO2 (aOR, 0.49 [95% CI, .26–.91]) and wheezing (aOR, 0.49 [95% CI, .28–.87]) than those caused by GAC1 (Table 2). However, they were borderline statistically significant when P values were adjusted for multiple comparisons (P = .054 and P = .242, respectively).
DISCUSSION
In this study, the proportion of severe illness episodes was higher for RV-C–positive cases than for RV-A– and RV-B–positive cases; however, the difference among the species did not reach the statistical significance when Bonferroni adjustment was applied. Previous research has also indicated that RV-C was associated with severe RTI and asthma exacerbation [3, 13–15]. Some differences might exist in the pathogenesis of RV-C infection compared with RV-A and RV-B infections. Because RV-C propagation remains difficult [4], there are no established in vivo and in vitro systems to analyze its pathogenesis. Other approaches, including proteomics studies, might provide some insights into RV-C pathogenesis.
The previous study classified RV-C into 2 major types; those with a 5′-UTR classified as RV-A (RV-Ca) and those with a 5′-UTR classified as RV-C (RV-Cc). Since RV-Ca has a capsid region classified as RV-C, RV-Ca was considered to have emerged due to recombination between RV-A and RV-C [8]. Kiang et al reported that RV-C is divided into GAC1, A2, GAC2, and C by phylogenetic analysis using the 5′-UTR [7], and strains previously classified as RV-Ca included GAC1, A2, and GAC2 [23]. All emerging RV-C clades (A2, GAC1, and GAC2) have 5′-UTRs derived from RV-A and capsid genes derived from RV-C. Interestingly, most RV-C specimens in this study (319/395 [80.8%]) were RV-Ca considered as recombinants with RV-A. Since RV-A and RV-C were more commonly detected than RV-B, most RVs detected in the present study probably possessed 5′-UTRs derived from RV-A. A similar pattern was observed in a different study site in the Philippines [19] and a study in Italy [24].
We also analyzed clinical symptoms and severity between RV-C clades and RV-A and RV-B. Episodes caused by each RV-C clade (C, A2, GAC1, and GAC2) had more severe symptoms, including low SpO2, wheezing, and chest indrawing, than those caused by RV-A and RV-B. Thus, all RV-C clades were linked to more severe LRTI than RV-A and RV-B. Interestingly, episodes caused by GAC1 and GAC2 were associated with more severe symptoms than those caused by RV-C (C) and A2. For episodes with GAC2 infection, in particular, more than half of them presented with severe symptoms, although the number of GAC2-positive episodes was too small to analyze statistical significance. Our previous study detected RV genome in some serum samples from pediatric patients with severe respiratory disease in the Philippines. Most positive serum samples had the capsid gene classified as RV-C and 5′-UTR as RV-A [19]. In vitro study revealed that 5′-UTR recombination and mutation in coxsackievirus were associated with increased viral replication and virulence [25]. Recombination in RV-C with 5′-UTRs derived from RV-A might have increased virulence or replication efficiency and contributed to increasing RV-C detection.
However, there are some limitations to the study. First, we only analyzed 5′UTR, and capsid and other regions were not analyzed. Therefore, we could not confirm the recombination. If the capsid region is also analyzed, it may differ from present classification. Second, we did not analyze effect of coinfection. We did not detect any bacteria in this study. Although other viruses were detected in the study, we excluded those positive for RV and other viruses from the analysis. Finally, clinical symptoms to separate the 2 sequential episodes and demographic data were obtained from the retrospective interview of the parents/guardians, which might be subject to a recall bias.
In conclusion, RV-C classified based on 5′-UTR sequences had an association with severe RTI among children <5 years of age with RV-associated RTI in 2 communities in the Philippines. Further analysis regarding the pathogenic mechanisms of RV-C with additional genomic analysis is needed.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We are grateful to Dr C. Balasbas and Dr D. Plaza, the medical health officers in the municipalities of Kawayan and Caibiran; Dr J. Caneja, the chief of Biliran Provincial Hospital; and Dr E. Veloso and the late Dr A. Veneracion, provincial health officers in that hospital, for their research cooperation. We also thank the staff of the Tohoku–RITM Collaborating Research Center on Emerging and Re-emerging Infectious Diseases in Biliran and RITM; Dr E. Segubre-Mercado, Ms M. Estanislao-Dueñas, Ms S. Abe, and Dr E. Nakagawa for administrative support; and Ms M. Kishi, Ms M. Venturina, and staff of Asian Foundation for Tropical Medicine (AFTM) for technical support.
Disclaimer. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the Japan Initiative for Global Research Network on Infectious Diseases of the Japan Agency for Medical Research and Development (AMED) (grant number JP19fm0108013); the Japan Program for Infectious Diseases Research and Infrastructure (AMED, grant number JP20wm0125001); the Science and Technology Research Partnership for Sustainable Development (AMED and the Japan International Cooperation Agency; grant number JP16jm0110001); the Japan Society for the Promotion of Science (KAKENHI grant number JP19KK0204); and the Takeda Science Foundation scholarship.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
World Health Organization.
Author notes
Potential conflicts of interest. The authors: No reported conflicts of interest.





Comments