-
PDF
- Split View
-
Views
-
Cite
Cite
Georgios Angelidakis, Roy F Chemaly, Partow Kebriaei, Nadim J Ajami, Micah M Bhatti, Elizabeth Shpall, Chitra Hosing, Preetesh Jain, Kris Michael Mahadeo, Fareed Khawaja, Jennifer Wargo, Robert Jenq, Ella Ariza Heredia, 15. Evaluation of Retained Immunity for Tetanus-Diphtheria and Pneumococcal Vaccines in Recipients of Cellular Therapies, Open Forum Infectious Diseases, Volume 8, Issue Supplement_1, November 2021, Pages S131–S132, https://doi.org/10.1093/ofid/ofab466.217
Close - Share Icon Share
Abstract
Infectious complications in cancer patients (pts) who have received T-cell therapies are similar to those in autologous hematopoietic stem cell transplant (HCT) recipients, who - because they lose prior acquired immunity after undergoing conditioning regimens and transplantation- may be at an increased risk for vaccine-preventable infections. We sought to determine seroprotection rates against pneumococcus and tetanus-diphtheria before and after cellular therapies.
In this ongoing prospective observational cohort study, we enrolled pts with any type of cancer who received cellular therapy with chimeric antigen receptor modified T cell (CAR-T), natural killer CAR-T, or T-cell receptor- directed immunotherapies at MD Anderson Cancer Center from January 2020 through May 2021. We performed antibody assays for diphtheria, tetanus, and pneumococcus before, at 1 month, and between 3-6 months after T-cell therapy for each pt regardless of vaccination history.
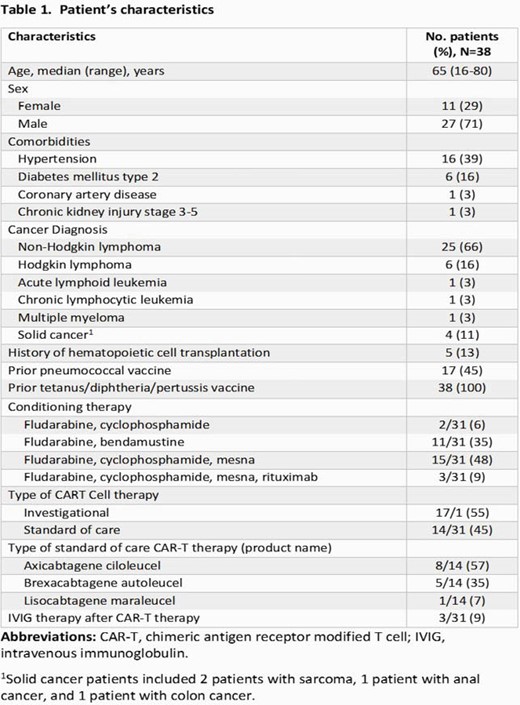
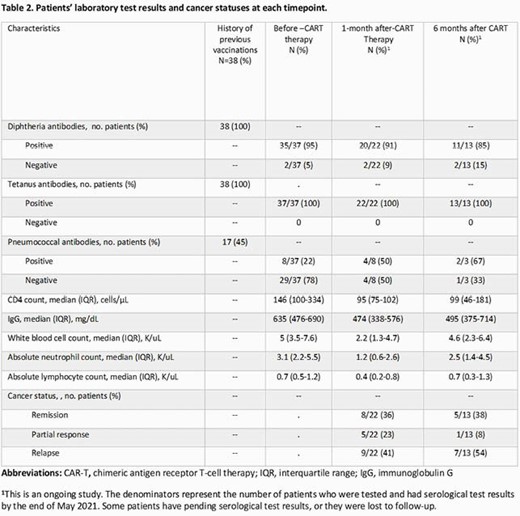
Of 38 pts enrolled, 27 (71%) were men and 25 (66%) had non-Hodgkin lymphoma (Table 1); 38 (100%) and 17 (45%) had a history of previous diphtheria-tetanus-acellular pertussis (Tdap) and pneumococcal vaccination, respectively (Table 2). Tetanus serologies were positive for all pts tested before, at 1 month and 3-6 months after T cell therapy (37/37 [100%], 22/22 [100%], and 13/13 [100%], respectively). Diphtheria serologies were positive for most pts tested before, at 1 month and 3-6 months after therapy (35/37 [95%], 20/22 [91%], and 11/13 [85%], respectively]. Pneumococcal serologies were positive for 8 out of 37 [22%] pts before therapy, among these 8 pts, 4 had positive serologies 1 month after therapy, and 2 of 3 tested 3-6 months after therapy had positive serologies. One pt received a pneumococcal vaccine 10 months after therapy but had negative serologies post-vaccination.
Most pts who received T-cell therapy retained their immunity for diphtheria and tetanus, but most also lost their immunity for pneumococcus. This suggests that the standard of care for pts receiving T-cell therapy should include more robust strategy for pneumococcal vaccination, but its timing, need for booster dosing, and antibody response needs to be determined in future trials.
Roy F. Chemaly, MD, MPH, FACP, FIDSA, AiCuris (Grant/Research Support)Ansun Biopharma (Consultant, Grant/Research Support)Chimerix (Consultant, Grant/Research Support)Clinigen (Consultant)Genentech (Consultant, Grant/Research Support)Janssen (Consultant, Grant/Research Support)Karius (Grant/Research Support)Merck (Consultant, Grant/Research Support)Molecular Partners (Consultant, Advisor or Review Panel member)Novartis (Grant/Research Support)Oxford Immunotec (Consultant, Grant/Research Support)Partner Therapeutics (Consultant)Pulmotec (Consultant, Grant/Research Support)Shire/Takeda (Consultant, Grant/Research Support)Viracor (Grant/Research Support)Xenex (Grant/Research Support) Fareed Khawaja, MBBS, Eurofins Viracor (Research Grant or Support) Ella Ariza Heredia, MD, Merck (Grant/Research Support)
- cancer
- antibody formation
- cancer care facilities
- conditioning (psychology)
- consultants
- disclosure
- immunity
- immunotherapy
- lymphoma, non-hodgkin
- pneumococcal vaccine
- serologic tests
- streptococcus pneumoniae
- t-lymphocytes
- vaccination
- vaccines
- infections
- antibodies
- diphtheria
- infection as complication of medical care
- tetanus
- transplantation
- pertussis
- t-cell therapy
- autologous hematopoietic stem cell transplant with purging
- autologous hematopoietic stem cell transplant without purging
- acquired immunity
- standard of care
- ulipristal
- chimeric antigen receptors
- cell therapy





Comments