-
PDF
- Split View
-
Views
-
Cite
Cite
Zachary A Yetmar, Supavit Chesdachai, Tarek Kashour, Muhammad Riaz, Danielle J Gerberi, Andrew D Badley, Elie F Berbari, Imad M Tleyjeh, Prior Statin Use and Risk of Mortality and Severe Disease From Coronavirus Disease 2019: A Systematic Review and Meta-analysis, Open Forum Infectious Diseases, Volume 8, Issue 7, July 2021, ofab284, https://doi.org/10.1093/ofid/ofab284
Close - Share Icon Share
Abstract
Statins up-regulate angiotensin-converting enzyme 2, the receptor of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), while also exhibiting pleiotropic antiviral, antithrombotic, and anti-inflammatory properties. Uncertainties exist about their effect on the course of SARS-CoV-2 infection. We sought to systematically review the literature and perform a meta-analysis to examine the association between prior statin use and outcomes of patients with coronavirus disease 2019 (COVID-19).
We searched Ovid Medline, Web of Science, Scopus, and the preprint server medRxiv from inception to December 2020. We assessed the quality of eligible studies with the Newcastle-Ottawa quality scale. We pooled adjusted relative risk (aRRs) of the association between prior statin use and outcomes of patients with COVID-19 using the DerSimonian-Laird random-effects model and assessed heterogeneity using the I2 index.
Overall, 19 (16 cohorts and 3 case-control) studies were eligible, with a total of 395 513 patients. Sixteen of 19 studies had low or moderate risk of bias. Among 109 080 patients enrolled in 13 separate studies, prior statin use was associated with a lower risk of mortality (pooled aRR, 0.65 [95% confidence interval {CI}, .56–.77], I2 = 84.1%) and a reduced risk of severe COVID-19 was also observed in 48 110 patients enrolled in 9 studies (pooled aRR, 0.73 [95% CI, .57–.94], I2 = 82.8%), with no evidence of publication bias.
Cumulative evidence suggests that prior statin use is associated with lower risks of mortality or severe disease in patients with COVID-19. These data support the continued use of statins medications in patients with an indication for lipid-lowering therapy during the COVID-19 pandemic.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the viral agent responsible for coronavirus disease 2019 (COVID-19), was identified in December 2019 and quickly spread globally. As of 8 February 2021, >113 million people have been infected, resulting in 2.5 million deaths [1]. Due to the scope of this pandemic, assessment of predictors associated with increased morbidity and mortality of COVID-19 are warranted.
There is an ongoing controversy on the impact of statins, a commonly used class of lipid-lowering medications working through inhibition of 3-hydroxy-3-methyl-glutaryl coenzyme A reductase, on patients with COVID-19. Statins are known to possess pleiotropic characteristics such as anti-inflammatory and immunomodulatory properties. They have been shown to reduce Toll-like receptor (TLR) expression and cytokine levels and modulate T-cell responses [2–4]. Furthermore, COVID-19 has been associated with significant thromboembolic complications and statins have been posited to have antithrombotic effects [5, 6].
Despite of these beneficial properties, statins are thought to up-regulate expression of angiotensin-converting enzyme 2 (ACE2) receptor, the target for SARS-CoV-2 [7–9]. This may theoretically increase the risk of SARS-CoV-2 infectivity. However, ACE2 has been shown to be protective against acute lung injury (ALI) in the murine sepsis and acid aspiration model [10]. This observation, along with the documented protective effects of statins against ALI, may indicate that the net effect of statins in COVID-19 disease may be favorable [11–16].
It is also well established that patients with underlying cardiovascular diseases are at higher risk for developing severe COVID-19 and death [17, 18]. Furthermore, COVID-19 disease is associated with a higher incidence of cardiovascular complications, where statins could play an important preventive role [19].
Statins have been previously evaluated in other infectious and inflammatory syndromes. A meta-analysis of observational studies of prevention or treatment of infections showed that prior statin use is associated with a lower risk of infections and mortality [20]. Other meta-analyses of statins in community-acquired pneumonia, influenza, and sepsis showed similar associations with statins [21–24]. However, a meta-analysis of randomized controlled trials of de novo use of statins in sepsis and a randomized controlled trial for simvastatin in acute respiratory distress syndrome (ARDS) did not show an impact on mortality or progression to severe sepsis [25, 26].
Therefore, we sought to systematically review the literature and perform a meta-analysis to examine the association between prior statin use and outcomes of patients with COVID-19.
METHODS
A comprehensive systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [27] and Meta-Analysis of Observational Studies in Epidemiology [28] guidelines. This study was registered in the International Prospective Register of Systematic Reviews (PROSPERO, registration number CRD42021227604).
Study Selection
Studies reporting an association between prior use of statins medications and outcomes of patients with COVID-19 were eligible for inclusion. Examined outcomes included mortality, severe COVID-19, hospitalization, and diagnosis of COVID-19. Two reviewers (Z. A. Y. and S. C.) independently examined eligible studies. Unresolved disagreements after discussion were addressed by a third reviewer (I. M. T.).
Data Sources and Search Strategy
The literature was searched by an experienced medical librarian (D. J. G.) for the concepts of COVID-19 and statin medications. Search strategies were created using a combination of keywords and standardized index terms. Searches were run in December 2020 in Ovid EBM Reviews, Ovid Embase (1974 and later), Ovid Medline (1946 and later including epub ahead of print, in-process, and other nonindexed citations), Scopus (1970 and later), and Web of Science Core Collection (1975 and later). Search results were limited to English-language articles. All results were exported to EndNote where duplicates were removed, leaving 427 citations. Search strategies are provided in the Supplementary Materials. We also queried medRxiv, a preprint server for health sciences, for non-peer-reviewed articles that were not captured by the preceding search strategy.
Data Collection and Quality Assessment
Data on study population demographics, comorbidities, statin exposure, and outcomes were extracted by 2 reviewers (Z. A. Y. and S. C.) independently. This was subsequently reviewed by the senior reviewer (I. M. T.). Mortality outcomes included in-hospital mortality, 28-day mortality, and 30-day mortality. We defined severe COVID-19 as any of the following: requirement of advanced ventilator support, intensive care unit (ICU) admission, or ARDS, either alone or as a composite with mortality. In the event where studies were missing required data for our analyses, we made inquiries to respective corresponding authors.
Eligible studies were assessed by the Newcastle-Ottawa quality assessment scale (NOS) [29, 30]. The NOS rates observational studies based on 3 parameters: selection, comparability between exposed and unexposed groups, and exposure and outcome assessment. These 3 domains can have a maximum score of 4, 2, and 3 stars, respectively. Studies with <5 stars are considered low quality, 5–7 stars moderate quality, and >7 stars high quality. Studies deemed at high risk of bias (low-quality studies) were excluded from quantitative analysis. Two reviewers (Z. A. Y. and S. C.) assessed quality of included studies with NOS.
Statistical Analysis
We extracted data on the effect estimates in the form of adjusted odds ratios (ORs), relative risks (RRs), and hazard ratios (HRs). Before conducting the meta-analysis and pooling the effect estimates, we converted adjusted ORs to RRs, if appropriate information was available [31]. To pool the effect estimates, we used the DerSimonian-Laird random-effects model [32] with the inverse variance weighting method (and constructed corresponding Forest plots) that recognizes studies as a sample of all potential studies and incorporates a between-study random-effects component to allow for between study heterogeneity [33]. If the effect estimates from both multivariable and propensity-matched analyses were available, we included the estimates from the multivariable analyses.
Heterogeneity among included studies was assessed using the I2 index [34]. To explore sources of heterogeneity, we conducted univariable meta-regressions to assess the impact of different variables on the overall estimate of effect. Our regression covariates were chosen a priori and consisted of study-level variables (when available): mean/median age, sex, proportion of patients with coronary artery disease, diabetes mellitus, smoking, hypertension, dyslipidemia, lung disease, stroke, peripheral vascular disease, malignancy, statin dose intensity, and preprint vs published studies.
We constructed contour-enhanced funnel plots [35] and performed an Egger precision-weighted linear regression test as a statistical test of funnel plot asymmetry and publication bias [36]. By identifying the regions of the funnel plot that correspond to statistically significant effects, an assessment can be made as to whether the location of the perceived missing studies is in significant or nonsignificant regions. All analyses were conducted using Stata statistical software (StataCorp 2011, release 12, College Station, Texas).
RESULTS
The results of our literature search are presented in Figure 1. Nineteen studies met a priori–outlined eligibility criteria with a total of 395 513 patients (Table 1) [37–55].
| Study . | Country . | Publication Status . | Study Type . | Age, y . | Male Sex, % . | Statin, No. . | Nonstatin, No. . | Mortality HR, RR, OR (95% CI) . | Severe COVID-19 HR, RR, OR (95% CI) . | Incidence HR, RR, OR (95% CI) . |
|---|---|---|---|---|---|---|---|---|---|---|
| Bifulco et al [37] | Italy | Published | Retrospective cohort | 65 (mean) | 63 | 117 | 424 | 0.75 (.26–2.17) | NR | NR |
| Butt et al [38] | Denmark | Published | Retrospective cohort | 54 (median) | 47.1 | 843 | 3999 | 0.96 (.78–1.18) | 1.16 (.95–1.41) | NR |
| Cariou et al [39] | France | Published | Retrospective cohort | 70.9 (mean) | 64 | 1192 | 1257 | 1.35 (1.07–1.66) | 1.1 (.86–1.39) | NR |
| Daniels et al [40] | USA | Published | Retrospective cohort | 59 (mean) | 58 | 46 | 124 | NR | 0.48 (.22–.85) | NR |
| De Spiegeleer et al [41] | Belgium | Published | Retrospective cohort | 85.9 (mean) | 33.1 | 31 | 123 | NR | 0.89 (.31–1.81) | NR |
| Fan et al [42] | China | Published | Retrospective cohort | 58 (median) | 48.8 | 250 | 1897 | 0.428 (.169–.907) | 0.319 (.27–.945) | NR |
| Gupta et al [43] | USA | Preprint | Retrospective cohort | NR | 57 | 951 | 1675 | 0.49 (.36–.63) | 0.54 (.44–.67) | NR |
| Ho et al [44] | UK | Published | Retrospective cohort | 66.5 (mean) | 47.2 | 36412 | 199516 | NR | NR | 1.24 (.97–1.59) |
| Israel et al [45] | Israel | Preprint | Retrospective case-control | 59.1 (mean) | 49.5 | 947 | 19810 | 0.53 (.36–.766) | 0.687 (.555–.845) | 0.746 (.645–.858) |
| Izzi-Engbeaya et al [46] | UK | Published | Retrospective cohort | 65.8 (mean) | 60 | 373 | 516 | NR | 0.733 (.417–1.39) | NR |
| Mallow et al [47] | USA | Published | Retrospective cohort | 64.9 (mean) | 52.8 | 5313 | 16363 | 0.61 (.56–.66) | NR | NR |
| Masana et al [48] | Spain | Published | Retrospective case-control | 67 (median) | 57.2 | 581 | 581 | 0.60 (.39–.92) | NR | NR |
| Nicholson et al [49] | USA | Preprint | Retrospective cohort | 64 (median) | 56.8 | 511 | 531 | 0.548 (.311–.938) | NR | NR |
| Rodriguez-Nava et al [50] | USA | Published | Retrospective cohort | 68 (median) | 64.4 | 47 | 40 | 0.38 (.18–.77) | NR | NR |
| Rosenthal et al [51] | USA | Published | Retrospective cohort | 56.1 (mean) | 49.3 | 11970 | 23332 | 0.62 (.58–.67) | NR | NR |
| Saeed et al [52] | USA | Published | Retrospective cohort | 65 (mean) | 53 | 983 | 1283 | 0.51 (.43–.61) | NR | NR |
| Song et al [53] | USA | Published | Retrospective cohort | 62 (median) | 57 | 123 | 126 | 0.88 (.37–2.08) | 0.90 (.49–1.67) | NR |
| Yan et al [54] | Singapore | Published | Retrospcetive case-control | 50 (mean) | 48.3 | 458 | 48787 | NR | 1.52 (.60–2.70) | 2.46 (1.47–4.06) |
| Zhang et al [55] | China | Published | Retrospective cohort | NR | 48.9 | 1219 | 12762 | 0.63 (.48–.84) | 0.80 (.62–1.05) | NR |
| Study . | Country . | Publication Status . | Study Type . | Age, y . | Male Sex, % . | Statin, No. . | Nonstatin, No. . | Mortality HR, RR, OR (95% CI) . | Severe COVID-19 HR, RR, OR (95% CI) . | Incidence HR, RR, OR (95% CI) . |
|---|---|---|---|---|---|---|---|---|---|---|
| Bifulco et al [37] | Italy | Published | Retrospective cohort | 65 (mean) | 63 | 117 | 424 | 0.75 (.26–2.17) | NR | NR |
| Butt et al [38] | Denmark | Published | Retrospective cohort | 54 (median) | 47.1 | 843 | 3999 | 0.96 (.78–1.18) | 1.16 (.95–1.41) | NR |
| Cariou et al [39] | France | Published | Retrospective cohort | 70.9 (mean) | 64 | 1192 | 1257 | 1.35 (1.07–1.66) | 1.1 (.86–1.39) | NR |
| Daniels et al [40] | USA | Published | Retrospective cohort | 59 (mean) | 58 | 46 | 124 | NR | 0.48 (.22–.85) | NR |
| De Spiegeleer et al [41] | Belgium | Published | Retrospective cohort | 85.9 (mean) | 33.1 | 31 | 123 | NR | 0.89 (.31–1.81) | NR |
| Fan et al [42] | China | Published | Retrospective cohort | 58 (median) | 48.8 | 250 | 1897 | 0.428 (.169–.907) | 0.319 (.27–.945) | NR |
| Gupta et al [43] | USA | Preprint | Retrospective cohort | NR | 57 | 951 | 1675 | 0.49 (.36–.63) | 0.54 (.44–.67) | NR |
| Ho et al [44] | UK | Published | Retrospective cohort | 66.5 (mean) | 47.2 | 36412 | 199516 | NR | NR | 1.24 (.97–1.59) |
| Israel et al [45] | Israel | Preprint | Retrospective case-control | 59.1 (mean) | 49.5 | 947 | 19810 | 0.53 (.36–.766) | 0.687 (.555–.845) | 0.746 (.645–.858) |
| Izzi-Engbeaya et al [46] | UK | Published | Retrospective cohort | 65.8 (mean) | 60 | 373 | 516 | NR | 0.733 (.417–1.39) | NR |
| Mallow et al [47] | USA | Published | Retrospective cohort | 64.9 (mean) | 52.8 | 5313 | 16363 | 0.61 (.56–.66) | NR | NR |
| Masana et al [48] | Spain | Published | Retrospective case-control | 67 (median) | 57.2 | 581 | 581 | 0.60 (.39–.92) | NR | NR |
| Nicholson et al [49] | USA | Preprint | Retrospective cohort | 64 (median) | 56.8 | 511 | 531 | 0.548 (.311–.938) | NR | NR |
| Rodriguez-Nava et al [50] | USA | Published | Retrospective cohort | 68 (median) | 64.4 | 47 | 40 | 0.38 (.18–.77) | NR | NR |
| Rosenthal et al [51] | USA | Published | Retrospective cohort | 56.1 (mean) | 49.3 | 11970 | 23332 | 0.62 (.58–.67) | NR | NR |
| Saeed et al [52] | USA | Published | Retrospective cohort | 65 (mean) | 53 | 983 | 1283 | 0.51 (.43–.61) | NR | NR |
| Song et al [53] | USA | Published | Retrospective cohort | 62 (median) | 57 | 123 | 126 | 0.88 (.37–2.08) | 0.90 (.49–1.67) | NR |
| Yan et al [54] | Singapore | Published | Retrospcetive case-control | 50 (mean) | 48.3 | 458 | 48787 | NR | 1.52 (.60–2.70) | 2.46 (1.47–4.06) |
| Zhang et al [55] | China | Published | Retrospective cohort | NR | 48.9 | 1219 | 12762 | 0.63 (.48–.84) | 0.80 (.62–1.05) | NR |
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; HR, hazard ratio; NR, not reported; OR, odds ratio; RR, rate ratio; USA, United States.
| Study . | Country . | Publication Status . | Study Type . | Age, y . | Male Sex, % . | Statin, No. . | Nonstatin, No. . | Mortality HR, RR, OR (95% CI) . | Severe COVID-19 HR, RR, OR (95% CI) . | Incidence HR, RR, OR (95% CI) . |
|---|---|---|---|---|---|---|---|---|---|---|
| Bifulco et al [37] | Italy | Published | Retrospective cohort | 65 (mean) | 63 | 117 | 424 | 0.75 (.26–2.17) | NR | NR |
| Butt et al [38] | Denmark | Published | Retrospective cohort | 54 (median) | 47.1 | 843 | 3999 | 0.96 (.78–1.18) | 1.16 (.95–1.41) | NR |
| Cariou et al [39] | France | Published | Retrospective cohort | 70.9 (mean) | 64 | 1192 | 1257 | 1.35 (1.07–1.66) | 1.1 (.86–1.39) | NR |
| Daniels et al [40] | USA | Published | Retrospective cohort | 59 (mean) | 58 | 46 | 124 | NR | 0.48 (.22–.85) | NR |
| De Spiegeleer et al [41] | Belgium | Published | Retrospective cohort | 85.9 (mean) | 33.1 | 31 | 123 | NR | 0.89 (.31–1.81) | NR |
| Fan et al [42] | China | Published | Retrospective cohort | 58 (median) | 48.8 | 250 | 1897 | 0.428 (.169–.907) | 0.319 (.27–.945) | NR |
| Gupta et al [43] | USA | Preprint | Retrospective cohort | NR | 57 | 951 | 1675 | 0.49 (.36–.63) | 0.54 (.44–.67) | NR |
| Ho et al [44] | UK | Published | Retrospective cohort | 66.5 (mean) | 47.2 | 36412 | 199516 | NR | NR | 1.24 (.97–1.59) |
| Israel et al [45] | Israel | Preprint | Retrospective case-control | 59.1 (mean) | 49.5 | 947 | 19810 | 0.53 (.36–.766) | 0.687 (.555–.845) | 0.746 (.645–.858) |
| Izzi-Engbeaya et al [46] | UK | Published | Retrospective cohort | 65.8 (mean) | 60 | 373 | 516 | NR | 0.733 (.417–1.39) | NR |
| Mallow et al [47] | USA | Published | Retrospective cohort | 64.9 (mean) | 52.8 | 5313 | 16363 | 0.61 (.56–.66) | NR | NR |
| Masana et al [48] | Spain | Published | Retrospective case-control | 67 (median) | 57.2 | 581 | 581 | 0.60 (.39–.92) | NR | NR |
| Nicholson et al [49] | USA | Preprint | Retrospective cohort | 64 (median) | 56.8 | 511 | 531 | 0.548 (.311–.938) | NR | NR |
| Rodriguez-Nava et al [50] | USA | Published | Retrospective cohort | 68 (median) | 64.4 | 47 | 40 | 0.38 (.18–.77) | NR | NR |
| Rosenthal et al [51] | USA | Published | Retrospective cohort | 56.1 (mean) | 49.3 | 11970 | 23332 | 0.62 (.58–.67) | NR | NR |
| Saeed et al [52] | USA | Published | Retrospective cohort | 65 (mean) | 53 | 983 | 1283 | 0.51 (.43–.61) | NR | NR |
| Song et al [53] | USA | Published | Retrospective cohort | 62 (median) | 57 | 123 | 126 | 0.88 (.37–2.08) | 0.90 (.49–1.67) | NR |
| Yan et al [54] | Singapore | Published | Retrospcetive case-control | 50 (mean) | 48.3 | 458 | 48787 | NR | 1.52 (.60–2.70) | 2.46 (1.47–4.06) |
| Zhang et al [55] | China | Published | Retrospective cohort | NR | 48.9 | 1219 | 12762 | 0.63 (.48–.84) | 0.80 (.62–1.05) | NR |
| Study . | Country . | Publication Status . | Study Type . | Age, y . | Male Sex, % . | Statin, No. . | Nonstatin, No. . | Mortality HR, RR, OR (95% CI) . | Severe COVID-19 HR, RR, OR (95% CI) . | Incidence HR, RR, OR (95% CI) . |
|---|---|---|---|---|---|---|---|---|---|---|
| Bifulco et al [37] | Italy | Published | Retrospective cohort | 65 (mean) | 63 | 117 | 424 | 0.75 (.26–2.17) | NR | NR |
| Butt et al [38] | Denmark | Published | Retrospective cohort | 54 (median) | 47.1 | 843 | 3999 | 0.96 (.78–1.18) | 1.16 (.95–1.41) | NR |
| Cariou et al [39] | France | Published | Retrospective cohort | 70.9 (mean) | 64 | 1192 | 1257 | 1.35 (1.07–1.66) | 1.1 (.86–1.39) | NR |
| Daniels et al [40] | USA | Published | Retrospective cohort | 59 (mean) | 58 | 46 | 124 | NR | 0.48 (.22–.85) | NR |
| De Spiegeleer et al [41] | Belgium | Published | Retrospective cohort | 85.9 (mean) | 33.1 | 31 | 123 | NR | 0.89 (.31–1.81) | NR |
| Fan et al [42] | China | Published | Retrospective cohort | 58 (median) | 48.8 | 250 | 1897 | 0.428 (.169–.907) | 0.319 (.27–.945) | NR |
| Gupta et al [43] | USA | Preprint | Retrospective cohort | NR | 57 | 951 | 1675 | 0.49 (.36–.63) | 0.54 (.44–.67) | NR |
| Ho et al [44] | UK | Published | Retrospective cohort | 66.5 (mean) | 47.2 | 36412 | 199516 | NR | NR | 1.24 (.97–1.59) |
| Israel et al [45] | Israel | Preprint | Retrospective case-control | 59.1 (mean) | 49.5 | 947 | 19810 | 0.53 (.36–.766) | 0.687 (.555–.845) | 0.746 (.645–.858) |
| Izzi-Engbeaya et al [46] | UK | Published | Retrospective cohort | 65.8 (mean) | 60 | 373 | 516 | NR | 0.733 (.417–1.39) | NR |
| Mallow et al [47] | USA | Published | Retrospective cohort | 64.9 (mean) | 52.8 | 5313 | 16363 | 0.61 (.56–.66) | NR | NR |
| Masana et al [48] | Spain | Published | Retrospective case-control | 67 (median) | 57.2 | 581 | 581 | 0.60 (.39–.92) | NR | NR |
| Nicholson et al [49] | USA | Preprint | Retrospective cohort | 64 (median) | 56.8 | 511 | 531 | 0.548 (.311–.938) | NR | NR |
| Rodriguez-Nava et al [50] | USA | Published | Retrospective cohort | 68 (median) | 64.4 | 47 | 40 | 0.38 (.18–.77) | NR | NR |
| Rosenthal et al [51] | USA | Published | Retrospective cohort | 56.1 (mean) | 49.3 | 11970 | 23332 | 0.62 (.58–.67) | NR | NR |
| Saeed et al [52] | USA | Published | Retrospective cohort | 65 (mean) | 53 | 983 | 1283 | 0.51 (.43–.61) | NR | NR |
| Song et al [53] | USA | Published | Retrospective cohort | 62 (median) | 57 | 123 | 126 | 0.88 (.37–2.08) | 0.90 (.49–1.67) | NR |
| Yan et al [54] | Singapore | Published | Retrospcetive case-control | 50 (mean) | 48.3 | 458 | 48787 | NR | 1.52 (.60–2.70) | 2.46 (1.47–4.06) |
| Zhang et al [55] | China | Published | Retrospective cohort | NR | 48.9 | 1219 | 12762 | 0.63 (.48–.84) | 0.80 (.62–1.05) | NR |
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; HR, hazard ratio; NR, not reported; OR, odds ratio; RR, rate ratio; USA, United States.
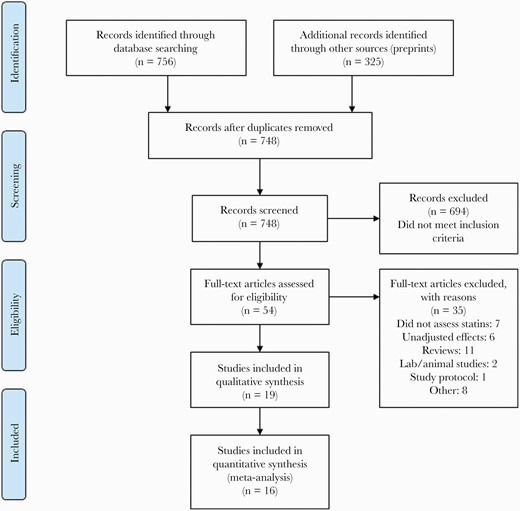
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of included studies.
All studies were observational, including 16 retrospective cohort studies and 3 case-control studies (Table 1). Most studies included all statins medications; however, 2 studies examined only rosuvastatin [45] and atorvastatin [50], respectively. No studies examined the effect of statins intensity or dose. Fourteen studies reported on associations of prior statin use with mortality [37–39, 42, 43, 45, 47–53, 55], 10 studies on associations with COVID-19 severity [38–40, 42, 43, 45, 46, 53–55], and 3 studies on associations with COVID-19 diagnosis [44, 45, 54]. Table 2 outlines the statistical methods utilized by individual studies and their risk of bias assessments. Sixteen studies were considered to be at low or moderate risk of bias and were eligible for quantitative analysis. Details of risk of bias assessment by NOS for cohort and case-control studies are shown in Supplementary Tables 1 and 2, respectively.
| Study . | Exposure . | Primary Outcome . | Secondary Outcome . | Statistical Method . | Factors Adjusted For . | Quality Assessment . |
|---|---|---|---|---|---|---|
| Bifulco et al [37] | Statin | In-hospital COVID-19-related mortality | NR | NR | Age, gender, smoking habit, preexisting comorbidities, indicators of disease severity and organ injuries, blood biomarkers | Low risk of bias |
| Butt et al [38] | Statin | 30-day all-cause mortality | Severe COVID-19; composite of mortality and severe COVID-19 | Adjusted Cox regression | Age, sex, ethnicity, education, income, comorbidity, concomitant medical treatment | Low risk of bias |
| Cariou et al [39] | Statin | 28-day mortality | MV | Logistic regression | Macrovascular complications, microvascular complications, diabetes treatments, antihypertensives, hypertension, ezetimibe, treated sleep apnea, gender, heart failure, COPD, age, BMI, corticosteroids, ethnicity, anticoagulants | Low risk of bias |
| Daniels et al [40] | Statin | Composite of ICU admission or mortality | NR | Multivariable Cox proportional hazards | Age, sex, and comorbid conditions including obesity, hypertension, diabetes, CVD, and CKD | Low risk of bias |
| De Spiegeleer et al [41] | Statin | Composite of 14-day mortality or hospital length of stay >7 days | Symptomatic COVID-19 | Logistic regression with Firth correction | Age, sex, functional status, diabetes mellitus, hypertension, method of COVID-19 diagnosis | High risk of bias |
| Fan et al [42] | Statin | In-hospital mortality | ARDS | Multivariable Cox regression | Age, gender, admitted hospital, comorbidities, in-hospital medication, blood lipids | Low risk of bias |
| Gupta et al [43] | Statin | 30-day mortality | Composite of in-hospital mortality or MV | Multivariable logistic regression | Age, sex, first BMI assessment, race and ethnicity, insurance, New York City borough of residence, hypertension, diabetes, coronary artery disease, heart failure, stroke/transient ischemic attack, atrial arrhythmias, chronic lung disease, CKD, liver disease, outpatient use of beta-blockers, ACEI, ARBs, oral anticoagulants, and P2Y12 receptor inhibitors | Low risk of bias |
| Ho et al [44] | Statin | Diagnosis of COVID-19 | NR | Poisson regression | Age, sex, ethnicity, deprivation index, smoking, alcohol use, adiposity, blood pressure, spirometry, physical capability | Moderate risk of bias |
| Israel et al [45] | Rosuvastatin | Hospitalization | Mortality; diagnosis of COVID-19 | Fisher exact test with Benjamini-Hochberg procedure | Age, gender, smoking, Adjusted Clinical Group measure of comorbidity, obesity | Low risk of bias |
| Izzi-Engbeaya et al [46] | Statin | Composite of ICU admission and 30-day mortality | NR | Multivariable logistic regression | Age, gender, ethnicity, diabetes mellitus, stroke, hyperlipidemia, ischemic heart disease, heart failure, hypertension, COPD, active cancer, ACEI, ARB, antiplatelet drug, Clinical Frailty Scale score, white cell count, hemoglobin, platelet count, neutrophils, lymphocytes, sodium, potassium, eGFR on diagnosis, CRP, temperature, respiratory rate, heart rate, systolic blood pressure, diastolic blood pressure, National Early Warning Score, inspired oxygen delivered on diagnosis, oxygen saturation on diagnosis, maximum inspired oxygen required during admission | Low risk of bias |
| Mallow et al [47] | Statin | In-hospital mortality | ICU admission, ICU LOS, hospital LOS | Logistic regression | CDC risk factors, gender, Medicaid insurance, hospital teaching status, hospital bed size, chronic lung disease, moderate-to-severe asthma, heart condition, immunocompromised, obesity, diabetes, hemodialysis, liver disease, hypertension, do-not-resuscitate status | Low risk of bias |
| Masana et al [48] | Statin | In-hospital COVID-19-related mortality | NR | Cox proportional hazards model | Age, gender, smoking, high blood pressure, hyperlipidemia, diabetes, obesity, coronary heart disease, stroke, PAD, heart failure, COPD/asthma, chronic liver disease, chronic kidney disease, rheumatic disease, cancer | Low risk of bias |
| Nicholson et al [49] | Statin | In-hospital mortality | MV | Multivariable logistic regression | Age, gender, diabetes mellitus, albumin, CRP, MCV, neutrophil:lymphocyte ratio, platelets, procalcitonin | High risk of bias |
| Rodriguez-Nava et al [50] | Atorvastatin | In-hospital mortality | NR | Cox proportional hazards regression | Age, hypertension, CVD, MV, severity according to the National Institutes of Health criteria, number of comorbidities, adjuvant therapies | Low risk of bias |
| Rosenthal et al [51] | Statin | In-hospital mortality | ICU admission; MV; hospital LOS, ICU LOS | Multivariable logistic regression | Age, sex, race, ethnicity, payer type, type of admission, admission point of origin, geographic region, hospital size, rural/urban hospital status, teaching hospital status, comorbidities, complications, ACEI, statins, hydroxychloroquine, azithromycin, beta-blockers, calcium channel blockers, vitamin C use, vitamin D use, zinc use | Low risk of bias |
| Saeed et al 2020 [52] | Statin | In-hospital mortality | NR | Multivariable regression | Age, sex, history of atherosclerotic heart disease, Charlson comorbidity index, presenting diastolic blood pressure, respiratory rate, pulse oximetry measurement, serum glucose, serum lactic acid, serum creatinine, and intravenous antibiotic use during hospitalization | Low risk of bias |
| Song et al [53] | Statin | In-hospital mortality | ICU admission, MV | Multivariable logistic regression | Age, sex, race, CVD, chronic pulmonary disease, diabetes, obesity | Low risk of bias |
| Yan et al [54] | Statin | Severe COVID-19 | NR | Logistic regression | Age, sex, BMI | High risk of bias |
| Zhang et al [55] | Statin | 28-day mortality | ICU admission | Time-varying Cox model | Age, gender, systolic blood pressure, preexisting comorbidities, medications at admission, MV, number of antihypertensive drugs | Low risk of bias |
| Study . | Exposure . | Primary Outcome . | Secondary Outcome . | Statistical Method . | Factors Adjusted For . | Quality Assessment . |
|---|---|---|---|---|---|---|
| Bifulco et al [37] | Statin | In-hospital COVID-19-related mortality | NR | NR | Age, gender, smoking habit, preexisting comorbidities, indicators of disease severity and organ injuries, blood biomarkers | Low risk of bias |
| Butt et al [38] | Statin | 30-day all-cause mortality | Severe COVID-19; composite of mortality and severe COVID-19 | Adjusted Cox regression | Age, sex, ethnicity, education, income, comorbidity, concomitant medical treatment | Low risk of bias |
| Cariou et al [39] | Statin | 28-day mortality | MV | Logistic regression | Macrovascular complications, microvascular complications, diabetes treatments, antihypertensives, hypertension, ezetimibe, treated sleep apnea, gender, heart failure, COPD, age, BMI, corticosteroids, ethnicity, anticoagulants | Low risk of bias |
| Daniels et al [40] | Statin | Composite of ICU admission or mortality | NR | Multivariable Cox proportional hazards | Age, sex, and comorbid conditions including obesity, hypertension, diabetes, CVD, and CKD | Low risk of bias |
| De Spiegeleer et al [41] | Statin | Composite of 14-day mortality or hospital length of stay >7 days | Symptomatic COVID-19 | Logistic regression with Firth correction | Age, sex, functional status, diabetes mellitus, hypertension, method of COVID-19 diagnosis | High risk of bias |
| Fan et al [42] | Statin | In-hospital mortality | ARDS | Multivariable Cox regression | Age, gender, admitted hospital, comorbidities, in-hospital medication, blood lipids | Low risk of bias |
| Gupta et al [43] | Statin | 30-day mortality | Composite of in-hospital mortality or MV | Multivariable logistic regression | Age, sex, first BMI assessment, race and ethnicity, insurance, New York City borough of residence, hypertension, diabetes, coronary artery disease, heart failure, stroke/transient ischemic attack, atrial arrhythmias, chronic lung disease, CKD, liver disease, outpatient use of beta-blockers, ACEI, ARBs, oral anticoagulants, and P2Y12 receptor inhibitors | Low risk of bias |
| Ho et al [44] | Statin | Diagnosis of COVID-19 | NR | Poisson regression | Age, sex, ethnicity, deprivation index, smoking, alcohol use, adiposity, blood pressure, spirometry, physical capability | Moderate risk of bias |
| Israel et al [45] | Rosuvastatin | Hospitalization | Mortality; diagnosis of COVID-19 | Fisher exact test with Benjamini-Hochberg procedure | Age, gender, smoking, Adjusted Clinical Group measure of comorbidity, obesity | Low risk of bias |
| Izzi-Engbeaya et al [46] | Statin | Composite of ICU admission and 30-day mortality | NR | Multivariable logistic regression | Age, gender, ethnicity, diabetes mellitus, stroke, hyperlipidemia, ischemic heart disease, heart failure, hypertension, COPD, active cancer, ACEI, ARB, antiplatelet drug, Clinical Frailty Scale score, white cell count, hemoglobin, platelet count, neutrophils, lymphocytes, sodium, potassium, eGFR on diagnosis, CRP, temperature, respiratory rate, heart rate, systolic blood pressure, diastolic blood pressure, National Early Warning Score, inspired oxygen delivered on diagnosis, oxygen saturation on diagnosis, maximum inspired oxygen required during admission | Low risk of bias |
| Mallow et al [47] | Statin | In-hospital mortality | ICU admission, ICU LOS, hospital LOS | Logistic regression | CDC risk factors, gender, Medicaid insurance, hospital teaching status, hospital bed size, chronic lung disease, moderate-to-severe asthma, heart condition, immunocompromised, obesity, diabetes, hemodialysis, liver disease, hypertension, do-not-resuscitate status | Low risk of bias |
| Masana et al [48] | Statin | In-hospital COVID-19-related mortality | NR | Cox proportional hazards model | Age, gender, smoking, high blood pressure, hyperlipidemia, diabetes, obesity, coronary heart disease, stroke, PAD, heart failure, COPD/asthma, chronic liver disease, chronic kidney disease, rheumatic disease, cancer | Low risk of bias |
| Nicholson et al [49] | Statin | In-hospital mortality | MV | Multivariable logistic regression | Age, gender, diabetes mellitus, albumin, CRP, MCV, neutrophil:lymphocyte ratio, platelets, procalcitonin | High risk of bias |
| Rodriguez-Nava et al [50] | Atorvastatin | In-hospital mortality | NR | Cox proportional hazards regression | Age, hypertension, CVD, MV, severity according to the National Institutes of Health criteria, number of comorbidities, adjuvant therapies | Low risk of bias |
| Rosenthal et al [51] | Statin | In-hospital mortality | ICU admission; MV; hospital LOS, ICU LOS | Multivariable logistic regression | Age, sex, race, ethnicity, payer type, type of admission, admission point of origin, geographic region, hospital size, rural/urban hospital status, teaching hospital status, comorbidities, complications, ACEI, statins, hydroxychloroquine, azithromycin, beta-blockers, calcium channel blockers, vitamin C use, vitamin D use, zinc use | Low risk of bias |
| Saeed et al 2020 [52] | Statin | In-hospital mortality | NR | Multivariable regression | Age, sex, history of atherosclerotic heart disease, Charlson comorbidity index, presenting diastolic blood pressure, respiratory rate, pulse oximetry measurement, serum glucose, serum lactic acid, serum creatinine, and intravenous antibiotic use during hospitalization | Low risk of bias |
| Song et al [53] | Statin | In-hospital mortality | ICU admission, MV | Multivariable logistic regression | Age, sex, race, CVD, chronic pulmonary disease, diabetes, obesity | Low risk of bias |
| Yan et al [54] | Statin | Severe COVID-19 | NR | Logistic regression | Age, sex, BMI | High risk of bias |
| Zhang et al [55] | Statin | 28-day mortality | ICU admission | Time-varying Cox model | Age, gender, systolic blood pressure, preexisting comorbidities, medications at admission, MV, number of antihypertensive drugs | Low risk of bias |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARDS, acute respiratory distress syndrome; BMI, body mass index; CDC, Centers for Disease Control and Prevention; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disorder; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; ICU, intensive care unit; LOS, length of stay; MCV, mean corpuscular volume; MV, mechanical ventilation; NR, not reported; PAD, peripheral artery disease.
| Study . | Exposure . | Primary Outcome . | Secondary Outcome . | Statistical Method . | Factors Adjusted For . | Quality Assessment . |
|---|---|---|---|---|---|---|
| Bifulco et al [37] | Statin | In-hospital COVID-19-related mortality | NR | NR | Age, gender, smoking habit, preexisting comorbidities, indicators of disease severity and organ injuries, blood biomarkers | Low risk of bias |
| Butt et al [38] | Statin | 30-day all-cause mortality | Severe COVID-19; composite of mortality and severe COVID-19 | Adjusted Cox regression | Age, sex, ethnicity, education, income, comorbidity, concomitant medical treatment | Low risk of bias |
| Cariou et al [39] | Statin | 28-day mortality | MV | Logistic regression | Macrovascular complications, microvascular complications, diabetes treatments, antihypertensives, hypertension, ezetimibe, treated sleep apnea, gender, heart failure, COPD, age, BMI, corticosteroids, ethnicity, anticoagulants | Low risk of bias |
| Daniels et al [40] | Statin | Composite of ICU admission or mortality | NR | Multivariable Cox proportional hazards | Age, sex, and comorbid conditions including obesity, hypertension, diabetes, CVD, and CKD | Low risk of bias |
| De Spiegeleer et al [41] | Statin | Composite of 14-day mortality or hospital length of stay >7 days | Symptomatic COVID-19 | Logistic regression with Firth correction | Age, sex, functional status, diabetes mellitus, hypertension, method of COVID-19 diagnosis | High risk of bias |
| Fan et al [42] | Statin | In-hospital mortality | ARDS | Multivariable Cox regression | Age, gender, admitted hospital, comorbidities, in-hospital medication, blood lipids | Low risk of bias |
| Gupta et al [43] | Statin | 30-day mortality | Composite of in-hospital mortality or MV | Multivariable logistic regression | Age, sex, first BMI assessment, race and ethnicity, insurance, New York City borough of residence, hypertension, diabetes, coronary artery disease, heart failure, stroke/transient ischemic attack, atrial arrhythmias, chronic lung disease, CKD, liver disease, outpatient use of beta-blockers, ACEI, ARBs, oral anticoagulants, and P2Y12 receptor inhibitors | Low risk of bias |
| Ho et al [44] | Statin | Diagnosis of COVID-19 | NR | Poisson regression | Age, sex, ethnicity, deprivation index, smoking, alcohol use, adiposity, blood pressure, spirometry, physical capability | Moderate risk of bias |
| Israel et al [45] | Rosuvastatin | Hospitalization | Mortality; diagnosis of COVID-19 | Fisher exact test with Benjamini-Hochberg procedure | Age, gender, smoking, Adjusted Clinical Group measure of comorbidity, obesity | Low risk of bias |
| Izzi-Engbeaya et al [46] | Statin | Composite of ICU admission and 30-day mortality | NR | Multivariable logistic regression | Age, gender, ethnicity, diabetes mellitus, stroke, hyperlipidemia, ischemic heart disease, heart failure, hypertension, COPD, active cancer, ACEI, ARB, antiplatelet drug, Clinical Frailty Scale score, white cell count, hemoglobin, platelet count, neutrophils, lymphocytes, sodium, potassium, eGFR on diagnosis, CRP, temperature, respiratory rate, heart rate, systolic blood pressure, diastolic blood pressure, National Early Warning Score, inspired oxygen delivered on diagnosis, oxygen saturation on diagnosis, maximum inspired oxygen required during admission | Low risk of bias |
| Mallow et al [47] | Statin | In-hospital mortality | ICU admission, ICU LOS, hospital LOS | Logistic regression | CDC risk factors, gender, Medicaid insurance, hospital teaching status, hospital bed size, chronic lung disease, moderate-to-severe asthma, heart condition, immunocompromised, obesity, diabetes, hemodialysis, liver disease, hypertension, do-not-resuscitate status | Low risk of bias |
| Masana et al [48] | Statin | In-hospital COVID-19-related mortality | NR | Cox proportional hazards model | Age, gender, smoking, high blood pressure, hyperlipidemia, diabetes, obesity, coronary heart disease, stroke, PAD, heart failure, COPD/asthma, chronic liver disease, chronic kidney disease, rheumatic disease, cancer | Low risk of bias |
| Nicholson et al [49] | Statin | In-hospital mortality | MV | Multivariable logistic regression | Age, gender, diabetes mellitus, albumin, CRP, MCV, neutrophil:lymphocyte ratio, platelets, procalcitonin | High risk of bias |
| Rodriguez-Nava et al [50] | Atorvastatin | In-hospital mortality | NR | Cox proportional hazards regression | Age, hypertension, CVD, MV, severity according to the National Institutes of Health criteria, number of comorbidities, adjuvant therapies | Low risk of bias |
| Rosenthal et al [51] | Statin | In-hospital mortality | ICU admission; MV; hospital LOS, ICU LOS | Multivariable logistic regression | Age, sex, race, ethnicity, payer type, type of admission, admission point of origin, geographic region, hospital size, rural/urban hospital status, teaching hospital status, comorbidities, complications, ACEI, statins, hydroxychloroquine, azithromycin, beta-blockers, calcium channel blockers, vitamin C use, vitamin D use, zinc use | Low risk of bias |
| Saeed et al 2020 [52] | Statin | In-hospital mortality | NR | Multivariable regression | Age, sex, history of atherosclerotic heart disease, Charlson comorbidity index, presenting diastolic blood pressure, respiratory rate, pulse oximetry measurement, serum glucose, serum lactic acid, serum creatinine, and intravenous antibiotic use during hospitalization | Low risk of bias |
| Song et al [53] | Statin | In-hospital mortality | ICU admission, MV | Multivariable logistic regression | Age, sex, race, CVD, chronic pulmonary disease, diabetes, obesity | Low risk of bias |
| Yan et al [54] | Statin | Severe COVID-19 | NR | Logistic regression | Age, sex, BMI | High risk of bias |
| Zhang et al [55] | Statin | 28-day mortality | ICU admission | Time-varying Cox model | Age, gender, systolic blood pressure, preexisting comorbidities, medications at admission, MV, number of antihypertensive drugs | Low risk of bias |
| Study . | Exposure . | Primary Outcome . | Secondary Outcome . | Statistical Method . | Factors Adjusted For . | Quality Assessment . |
|---|---|---|---|---|---|---|
| Bifulco et al [37] | Statin | In-hospital COVID-19-related mortality | NR | NR | Age, gender, smoking habit, preexisting comorbidities, indicators of disease severity and organ injuries, blood biomarkers | Low risk of bias |
| Butt et al [38] | Statin | 30-day all-cause mortality | Severe COVID-19; composite of mortality and severe COVID-19 | Adjusted Cox regression | Age, sex, ethnicity, education, income, comorbidity, concomitant medical treatment | Low risk of bias |
| Cariou et al [39] | Statin | 28-day mortality | MV | Logistic regression | Macrovascular complications, microvascular complications, diabetes treatments, antihypertensives, hypertension, ezetimibe, treated sleep apnea, gender, heart failure, COPD, age, BMI, corticosteroids, ethnicity, anticoagulants | Low risk of bias |
| Daniels et al [40] | Statin | Composite of ICU admission or mortality | NR | Multivariable Cox proportional hazards | Age, sex, and comorbid conditions including obesity, hypertension, diabetes, CVD, and CKD | Low risk of bias |
| De Spiegeleer et al [41] | Statin | Composite of 14-day mortality or hospital length of stay >7 days | Symptomatic COVID-19 | Logistic regression with Firth correction | Age, sex, functional status, diabetes mellitus, hypertension, method of COVID-19 diagnosis | High risk of bias |
| Fan et al [42] | Statin | In-hospital mortality | ARDS | Multivariable Cox regression | Age, gender, admitted hospital, comorbidities, in-hospital medication, blood lipids | Low risk of bias |
| Gupta et al [43] | Statin | 30-day mortality | Composite of in-hospital mortality or MV | Multivariable logistic regression | Age, sex, first BMI assessment, race and ethnicity, insurance, New York City borough of residence, hypertension, diabetes, coronary artery disease, heart failure, stroke/transient ischemic attack, atrial arrhythmias, chronic lung disease, CKD, liver disease, outpatient use of beta-blockers, ACEI, ARBs, oral anticoagulants, and P2Y12 receptor inhibitors | Low risk of bias |
| Ho et al [44] | Statin | Diagnosis of COVID-19 | NR | Poisson regression | Age, sex, ethnicity, deprivation index, smoking, alcohol use, adiposity, blood pressure, spirometry, physical capability | Moderate risk of bias |
| Israel et al [45] | Rosuvastatin | Hospitalization | Mortality; diagnosis of COVID-19 | Fisher exact test with Benjamini-Hochberg procedure | Age, gender, smoking, Adjusted Clinical Group measure of comorbidity, obesity | Low risk of bias |
| Izzi-Engbeaya et al [46] | Statin | Composite of ICU admission and 30-day mortality | NR | Multivariable logistic regression | Age, gender, ethnicity, diabetes mellitus, stroke, hyperlipidemia, ischemic heart disease, heart failure, hypertension, COPD, active cancer, ACEI, ARB, antiplatelet drug, Clinical Frailty Scale score, white cell count, hemoglobin, platelet count, neutrophils, lymphocytes, sodium, potassium, eGFR on diagnosis, CRP, temperature, respiratory rate, heart rate, systolic blood pressure, diastolic blood pressure, National Early Warning Score, inspired oxygen delivered on diagnosis, oxygen saturation on diagnosis, maximum inspired oxygen required during admission | Low risk of bias |
| Mallow et al [47] | Statin | In-hospital mortality | ICU admission, ICU LOS, hospital LOS | Logistic regression | CDC risk factors, gender, Medicaid insurance, hospital teaching status, hospital bed size, chronic lung disease, moderate-to-severe asthma, heart condition, immunocompromised, obesity, diabetes, hemodialysis, liver disease, hypertension, do-not-resuscitate status | Low risk of bias |
| Masana et al [48] | Statin | In-hospital COVID-19-related mortality | NR | Cox proportional hazards model | Age, gender, smoking, high blood pressure, hyperlipidemia, diabetes, obesity, coronary heart disease, stroke, PAD, heart failure, COPD/asthma, chronic liver disease, chronic kidney disease, rheumatic disease, cancer | Low risk of bias |
| Nicholson et al [49] | Statin | In-hospital mortality | MV | Multivariable logistic regression | Age, gender, diabetes mellitus, albumin, CRP, MCV, neutrophil:lymphocyte ratio, platelets, procalcitonin | High risk of bias |
| Rodriguez-Nava et al [50] | Atorvastatin | In-hospital mortality | NR | Cox proportional hazards regression | Age, hypertension, CVD, MV, severity according to the National Institutes of Health criteria, number of comorbidities, adjuvant therapies | Low risk of bias |
| Rosenthal et al [51] | Statin | In-hospital mortality | ICU admission; MV; hospital LOS, ICU LOS | Multivariable logistic regression | Age, sex, race, ethnicity, payer type, type of admission, admission point of origin, geographic region, hospital size, rural/urban hospital status, teaching hospital status, comorbidities, complications, ACEI, statins, hydroxychloroquine, azithromycin, beta-blockers, calcium channel blockers, vitamin C use, vitamin D use, zinc use | Low risk of bias |
| Saeed et al 2020 [52] | Statin | In-hospital mortality | NR | Multivariable regression | Age, sex, history of atherosclerotic heart disease, Charlson comorbidity index, presenting diastolic blood pressure, respiratory rate, pulse oximetry measurement, serum glucose, serum lactic acid, serum creatinine, and intravenous antibiotic use during hospitalization | Low risk of bias |
| Song et al [53] | Statin | In-hospital mortality | ICU admission, MV | Multivariable logistic regression | Age, sex, race, CVD, chronic pulmonary disease, diabetes, obesity | Low risk of bias |
| Yan et al [54] | Statin | Severe COVID-19 | NR | Logistic regression | Age, sex, BMI | High risk of bias |
| Zhang et al [55] | Statin | 28-day mortality | ICU admission | Time-varying Cox model | Age, gender, systolic blood pressure, preexisting comorbidities, medications at admission, MV, number of antihypertensive drugs | Low risk of bias |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARDS, acute respiratory distress syndrome; BMI, body mass index; CDC, Centers for Disease Control and Prevention; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disorder; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; ICU, intensive care unit; LOS, length of stay; MCV, mean corpuscular volume; MV, mechanical ventilation; NR, not reported; PAD, peripheral artery disease.
Mortality
Thirteen studies at low or moderate risk of bias were included in the quantitative mortality analysis, including 109 080 patients [37–39, 42, 43, 45, 47, 48, 50–53, 55]. Use of statins prior to diagnosis of COVID-19 was associated with reduced mortality, with a pooled adjusted RR of 0.65 (95% confidence interval [CI], .56–.77, I2 = 84.1%) as shown in Figure 2. To explore the source of this heterogeneity, we performed a meta-regression analysis. However, we could not identify any statistically significant moderator of effect on the pooled RR. A contoured-enhanced funnel plot did not reveal evidence of publication bias (P = .788) (Supplementary Figure 1). Because studies appear to be missing in areas of high statistical significance in the funnel plot, publication bias is a less likely cause of the funnel asymmetry.
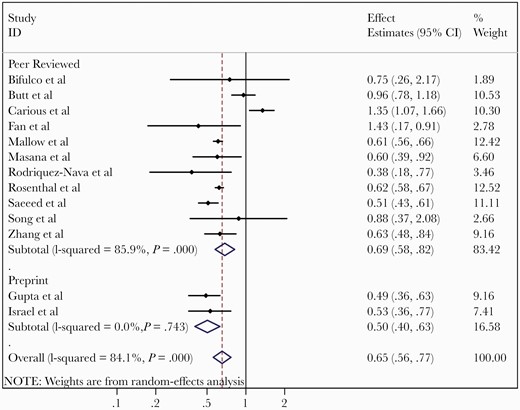
Meta-analysis of adjusted effects estimates of association between statin use and mortality from coronavirus disease 2019. Abbreviations: CI, confidence interval; ID, identification.
Severe COVID-19
Nine studies at low or moderate risk of bias were included in the quantitative analysis of the risk of developing severe COVID-19 disease (as defined above) with a total of 48 110 patients [38–40, 42, 43, 45, 46, 53, 55]. Statins use was associated with a reduced risk of severe COVID-19 with pooled RR of 0.73 (95% CI, .57–.94, I2 = 82.8%) (Figure 3). Meta-regression analyses did not identify any statistically significant moderators of effect on the pooled RR. The funnel plot is displayed in Supplementary Figure 2, with no evidence of publication bias (P = .531).
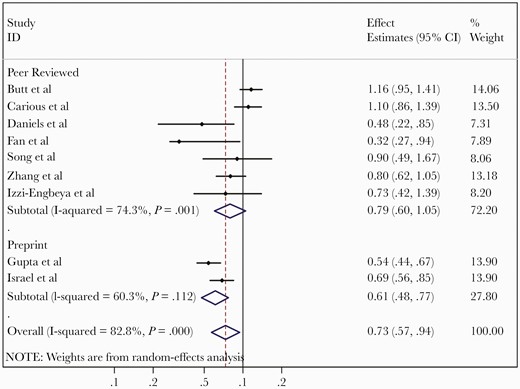
Meta-analysis of adjusted effects estimates of association between statin use and severe coronavirus disease 2019.
COVID-19 Diagnosis
Our systematic review identified 3 studies reporting on the association between statin use and COVID-19 diagnosis [44, 45, 54], with 2 being at low or moderate risk of bias [44, 45]. The remaining study was considered at risk of bias due to inadequate adjustment for confounding [54]. One study of 37 212 patients found that prior rosuvastatin use was associated with a lower risk of COVID-19 infection after extensive matching based on age, gender, body mass index, smoking, socioeconomic status, and multiple comorbidities, with OR of 0.746 (95% CI, .645–.858) [45]. However, another study of 49 245 patients showed that prior statin use was associated with an increased risk of acquiring COVID-19 with an OR of 2.50 (95% CI, 1.48–4.21), after adjusting for age, gender, and body mass index [54]. The third study of 235 928 patients included 2 statistical models that first accounted for age, gender, ethnicity, and deprivation index, followed by additional adjustment for smoking, alcohol use, adiposity, blood pressure, spirometry, and physical capability. Though the first model showed a statistically significant increased risk of COVID-19, this lost significance in the second model [44]. Due to limited data, quantitative analysis of the association between statin use and the risk of developing COVID-19 disease could not be performed.
DISCUSSION
Findings
Our systematic review and meta-analysis suggests that prior statin use was associated with reduced mortality and lower risk of severe disease among COVID-19 patients. The pooled effect estimates of several studies at low to moderate risk of bias, which enrolled large numbers of patients and adjusted for many important confounders, strengthen the findings of our systematic review. Moreover, data from a single well-designed and large study of 37 212 patients found that prior rosuvastatin use was associated with a lower risk of COVID-19 infection after extensive confounder adjustment [45]. Our results are in line with prior analyses of prior statin use and outcomes in other respiratory infections [21–23].
Mechanisms
These findings might be explained by several mechanisms including immune modulation, anti-inflammatory effects, and antithrombotic properties. Statins have been demonstrated to reduce endothelial cell injury involved in thromboembolism formation, which may influence outcomes by reducing thromboembolic complications [56]. Statins have also been shown to reduce expression of TLR and cytokines, which are important mediators in host antiviral response [57]. Statins also inhibit T-cell activation and proliferation, further modulating the immune response [4]. It is theorized that these mechanisms may contribute to reduced inflammation and improved outcomes in those taking statins.
Statins have been shown to reduce ALI in different animal models through reduction in TLRs, cytokine expression, inflammatory cell infiltration, vascular permeability, and others [11–15]. Similar anti-inflammatory effects have been demonstrated in a human experiment of lipopolysaccharide inhalation in healthy volunteers [16]. In a clinical trial of ARDS, statin therapy was associated with better outcomes in the subset of patients with hyperinflammatory phenotype [58]. Severe COVID-19 disease is associated with a similar hyperinflammatory response; hence, statin therapy may reduce disease severity by similar mechanisms.
Patients with COVID-19 are also at high risk for cardiac complications, with as many as 23% of hospitalized patients with COVID-19 experiencing a major adverse cardiovascular event [19]. A large body of evidence indicates that statins improve cardiovascular outcomes through lipid-lowering and non-lipid-lowering effects [59, 60]. Prior studies have also shown improved outcomes in patients taking indicated drug therapy for hypertension [61] and diabetes mellitus [62]. Hence, the observed reduced mortality with statins in COVID-19 could be explained at least in part through their favorable effects on the cardiovascular system. Furthermore, statin use has previously been identified as a marker for undergoing other health maintenance measures, such as disease screening and vaccinations, which could account for some of the effect seen in COVID-19 [63].
We did not identify enough studies to pool the effect of statins on acquiring COVID-19. Studies included for qualitative analysis reported conflicting findings with variable risk of bias. Prior studies have revealed an association between statin use and lower odds of cardiovascular implantable electronic device infections [64] and nosocomial infection following stroke [65]. While the theorized mechanisms in these studies relied on anti-inflammatory and antibacterial properties, statins have a more complex relationship with SARS-CoV-2 due to its up-regulation of the virus’s target receptor, ACE2. This could theoretically increase the likelihood of acquiring SARS-CoV-2 among exposed individuals. However, ACE2 expression and cell membrane ACE2 density are not the major factors that determine SARS-CoV and SARS-CoV-2 cell entry [66]. A similar concern regarding increased ACE2 expression has also been raised with angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs). However, recent studies indicate that ACEI and ARB therapy is actually associated with better survival in patients with COVID-19 disease [67]. Additionally, ACEIs and ARBs were not associated with increased risk of acquiring SARS-CoV-2 infection [68]. However, the effect statins may have on acquiring SARS-CoV-2 is unclear.
Although we observed that statin therapy was associated with better outcomes among patients with COVID-19 disease, the current data regarding the effect of statins on the risk of acquiring SARS-CoV-2 infection are conflicting and therefore, further studies are required to resolve this controversy.
Limitations
Our study has several limitations of note. First, there was variability between studies’ outcomes. This is most relevant to the analysis of severe COVID-19, as several different definitions of severe infection were used such as ICU admission or ARDS. Furthermore, 3 pooled studies for the severe COVID-19 analysis utilized a composite outcome of severe COVID-19 and mortality. There are also important limitations inherent to individual observational studies included in our meta-analysis. These include residual confounding such as the healthy user effect whereby patients taking statins are more likely to adhere to healthier lifestyles and preventive measures [63]. Moreover, associations do not always imply causation. Although the effect of statins has biological plausibility, included studies have not examined the duration of prior statins intake, adherence to statins, or a dose-effect relationship to support causal effect. Additionally, our pooled effect estimates had significant heterogeneity and we were unable to definitively identify the source for this heterogeneity, despite meta-regression analyses of study-level factors. The meta-regression analyses could be underpowered. This heterogeneity could be partially explained by differences in outcome definitions, particularly in the severe COVID-19 analysis. Finally, this analysis does not specifically address initiation of statins in those diagnosed with COVID-19, rather, only whether preexisting statin use affects outcomes. Ongoing randomized trials are examining the effect of de novo initiation of statins on outcomes of COVID-19. One of the important trials include the Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community- Acquired Pneumonia (REMAP-CAP; NCT02735707) platform where patients are randomized to receive simvastatin (80 mg once daily) for up to 28 days while the patient remains in hospital vs no simvastatin.
Conclusions
The findings of this systematic review and meta-analysis indicate that prior statin use is associated with reduced mortality and risk of severe COVID-19 disease among SARS-CoV-2–infected patients. These data do not address the impact of de novo initiation of statin therapy in patients with COVID-19. Interventional clinical trials are underway to answer this question. Continuation of statins medications in patients with recognized indications should be encouraged since statins are not associated with worse outcomes should they develop COVID-19.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Figure 1: Contour funnel plot for association of statin use and mortality from COVID-19, examining the relationship between the effect estimates and their standard errors to assess for plot asymmetry as a sign of publication bias. The contour lines differentiate the significance and non-significance regions in the plot at 1%, 5% and 10% significance levels. X-axis: effect estimate (adjusted RR or HR or OR), Y-axis: Standard error (SE) of effect estimate. Because studies appear to be missing in areas of high statistical significance, publication bias is a less likely cause of the funnel asymmetry.
Supplementary Figure 2: Contour funnel plot for association of statin use and severe COVID-19, examining the relationship between the effect estimates and their standard errors to assess for plot asymmetry as a sign of publication bias. The contour lines differentiate the significance and non-significance regions in the plot at 1%, 5% and 10% significance levels. X-axis: effect estimate (adjusted RR or HR or OR), Y-axis: Standard error (SE) of effect estimate.
Notes
Patient consent statement. The design of this work was approved by our local institutional review board and a waiver of informed consent was obtained.
Potential conflicts of interest. A. D. B. is a paid consultant for AbbVie and a paid member of the data and safety monitoring board for Corvus Pharmaceuticals; owns equity for scientific advisory work in Zentalis and Nference; is founder and President of Splissen Therapeutics; and is supported by grants from the National Institute of Allergy and Infectious Diseases (grant numbers AI110173 and AI120698), Amfar (grant number 109593), and Mayo Clinic (HH Sheikh Khalifa Bib Zayed Al-Nahyan Named Professorship of Infectious Diseases). All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
REFERENCES
World Health Organization.





Comments