-
PDF
- Split View
-
Views
-
Cite
Cite
Julio Ramirez, Daniel H Deck, Paul B Eckburg, Marla Curran, Anita F Das, Courtney Kirsch, Amy Manley, Evan Tzanis, Paul C McGovern, Efficacy of Omadacycline Versus Moxifloxacin in the Treatment of Community-Acquired Bacterial Pneumonia by Disease Severity: Results From the OPTIC Study, Open Forum Infectious Diseases, Volume 8, Issue 6, June 2021, ofab135, https://doi.org/10.1093/ofid/ofab135
Close - Share Icon Share
Abstract
Severity/mortality risk scores and disease characteristics may assist in deciding whether patients with community-acquired bacterial pneumonia (CABP) require outpatient treatment or hospitalization. The phase 3 OPTIC (Omadacycline for Pneumonia Treatment In the Community) study enrolled patients with Pneumonia Outcomes Research Team (PORT) risk class II–IV. Omadacycline demonstrated noninferiority to moxifloxacin in adults with CABP, at early clinical response (ECR) and posttreatment evaluation (PTE). We assessed efficacy of omadacycline versus moxifloxacin in these patients based on disease severity.
Patients were randomized 1:1 to receive intravenous (IV) omadacycline (100 mg every 12 hours for 2 doses followed by 100 mg daily [q24h], with optional transition to omadacycline 300 mg orally q24h after 3 days of IV treatment) or moxifloxacin IV 400 mg q24h (with optional transition to 400 mg orally q24h after 3 days of IV treatment). Total treatment duration was 7–14 days. We compared rates of early clinical success (72–120 hours after first dose) and investigator-assessed clinical success at PTE (5–10 days after last dose) in subgroups based (1) on severity/mortality risk scores (PORT, CURB-65, systemic inflammatory response syndrome, quick Sequential [Sepsis-related] Organ Failure Assessment, modified ATS, SMART-COP) and (2) on presence of baseline radiographic characteristics, chronic obstructive pulmonary disease (COPD)/asthma, or bacteremia.
Altogether, 774 patients (omadacycline, n = 386; moxifloxacin, n = 388) were randomized. Clinical success rates (ECR/PTE) were similar between treatment groups (across all subgroups). Efficacy across treatment groups was similar in patients with baseline radiographic characteristics or COPD/asthma, but moxifloxacin had higher clinical success rates in patients with bacteremia.
Efficacy of omadacycline was similar to that of moxifloxacin, regardless of disease severity/mortality risk and disease characteristics.
Together with influenza, community-acquired pneumonia (CAP) is the eighth leading cause of death in the United States, with an estimated hospitalization incidence of 649 cases per 100 000 adults per year [1, 2]. Community-acquired pneumonia presents a huge economic burden to healthcare services, with an estimated US$18 000 spent per inpatient treatment, and an overall annual burden of US $13 million in Medicare fee-for-service patients [3]. Streptococcus pneumoniae is the most common causative pathogen of CAP worldwide, estimated to have been responsible for 1.1 million deaths globally in 2016 alone [4]. However, many other bacterial organisms are known to cause CAP, including Haemophilus influenzae, Staphylococcus aureus, and the atypical pathogens Mycoplasma pneumoniae, Chlamydophila pneumoniae, and Legionella pneumophila [5].
The Pneumonia Outcomes Research Team (PORT) score (also known as the Pneumonia Severity Index) and CURB-65 are validated clinical prediction rules that were developed to predict mortality risk in patients with CAP [6, 7]. Current treatment guidelines recommend that these tools should be used by clinicians to assist in choosing the optimal initial site of care for CAP patients. The PORT and CURB-65 severity scoring systems are particularly helpful for identifying patients who may be safely managed in the outpatient setting or quickly discharged from hospital after stabilization. In clinical research, these scoring systems have also been used as stratification factors and for adjustment by baseline classification of illness severity [6–8]. However, PORT and CURB-65 were not designed to help select the optimal level of care (intensive care unit [ICU], non-ICU) for patients admitted to the hospital with CAP. Prognostic models (2007 American Thoracic Society [ATS] criteria, SMART-COP) have been designed to predict the need for higher levels of inpatient treatment intensity using severity-of-illness parameters based on patient outcomes. These tools help to define severely ill CAP patients and can assist clinicians in determining the need for intensive care unit (ICU) care along with clinical assessment [9, 10]. Other scoring systems that help to define patients with sepsis (eg, systemic inflammatory response syndrome [SIRS] [11] and the quick Sequential [Sepsis-related] Organ Failure Assessment [qSOFA]) have also been explored in various infectious disease settings, including CAP [12, 13]. In addition, the presence of radiographic characteristics, comorbidities, and bacteremia may be associated with CAP severity, and this can also impact site of care and treatment decisions [9, 10].
In the phase 3 Omadacycline for Pneumonia Treatment In the Community (OPTIC) study, omadacycline demonstrated noninferiority to moxifloxacin at the primary endpoint of early clinical response (ECR) in patients with community-acquired bacterial pneumonia (CABP) [14]. Clinical response was similar across patient mortality risk levels defined by PORT risk class. Because scoring systems differ in the parameters included to assess patients, we performed additional analyses of omadacycline efficacy using 5 other severity or mortality risk measures (CURB-65, SIRS, qSOFA, modified ATS, SMART-COP), as well as in subgroups of patients based on baseline radiographic findings, and with chronic obstructive pulmonary disease (COPD)/asthma, and bacteremia.
METHODS
Study Design and Participants
Full details of the phase 3, double-blind, multicenter, randomized, controlled OPTIC study methodology have been published [14]. In brief, adults (≥18 years) with CABP PORT risk class II, III, or IV and ≥3 pneumonia symptoms (cough, purulent sputum production, dyspnea, or pleuritic chest pain) were randomized 1:1 to receive either intravenous (IV) omadacycline 100 mg every 12 hours for 2 doses, followed by 100 mg daily (q24h), with optional transition to omadacycline 300 mg orally q24h after 3 days of IV treatment, or moxifloxacin IV 400 mg q24h, with optional transition to 400 mg orally q24h after 3 days of IV treatment. Patients were eligible for transition to oral treatment if protocol-defined stability criteria were met. The total treatment duration for both groups was 7–14 days. Recruitment of PORT risk class II patients was capped at 15%, and patients with risk class I or V, septic shock, empyema, or immunosuppression were excluded from enrollment. Full inclusion and exclusion criteria have been reported previously [14].
Patient Consent Statement
Written informed consent was obtained from each patient before participation, and the study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. A full list of the institutional review boards that approved the study protocol is provided in Supplementary Table 1.
Endpoints and Analysis
The primary endpoint was clinical response, measured at the ECR time point (72–120 hours after receipt of the first dose). Clinical success at ECR was defined as survival, investigator-assessed symptom improvement, and no use of rescue antibacterial therapy. Symptom improvement was defined as ≥1 level improvement (categorized as absent, mild, moderate, or severe by investigator assessment) in ≥2 CABP symptoms, with no worsening by ≥1 level in any of the other CABP symptoms. Investigator-assessed clinical response at posttreatment evaluation (PTE), measured 5–10 days after receipt of the last dose of the study drug, was included as a secondary endpoint. Clinical success at PTE was defined as survival with improvement in signs or symptoms so that no further antibacterial therapy was required. Full details of safety endpoints have been presented previously [14].
Analysis of clinical response at ECR and PTE was performed for patients in subgroups defined by PORT risk class. Post hoc analysis of clinical response at the 2 time points was performed on subgroups based on the following measures of disease severity and mortality risk: CURB-65 score; SIRS criteria; modified ATS criteria; qSOFA criteria; and SMART-COP risk criteria. Table 1 outlines the parameters that contribute to each scoring system. In addition, clinical success was analyzed in patients with pleural effusion, multilobar infiltrates, COPD/asthma, or bacteremia. The difference between treatment arms in percentage of participants achieving clinical success at each time point was determined for each subgroup, with associated 95% confidence intervals. No formal statistical hypotheses were tested.
Parameters Considered in the Severity and Mortality Risk Scoring Systems for Community-Acquired Pneumonia (Adapted from Marti et al [15])
| Parameter . | PORT . | CURB-65 . | SMART-COP . | Modified ATS . | SIRS . | qSOFA . |
|---|---|---|---|---|---|---|
| Confusion | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Heart rate | ≥125 bpm | ≥125 bpm | >90 bpm | |||
| Blood pressure | Systolic <90 mmHg | Systolic <90 or diastolic ≤60 mmHg | Systolic <90 mmHg | Hypotension requiring aggressive fluid resuscitation | Systolic ≤100 mmHg | |
| Respiration rate | ≥30 breaths/min | ≥30 breaths/min | ≥25 breaths/min (≥30 in patients aged >50 y) | ≥30 breaths/min | ≥20 breaths/min | ≥21 breaths/min |
| PaO2/FiO2 ratio | PaO2 <60 mmHg | Age-dependenta | PaO2/FiO2 ratio ≤250 | PaO2 <32 mmHg | ||
| Radiography | Pleural effusion | Multilobar infiltrates | Multilobar infiltrates | |||
| Urea | ≥30 mg/dL | >7 mmol/L | ≥20 mg/dL | |||
| Age | ✓ | ✓ | ✓ | |||
| Body temperature | <35°C or ≥40°C | <36°C | <36°C or >38°C | |||
| Arterial pH | <7.35 | <7.35 | ||||
| Other criteria | Sex | Albumin <35 g/L | Mechanical ventilation | Leucocytes <4000 or >12 000 cells/μL | ||
| Comorbid disease | Septic shock requiring vasopressors | |||||
| Hematocrit <30% | Leucocytes <4000 cells/μL | |||||
| Sodium <130 mmol/dL | Thrombocytes <100 000 cells/μL | |||||
| Glycemia ≥250 mg/dL | ||||||
| Subgroups by Scoring System | ||||||
| Low risk/severity | PORT II Low risk | <2 Low risk | <3 Low severity/risk of ICU | <3 Not severe | <2 No sepsis | <2 No increased mortality risk |
| Increased risk/severity | PORT III moderate risk PORT IV high risk | ≥2 Moderate to high risk | ≥3 Moderate to very high risk of ICU | ≥3 Severe | ≥2 Sepsis | ≥2 Increased mortality risk |
| Parameter . | PORT . | CURB-65 . | SMART-COP . | Modified ATS . | SIRS . | qSOFA . |
|---|---|---|---|---|---|---|
| Confusion | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Heart rate | ≥125 bpm | ≥125 bpm | >90 bpm | |||
| Blood pressure | Systolic <90 mmHg | Systolic <90 or diastolic ≤60 mmHg | Systolic <90 mmHg | Hypotension requiring aggressive fluid resuscitation | Systolic ≤100 mmHg | |
| Respiration rate | ≥30 breaths/min | ≥30 breaths/min | ≥25 breaths/min (≥30 in patients aged >50 y) | ≥30 breaths/min | ≥20 breaths/min | ≥21 breaths/min |
| PaO2/FiO2 ratio | PaO2 <60 mmHg | Age-dependenta | PaO2/FiO2 ratio ≤250 | PaO2 <32 mmHg | ||
| Radiography | Pleural effusion | Multilobar infiltrates | Multilobar infiltrates | |||
| Urea | ≥30 mg/dL | >7 mmol/L | ≥20 mg/dL | |||
| Age | ✓ | ✓ | ✓ | |||
| Body temperature | <35°C or ≥40°C | <36°C | <36°C or >38°C | |||
| Arterial pH | <7.35 | <7.35 | ||||
| Other criteria | Sex | Albumin <35 g/L | Mechanical ventilation | Leucocytes <4000 or >12 000 cells/μL | ||
| Comorbid disease | Septic shock requiring vasopressors | |||||
| Hematocrit <30% | Leucocytes <4000 cells/μL | |||||
| Sodium <130 mmol/dL | Thrombocytes <100 000 cells/μL | |||||
| Glycemia ≥250 mg/dL | ||||||
| Subgroups by Scoring System | ||||||
| Low risk/severity | PORT II Low risk | <2 Low risk | <3 Low severity/risk of ICU | <3 Not severe | <2 No sepsis | <2 No increased mortality risk |
| Increased risk/severity | PORT III moderate risk PORT IV high risk | ≥2 Moderate to high risk | ≥3 Moderate to very high risk of ICU | ≥3 Severe | ≥2 Sepsis | ≥2 Increased mortality risk |
Abbreviations: ATS, American Thoracic Society; bpm, beats per minute; FiO2, fraction inspired oxygen; ICU, intensive care unit; PaO2, partial pressure of arterial oxygen; PORT, Pneumonia Outcomes Research Team; qSOFA, quick Sequential [Sepsis-related] Organ Failure Assessment; SIRS, systemic inflammatory response syndrome; y, years.
aAge ≤50 years: PaO2 <70 mmHg, saturation ≤93%, or PaO2/FiO2 ratio <333; age >50 years: PaO2 <60 mmHg, saturation ≤90%, or PaO2/FiO2 ratio <250.
Parameters Considered in the Severity and Mortality Risk Scoring Systems for Community-Acquired Pneumonia (Adapted from Marti et al [15])
| Parameter . | PORT . | CURB-65 . | SMART-COP . | Modified ATS . | SIRS . | qSOFA . |
|---|---|---|---|---|---|---|
| Confusion | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Heart rate | ≥125 bpm | ≥125 bpm | >90 bpm | |||
| Blood pressure | Systolic <90 mmHg | Systolic <90 or diastolic ≤60 mmHg | Systolic <90 mmHg | Hypotension requiring aggressive fluid resuscitation | Systolic ≤100 mmHg | |
| Respiration rate | ≥30 breaths/min | ≥30 breaths/min | ≥25 breaths/min (≥30 in patients aged >50 y) | ≥30 breaths/min | ≥20 breaths/min | ≥21 breaths/min |
| PaO2/FiO2 ratio | PaO2 <60 mmHg | Age-dependenta | PaO2/FiO2 ratio ≤250 | PaO2 <32 mmHg | ||
| Radiography | Pleural effusion | Multilobar infiltrates | Multilobar infiltrates | |||
| Urea | ≥30 mg/dL | >7 mmol/L | ≥20 mg/dL | |||
| Age | ✓ | ✓ | ✓ | |||
| Body temperature | <35°C or ≥40°C | <36°C | <36°C or >38°C | |||
| Arterial pH | <7.35 | <7.35 | ||||
| Other criteria | Sex | Albumin <35 g/L | Mechanical ventilation | Leucocytes <4000 or >12 000 cells/μL | ||
| Comorbid disease | Septic shock requiring vasopressors | |||||
| Hematocrit <30% | Leucocytes <4000 cells/μL | |||||
| Sodium <130 mmol/dL | Thrombocytes <100 000 cells/μL | |||||
| Glycemia ≥250 mg/dL | ||||||
| Subgroups by Scoring System | ||||||
| Low risk/severity | PORT II Low risk | <2 Low risk | <3 Low severity/risk of ICU | <3 Not severe | <2 No sepsis | <2 No increased mortality risk |
| Increased risk/severity | PORT III moderate risk PORT IV high risk | ≥2 Moderate to high risk | ≥3 Moderate to very high risk of ICU | ≥3 Severe | ≥2 Sepsis | ≥2 Increased mortality risk |
| Parameter . | PORT . | CURB-65 . | SMART-COP . | Modified ATS . | SIRS . | qSOFA . |
|---|---|---|---|---|---|---|
| Confusion | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Heart rate | ≥125 bpm | ≥125 bpm | >90 bpm | |||
| Blood pressure | Systolic <90 mmHg | Systolic <90 or diastolic ≤60 mmHg | Systolic <90 mmHg | Hypotension requiring aggressive fluid resuscitation | Systolic ≤100 mmHg | |
| Respiration rate | ≥30 breaths/min | ≥30 breaths/min | ≥25 breaths/min (≥30 in patients aged >50 y) | ≥30 breaths/min | ≥20 breaths/min | ≥21 breaths/min |
| PaO2/FiO2 ratio | PaO2 <60 mmHg | Age-dependenta | PaO2/FiO2 ratio ≤250 | PaO2 <32 mmHg | ||
| Radiography | Pleural effusion | Multilobar infiltrates | Multilobar infiltrates | |||
| Urea | ≥30 mg/dL | >7 mmol/L | ≥20 mg/dL | |||
| Age | ✓ | ✓ | ✓ | |||
| Body temperature | <35°C or ≥40°C | <36°C | <36°C or >38°C | |||
| Arterial pH | <7.35 | <7.35 | ||||
| Other criteria | Sex | Albumin <35 g/L | Mechanical ventilation | Leucocytes <4000 or >12 000 cells/μL | ||
| Comorbid disease | Septic shock requiring vasopressors | |||||
| Hematocrit <30% | Leucocytes <4000 cells/μL | |||||
| Sodium <130 mmol/dL | Thrombocytes <100 000 cells/μL | |||||
| Glycemia ≥250 mg/dL | ||||||
| Subgroups by Scoring System | ||||||
| Low risk/severity | PORT II Low risk | <2 Low risk | <3 Low severity/risk of ICU | <3 Not severe | <2 No sepsis | <2 No increased mortality risk |
| Increased risk/severity | PORT III moderate risk PORT IV high risk | ≥2 Moderate to high risk | ≥3 Moderate to very high risk of ICU | ≥3 Severe | ≥2 Sepsis | ≥2 Increased mortality risk |
Abbreviations: ATS, American Thoracic Society; bpm, beats per minute; FiO2, fraction inspired oxygen; ICU, intensive care unit; PaO2, partial pressure of arterial oxygen; PORT, Pneumonia Outcomes Research Team; qSOFA, quick Sequential [Sepsis-related] Organ Failure Assessment; SIRS, systemic inflammatory response syndrome; y, years.
aAge ≤50 years: PaO2 <70 mmHg, saturation ≤93%, or PaO2/FiO2 ratio <333; age >50 years: PaO2 <60 mmHg, saturation ≤90%, or PaO2/FiO2 ratio <250.
Data Availability
Paratek Pharmaceuticals, Inc. has a commitment to ensure that access to clinical trial data is available to regulators, researchers, and trial participants, when permitted, feasible and appropriate. Requests for deidentified patient-level data may be submitted to [email protected] for review (Clinical Trial Registration: ClinicalTrials.gov, no. NCT02531438).
RESULTS
A total of 774 patients (386 in the omadacycline group and 388 in the moxifloxacin group) were randomized (intent-to-treat population) (Figure 1). Baseline demographics including age, sex, and body mass index were similar in the 2 treatment arms (Table 2). Patients with increased mortality risk and disease severity varied across scoring systems. The SIRS and qSOFA identified 74% and 77% of patients with suspected sepsis, respectively. Across both treatment groups, 27% of patients showed evidence of multilobar infiltrates at baseline, 16% had pleural effusion, 21% had COPD/asthma, and 4% had bacteremia (Table 2), for which the most common pathogen was S pneumoniae.
| . | Omadacycline . | Moxifloxacin . | All Patients . |
|---|---|---|---|
| Characteristic . | (N = 386) . | (N = 388) . | (N = 774) . |
| Male | 208 (53.9) | 219 (56.4) | 427 (55.2) |
| Age, mean (SD) | 60.9 (15.2) | 62.1 (15.2) | 61.5 (15.2) |
| Categorical age, years | |||
| 18–45 | 62 (16.1) | 61 (15.7) | 123 (15.9) |
| >45–65 | 172 (44.6) | 155 (39.9) | 327 (42.2) |
| >65a | 152 (39.4) | 172 (44.3) | 324 (41.9) |
| Mean BMI, kg/m2 (SD) | 27.23 (5.746) | 27.42 (5.791) | 27.33 (5.765) |
| PORT risk class (actual)b | |||
| II (PORT score 51–70)c | 57 (14.8) | 56 (14.4) | 109 (14.1) |
| III (PORT score 71–90) | 227 (58.8) | 216 (55.7) | 443 (57.2) |
| IV (PORT score 91–130) | 102 (26.4) | 116 (29.9) | 217 (28.0) |
| CURB-65 (≥2 criteria) | 53 (13.7) | 57 (14.7) | 110 (14.2) |
| SMART-COP (≥3 criteria) | 173 (44.8) | 182 (46.9) | 355 (45.9) |
| Modified ATS severity (≥3 minor criteria) | 49 (12.7) | 62 (16.0) | 111 (14.3) |
| SIRS criteria (≥2 criteria) | 288 (74.6) | 286 (73.7) | 574 (74.2) |
| qSOFA (≥2 criteria) | 296 (76.7) | 301 (77.6) | 597 (77.1) |
| COPD/asthmad | 83 (21.5) | 76 (19.6) | 159 (20.5) |
| Multilobar infiltrates | 93 (24.1) | 113 (29.1) | 206 (26.6) |
| Pleural effusion | 60 (15.5) | 65 (16.8) | 125 (16.1) |
| Bacteremia | 15 (3.9) | 18 (4.6) | 33 (4.3) |
| . | Omadacycline . | Moxifloxacin . | All Patients . |
|---|---|---|---|
| Characteristic . | (N = 386) . | (N = 388) . | (N = 774) . |
| Male | 208 (53.9) | 219 (56.4) | 427 (55.2) |
| Age, mean (SD) | 60.9 (15.2) | 62.1 (15.2) | 61.5 (15.2) |
| Categorical age, years | |||
| 18–45 | 62 (16.1) | 61 (15.7) | 123 (15.9) |
| >45–65 | 172 (44.6) | 155 (39.9) | 327 (42.2) |
| >65a | 152 (39.4) | 172 (44.3) | 324 (41.9) |
| Mean BMI, kg/m2 (SD) | 27.23 (5.746) | 27.42 (5.791) | 27.33 (5.765) |
| PORT risk class (actual)b | |||
| II (PORT score 51–70)c | 57 (14.8) | 56 (14.4) | 109 (14.1) |
| III (PORT score 71–90) | 227 (58.8) | 216 (55.7) | 443 (57.2) |
| IV (PORT score 91–130) | 102 (26.4) | 116 (29.9) | 217 (28.0) |
| CURB-65 (≥2 criteria) | 53 (13.7) | 57 (14.7) | 110 (14.2) |
| SMART-COP (≥3 criteria) | 173 (44.8) | 182 (46.9) | 355 (45.9) |
| Modified ATS severity (≥3 minor criteria) | 49 (12.7) | 62 (16.0) | 111 (14.3) |
| SIRS criteria (≥2 criteria) | 288 (74.6) | 286 (73.7) | 574 (74.2) |
| qSOFA (≥2 criteria) | 296 (76.7) | 301 (77.6) | 597 (77.1) |
| COPD/asthmad | 83 (21.5) | 76 (19.6) | 159 (20.5) |
| Multilobar infiltrates | 93 (24.1) | 113 (29.1) | 206 (26.6) |
| Pleural effusion | 60 (15.5) | 65 (16.8) | 125 (16.1) |
| Bacteremia | 15 (3.9) | 18 (4.6) | 33 (4.3) |
Abbreviations: ATS, American Thoracic Society; BMI, body mass index; COPD, chronic obstructive pulmonary disease; PORT, Pneumonia Outcomes Research Team; qSOFA, quick Sequential [Sepsis-related] Organ Failure Assessment; SD, standard deviation; SIRS, systemic inflammatory response syndrome.
NOTE: Data are n (%) unless otherwise indicated.
a20.4% of all patients were >75 years old.
bExcludes 5 patients with PORT risk class I and V (2 on omadacycline; 3 on moxifloxacin).
cPORT risk class II capped at 15% by protocol design.
dDefined as medical history of COPD, asthma, emphysema, or chronic bronchitis.
| . | Omadacycline . | Moxifloxacin . | All Patients . |
|---|---|---|---|
| Characteristic . | (N = 386) . | (N = 388) . | (N = 774) . |
| Male | 208 (53.9) | 219 (56.4) | 427 (55.2) |
| Age, mean (SD) | 60.9 (15.2) | 62.1 (15.2) | 61.5 (15.2) |
| Categorical age, years | |||
| 18–45 | 62 (16.1) | 61 (15.7) | 123 (15.9) |
| >45–65 | 172 (44.6) | 155 (39.9) | 327 (42.2) |
| >65a | 152 (39.4) | 172 (44.3) | 324 (41.9) |
| Mean BMI, kg/m2 (SD) | 27.23 (5.746) | 27.42 (5.791) | 27.33 (5.765) |
| PORT risk class (actual)b | |||
| II (PORT score 51–70)c | 57 (14.8) | 56 (14.4) | 109 (14.1) |
| III (PORT score 71–90) | 227 (58.8) | 216 (55.7) | 443 (57.2) |
| IV (PORT score 91–130) | 102 (26.4) | 116 (29.9) | 217 (28.0) |
| CURB-65 (≥2 criteria) | 53 (13.7) | 57 (14.7) | 110 (14.2) |
| SMART-COP (≥3 criteria) | 173 (44.8) | 182 (46.9) | 355 (45.9) |
| Modified ATS severity (≥3 minor criteria) | 49 (12.7) | 62 (16.0) | 111 (14.3) |
| SIRS criteria (≥2 criteria) | 288 (74.6) | 286 (73.7) | 574 (74.2) |
| qSOFA (≥2 criteria) | 296 (76.7) | 301 (77.6) | 597 (77.1) |
| COPD/asthmad | 83 (21.5) | 76 (19.6) | 159 (20.5) |
| Multilobar infiltrates | 93 (24.1) | 113 (29.1) | 206 (26.6) |
| Pleural effusion | 60 (15.5) | 65 (16.8) | 125 (16.1) |
| Bacteremia | 15 (3.9) | 18 (4.6) | 33 (4.3) |
| . | Omadacycline . | Moxifloxacin . | All Patients . |
|---|---|---|---|
| Characteristic . | (N = 386) . | (N = 388) . | (N = 774) . |
| Male | 208 (53.9) | 219 (56.4) | 427 (55.2) |
| Age, mean (SD) | 60.9 (15.2) | 62.1 (15.2) | 61.5 (15.2) |
| Categorical age, years | |||
| 18–45 | 62 (16.1) | 61 (15.7) | 123 (15.9) |
| >45–65 | 172 (44.6) | 155 (39.9) | 327 (42.2) |
| >65a | 152 (39.4) | 172 (44.3) | 324 (41.9) |
| Mean BMI, kg/m2 (SD) | 27.23 (5.746) | 27.42 (5.791) | 27.33 (5.765) |
| PORT risk class (actual)b | |||
| II (PORT score 51–70)c | 57 (14.8) | 56 (14.4) | 109 (14.1) |
| III (PORT score 71–90) | 227 (58.8) | 216 (55.7) | 443 (57.2) |
| IV (PORT score 91–130) | 102 (26.4) | 116 (29.9) | 217 (28.0) |
| CURB-65 (≥2 criteria) | 53 (13.7) | 57 (14.7) | 110 (14.2) |
| SMART-COP (≥3 criteria) | 173 (44.8) | 182 (46.9) | 355 (45.9) |
| Modified ATS severity (≥3 minor criteria) | 49 (12.7) | 62 (16.0) | 111 (14.3) |
| SIRS criteria (≥2 criteria) | 288 (74.6) | 286 (73.7) | 574 (74.2) |
| qSOFA (≥2 criteria) | 296 (76.7) | 301 (77.6) | 597 (77.1) |
| COPD/asthmad | 83 (21.5) | 76 (19.6) | 159 (20.5) |
| Multilobar infiltrates | 93 (24.1) | 113 (29.1) | 206 (26.6) |
| Pleural effusion | 60 (15.5) | 65 (16.8) | 125 (16.1) |
| Bacteremia | 15 (3.9) | 18 (4.6) | 33 (4.3) |
Abbreviations: ATS, American Thoracic Society; BMI, body mass index; COPD, chronic obstructive pulmonary disease; PORT, Pneumonia Outcomes Research Team; qSOFA, quick Sequential [Sepsis-related] Organ Failure Assessment; SD, standard deviation; SIRS, systemic inflammatory response syndrome.
NOTE: Data are n (%) unless otherwise indicated.
a20.4% of all patients were >75 years old.
bExcludes 5 patients with PORT risk class I and V (2 on omadacycline; 3 on moxifloxacin).
cPORT risk class II capped at 15% by protocol design.
dDefined as medical history of COPD, asthma, emphysema, or chronic bronchitis.
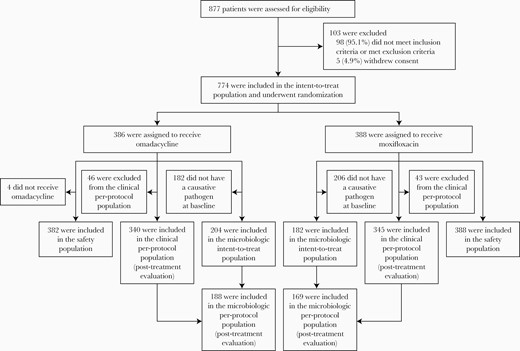
When assessed by PORT risk class, clinical success was achieved by 73%–87% of patients at ECR and 80%–91% at PTE across both treatment groups (Figure 2), with no differences between groups. Similar success rates were observed across treatment groups when assessed by CURB-65 criteria (omadacycline, 74%–84% at ECR and 81%–90% at PTE; moxifloxacin, 82%–83% at ECR and 84%–86% at PTE). Although 74% of omadacycline-treated patients who met CURB-65 ≥2 criteria had achieved clinical success at ECR, compared with 82% of moxifloxacin-treated patients, the differences were less pronounced at PTE (81% vs 84%).
![Clinical success at early clinical response (ECR) and posttreatment evaluation (PTE) stratified by severity and mortality risk scoring systems. ATS, American Thoracic Society; CI, confidence interval; ITT, intent to treat; PORT, Pneumonia Outcomes Research Team; qSOFA, Sequential [Sepsis-related] Organ Failure Assessment; SIRS, systemic inflammatory response syndrome.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ofid/8/6/10.1093_ofid_ofab135/1/m_ofab135_fig2.jpeg?Expires=1750887355&Signature=cP~iYC5udxt6gH8lXecMecsxIp3PAEzO3Oq7sgy-ncpVLU3uGp6ZUpk5TAYCv8Y-xuBHRMGwnqoMJuL4feTuESnKayhag4cdgDIr2pQ4aZqtE-Rs9TTGINfE22Dodab6DelkQ6qGSQlMWI2ayOrnrp~LqTHxQ5Et6A8OOcIH66mLCP0qGcdRFQKgqWSEqN74eDhS9FB6jEwYXsxqETJkXKwqKZcxlKFfB92bZYg0Wcct-fXgH6CPRRBsiJkOvl~cx2jIWBqKsq3GKYfNVy5B39yISeQKRgpQUeJagnSdq~dbWw-xE9N~N--Kh515cizDIPSSv1unp3os43MTqTfyBw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Clinical success at early clinical response (ECR) and posttreatment evaluation (PTE) stratified by severity and mortality risk scoring systems. ATS, American Thoracic Society; CI, confidence interval; ITT, intent to treat; PORT, Pneumonia Outcomes Research Team; qSOFA, Sequential [Sepsis-related] Organ Failure Assessment; SIRS, systemic inflammatory response syndrome.
When stratified by severity measures (modified ATS criteria, SMART-COP), similar percentages of patients achieved clinical success at ECR and PTE across both treatment groups, regardless of measure used or disease severity level (Figure 2). Numerical differences were observed between treatment groups at ECR and PTE using modified ATS criteria (for omadacycline vs moxifloxacin, clinical success rates in patients meeting ≥3 criteria were 71% vs 79% at ECR and 77% vs 83% at PTE), whereas no clear trends were seen in patients meeting SMART-COP ≥3 criteria (80% vs 82% at ECR and 88% vs 84% at PTE for omadacycline vs moxifloxacin, respectively).
Patients who met sepsis (SIRS) ≥2 criteria had similar clinical success rates at ECR and PTE (for omadacycline vs moxifloxacin, 80% vs 83% at ECR and 87% vs 84% at PTE). In patients who met qSOFA ≥2 criteria, for omadacycline vs moxifloxacin, clinical success rates were similar at ECR and were 89% vs 84% at PTE. In patients with qSOFA <2 criteria, both treatments resulted in similar rates of clinical success at ECR and PTE.
Clinical success rates were similar across treatment groups in patients with pleural effusion and multilobar infiltrates (Figure 3). When patients with COPD/asthma were considered, clinical success rates were also broadly similar across treatment groups at both time points (for omadacycline vs moxifloxacin, 76% vs 83% at ECR and 81% vs 85% at PTE). However, in patients with bacteremia, clinical success rates were numerically lower in the omadacycline group compared with the moxifloxacin group (67% vs 89% at ECR), although differences were less pronounced at PTE (73% vs 83%) (Figure 3). The most common cause of bacteremia in each treatment arm was S pneumoniae (omadacycline, 11 of 15 [73.3%]; moxifloxacin, 11 of 18 [61.1%]). For patients in whom S pneumoniae was identified in baseline blood cultures, clinical success at PTE was achieved in 9 of 11 (81.8%) in the omadacycline group and 11 of 11 (100%) in the moxifloxacin group.
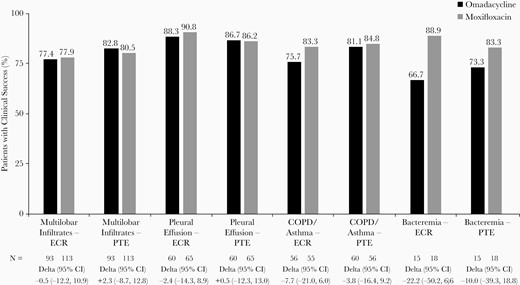
Clinical success at early clinical response (ECR) and posttreatment evaluation (PTE) in patients with radiographic characteristics, comorbidities, or bacteremia. CI, confidence interval; COPD, chronic obstructive pulmonary disease.
Full safety data for the OPTIC study have been published previously [14]. In brief, the most common adverse events (AEs) were gastrointestinal events (10% of omadacycline vs 29% of moxifloxacin patients); of these, in the omadacycline versus moxifloxacin patients, nausea (2.4% vs 5.4%), vomiting (2.6% vs 1.5%), diarrhea (1.0% vs 8.0%), and constipation (2.4% vs 1.5%) were the most frequently reported. Other AEs by Medical Dictionary for Regulatory Activities (MedDRA) preferred term reported by ≥2% of patients in either treatment group were, for omadacycline versus moxifloxacin, alanine aminotransferase increased (3.7% vs 4.6%), gamma-glutamyltransferase increased (2.6% vs 2.1%), aspartate aminotransferase increased (2.1% vs 3.6%), headache (2.1% vs 1.3%), insomnia (2.6% vs 2.1%), and hypertension (3.4% vs 2.8%). Eight cases of Clostridioides difficile infection were reported during the study, all of which occurred in the moxifloxacin group. The rate of treatment discontinuation due to AEs was low (5.5% of omadacycline vs 7.0% of moxifloxacin patients), and all deaths (2% in the omadacycline vs 1% in the moxifloxacin group) occurred in patients aged >65 years, most of whom had multiple comorbidities [16].
DISCUSSION
In the phase 3, randomized OPTIC clinical trial, the efficacy of omadacycline, as IV administration with the option to transition to oral administration, was noninferior to moxifloxacin for ECR, and it was generally well tolerated in patients with CABP [14]. When analyzed by additional measures of mortality and severity, as performed in this secondary analysis, no clinically relevant differences were observed in the efficacy of omadacycline compared with moxifloxacin. Although numerically different responses were observed in some indices (eg, CURB-65 ≥2 criteria at ECR), the point estimates of the differences were all <10%. The use of multiple CABP prognostic indices and characteristics to explore clinical response in this analysis suggests that there is no difference in clinical response between omadacycline and moxifloxacin in patients enrolled in the OPTIC trial including those with elevated severity or mortality risk. The clinical responses across the different mortality and severity measures were generally consistent with the overall clinical success rates observed in the primary analysis [14]. This analysis provides further confidence for the use of omadacycline in the treatment of patients with CABP.
In the current analysis, clinical response rates were similar (1) for the 2 treatments across the 3 PORT classes included and (2) across CURB-65 scores. The 2019 Infectious Diseases Society of America/ATS CAP guidelines recommend PORT as opposed to CURB-65 as a risk stratification and site-of-care decision-making tool. Compared with CURB-65, PORT “identifies larger proportions of patients as low risk and has a higher discriminative power in predicting mortality” [9]. However, PORT scoring is not frequently used in everyday clinical practice, possibly due to its complexity or lack of physician awareness [17, 18]. Therefore, the guidelines also acknowledge the simplicity of the CURB-65 score and recommend it as an alternative for site-of-care decision making, despite the lower quality of evidence regarding its use.
We observed similar response rates across the 2 treatment groups when clinical response was assessed by either SMART-COP or modified ATS criteria, although clinical success rates were higher for both treatments in patients meeting SMART-COP criteria than when grouped by modified ATS criteria. The current guideline recommends the 2007 ATS severity criteria for defining severe CAP, as opposed to other severity scoring systems, because of their easily measured parameters [9]. The SMART-COP and modified ATS criteria (in patients not meeting major criteria) appear to have similar predictive test characteristics to each other [19], but SMART-COP uses several criteria that are not always available for rapid decision making (eg, albumin and pH levels) [20]. When assessed by sepsis measures, qSOFA and SIRS criteria, similar clinical success was observed for omadacycline compared with moxifloxacin in patients for all categories including those meeting ≥2 criteria for the respective measures.
Finally, although clinical success rates were numerically lower for omadacycline compared with moxifloxacin at ECR and PTE in patients with bacteremia, the analysis was limited by the small sample size (15 and 18 patients for omadacycline and moxifloxacin, respectively). Additional details of clinical response in these patients have been described previously [21]. Pharmacokinetic sampling in this study was limited, and omadacycline concentrations in bacteremic patients are not available. However, the omadacycline MIC90 was 0.06 µg/mL for the 22 S pneumoniae isolates recovered from blood cultures in OPTIC [21]. Pharmacokinetic/pharmacodynamic models predict target attainment of 1-log10 colony-forming unit reduction in plasma for ≥90% of S pneumoniae isolates up to a minimum inhibitory concentration of 0.06 µg/mL (data on file). Similar findings have been observed in patients with secondary bacteremia treated with other tetracycline-class antibiotics [22, 23].
This analysis has several limitations. First, discussion of the imperfect test characteristics of published mortality and severity scoring systems and patient characteristics is beyond the scope of this paper. Second, assessments of mortality and severity by scoring systems and characteristics were mostly defined and performed post hoc. Analysis within subgroups has known limitations, including small sample sizes that are not powered for inferential testing, treatment groups that may be unbalanced for prognostic factors, and results that could be due to chance given the large number of subgroups examined. However, the tools were used solely to identify subsets of patients at potentially higher risk of poor outcomes, to explore the robustness of the OPTIC clinical trial data. In addition, the data are consistent across the subsets of OPTIC patients who are generally cared for in non-ICU settings in clinical practice, but identified with disease characteristics or score thresholds associated with potentially higher risk of mortality and disease severity. Therefore, another important limitation of the study was the exclusion of patients from the OPTIC trial who required vasopressor support, those with septic shock, those classified as PORT risk class V, and the immunosuppressed. Findings presented in this trial and this secondary analysis are not generalizable to these patient types.
CONCLUSIONS
In summary, omadacycline demonstrated consistently similar clinical outcomes to moxifloxacin in patients with characteristics or criteria for heightened disease severity or mortality risk. Additional data in at-risk patients receiving omadacycline therapy would be helpful to expand on these initial observations of similar clinical outcomes.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank Lynne Garrity-Ryan (Paratek Pharmaceuticals, Inc.). Medical writing assistance was provided by Dr. Jenny Engelmoer (Innovative Strategic Communications), funded by Paratek Pharmaceuticals, Inc.
Author contributions. All authors were involved in the conception of the work, interpretation of the data, and revising the manuscript critically for important intellectual content. All authors approved the final version of the manuscript for publication and agree to be accountable for the accuracy of the information presented herein.
Financial support. This work was funded by Paratek Pharmaceuticals, Inc.
Potential conflicts of interest. J. R. has received research grants from Pfizer, Inc. D. D., C. K., and A. M. are employees of Paratek Pharmaceuticals. P. B. E. is a consultant for Paratek Pharmaceuticals, Spero Therapeutics, AN2 Therapeutics, Bugworks Research, and SNIPR Biome. M. C. was an employee of Paratek Pharmaceuticals at the time of the study. A. F. D. is a consultant to AntibioTx, Achaogen, Boston Pharmaceuticals, Cempra, ContraFect, Iterum Therapeutics, Nabriva Therapeutics, Paratek Pharmaceuticals, Tetraphase, Theravance, UTILITY, Wockhardt, and Zavante. E. T. was an employee of Paratek Pharmaceuticals at the time of the study and is a consultant to UTILITY. P. C. M. was an employee of Paratek Pharmaceuticals at the time of the study; he is a stockholder and works as a consultant for Paratek Pharmaceuticals. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.





Comments