-
PDF
- Split View
-
Views
-
Cite
Cite
Ian A Strohbehn, Tianqi Ouyang, Meghan D Lee, Sophia Zhao, Destiny Harden, Sherley M Mejia, Andrew Cao, Roby P Bhattacharyya, Meghan E Sise, The Effect of Nirmatrelvir-Ritonavir on Short- and Long-term Adverse Outcomes From COVID-19 Among Patients With Kidney Disease: A Propensity Score–Matched Study, Open Forum Infectious Diseases, Volume 12, Issue 1, January 2025, ofae756, https://doi.org/10.1093/ofid/ofae756
Close - Share Icon Share
Abstract
Patients with kidney disease are at high risk for adverse outcomes after coronavirus disease 2019 (COVID-19) despite vaccination. Because patients with advanced chronic kidney disease (CKD) and kidney failure were excluded from registrational trials, the impact of the protease inhibitor nirmatrelvir-ritonavir in patients with kidney disease is unknown.
This was a cohort study evaluating adverse outcomes in patients with kidney disease who developed COVID-19. Patients prescribed nirmatrelvir-ritonavir for COVID-19 between March 16, 2022, and November 30, 2022, were propensity score–matched to comparators diagnosed with COVID-19 between July 15, 2021, and March 15, 2022 (before the use of nirmatrelvir-ritonavir in our health care network). We determined the association between nirmatrelvir-ritonavir and short- and long-term outcomes using Fine-Gray subdistribution hazard and Cox proportional hazard models, adjusting for potential confounders. Outcomes included 30-day risk of hospitalization and 1-year risk of a major adverse cardiovascular event (MACE), CKD progression, and death.
A total of 1095 nirmatrelvir-ritonavir-treated patients were matched to 584 comparators. Patients who received nirmatrelvir-ritonavir patients were less likely to be hospitalized within 30 days of diagnosis (adjusted subdistribution hazard ratio [sHR], 0.44; 95% CI, 0.26–0.73; P < .01). At 1 year, nirmatrelvir-ritonavir-treated patients had a lower risk of hospitalization for MACE (adjusted sHR, 0.49; 95% CI, 0.36–0.67; P < .01) and death (adjusted hazard ratio, 0.37; 95% CI, 0.21–0.65; P < .01). Use of nirmatrelvir-ritonavir was not associated with decreased risk of CKD progression or attenuation of estimated glomerular filtration rate decline slope in the year following infection.
Nirmatrelvir-ritonavir was associated with decreased risk of hospitalization within 30 days and 1-year risk of MACE and death in patients with CKD and kidney failure.
Patients with chronic kidney disease (CKD) and kidney failure remain at high risk for hospitalization and death from coronavirus disease 2019 (COVID-19) despite widespread vaccination [1]. In addition to the acute symptoms associated with COVID-19, survivors can experience long-term effects known as “long COVID” and are at higher risk of developing cardiovascular and kidney adverse events such as myocardial infarction, thromboembolic disease, and CKD progression [2, 3]. Major adverse cardiovascular events (MACE) are more likely for up to a year after COVID-19, even among patients who are not hospitalized during their acute illness [2]. As novel treatments for COVID-19 are approved, it is important to evaluate whether antiviral treatments reduce short- and long-term adverse outcomes in high-risk populations.
Nirmatrelvir-ritonavir, an inhibitor of the coronavirus protease Mpro, was the first oral antiviral therapy to receive Emergency Use Authorization (EUA) by the Food and Drug Administration for adults with mild to moderate COVID-19 who are at risk of progressing to severe disease [4]. Its use has been associated with decreased risk of hospitalization and death in trials in the prevaccine era [5] and in observational studies of vaccinated patients where it was mostly prescribed to individuals with multiple comorbidities [6–12], though its benefit in relatively low-risk vaccinated populations is uncertain [13]. However, patients with advanced CKD or kidney failure were excluded from randomized clinical trials testing the safety and efficacy of nirmatrelvir-ritonavir; thus its use has been restricted in this patient population. Patients with advanced CKD are also poorly represented in observational studies of nirmatrelvir-ritonavir [9, 12]. However, emerging “real-world” data suggest that nirmatrelvir-ritonavir is safe and well tolerated in patients with advanced CKD and kidney failure [14–19].
To assess the efficacy of nirmatrelvir-ritonavir in patients with CKD and kidney failure, we designed a propensity score–matched study to compare the risk of hospitalization within 30 days and the 1-year risks of hospitalization for MACE, CKD progression, and death. We compared patients with advanced CKD and kidney failure treated with nirmatrelvir-ritonavir with recent historical comparators who were diagnosed with COVID-19 in the period after widespread vaccination but before the initial EUA for nirmatrelvir-ritonavir.
METHODS
We included adult patients (≥18 years old) with baseline CKD or kidney failure diagnosed with COVID-19 between July 15, 2021, and November 30, 2022, in the Massachusetts General Brigham (MGB) health care system. Cases were prescribed nirmatrelvir-ritonavir for COVID-19 between March 16, 2022, and November 30, 2022, and potential historical comparators were patients who were diagnosed with COVID-19 as an outpatient via reverse transcriptase quantitative polymerase chain reaction (RT-qPCR) between July 15, 2021, and March 15, 2022. The comparator time window was chosen because it includes the period after widespread vaccination in Boston, MA, but was just before the first use of oral antivirals within our health care network; this minimized confounding by indication for eligible patients who were not selected for treatment. Baseline CKD was defined as baseline creatinine-based estimated glomerular filtration rate (eGFR) <60 mL/min per 1.73 m2. Baseline creatinine-based eGFR was calculated using the 2021 CKD-EPI race-free eGFR equation, using the median of all serum creatinine values obtained within 1 year before infection. Kidney failure was defined by presence of a diagnosis code for kidney failure, end-stage kidney disease, or receipt of kidney replacement therapy for ≥30 days before baseline. Exclusion criteria were (1) prior solid organ transplant recipients, (2) lacking a creatinine measurement within 1 year before baseline, and (3) having a median serum creatinine that corresponded to an eGFR ≥60 mL/min per 1.73 m2.
To control for treatment assignment and outcome-associated confounding variables, we performed propensity score matching between the patients who received nirmatrelvir-ritonavir and potential historical comparators [20]. We matched patients across variables that were identified a priori, including age, sex, history of hypertension, diabetes mellitus, coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, cancer, CKD stage, use of nonsteroidal anti-inflammatory drugs, beta-blockers, statins, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, proton pump inhibitors, diuretics, immunosuppressant medications, prior doses of COVID-19 vaccine received, time since last COVID-19 vaccination, number of serum creatinine measurements, and number of hospitalizations within the MGB health network within 1 year before baseline (Supplementary Methods and Supplementary Table 1). We performed 1:4 nearest-neighbor matching without replacement with a caliper of 0.2 standard deviations of the propensity score. Using standardized mean differences, we assessed the balance between nirmatrelvir-ritonavir-treated and -untreated patients before and after matching.
We investigated both short-term and long-term outcomes in nirmatrelvir-ritonavir-treated patients and historical comparators. Short-term outcomes included hospitalization and death within 30 days following baseline. Long-term outcomes included hospitalization for MACE; a composite outcome of CKD progression, defined as a doubling of serum creatinine, receiving dialysis, or being diagnosed with kidney failure (among patients without kidney failure at baseline); and death within 1 year. Hospitalization for MACE was determined by diagnosis codes linked to hospital encounters within MGB and included acute coronary disease, myocardial infarction, ischemic cardiomyopathy, angina, congestive heart failure, cardiomyopathy, cardiac arrest, cardiogenic shock, cardiac dysrhythmia (atrial fibrillation/flutter, sinus tachycardia, sinus bradycardia, ventricular arrhythmias), pericarditis, myocarditis, stroke, transient ischemic attack, pulmonary embolism, and deep or superficial vein thrombosis.
We used Fine-Gray subdistribution hazard models to compare the short- and long-term outcomes between the matched groups (nirmatrelvir-ritonavir-treated patients and matched historical comparators) accounting for the competing risk of death. A Cox proportional hazard model was used for the long-term death outcome, adjusting for potential confounders. We adjusted for age, sex, number of COVID-19 vaccination doses received, time since last COVID-19 vaccination dose, documented prior infections, and relevant comorbidities including hypertension, diabetes, coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, and CKD stage to account for any residual confounding bias (Supplementary Methods). We evaluated survival at 1 year using Kaplan-Meier (KM) statistics and plotted the cumulative incidence of hospitalization at 30 days, hospitalization for MACE, and CKD progression within 1 year. As a sensitivity analysis, we estimated the association between nirmatrelvir-ritonavir use and chronic eGFR slope using linear mixed-effects models with random intercepts and random slopes, accounting for repeated observations within each patient without baseline kidney failure from day 30 through 1 year.
We performed a subgroup analysis to determine the consistency of nirmatrelvir-ritonavir effect by kidney disease severity (predialysis CKD vs kidney failure). Because 2 distinct variants were captured in the historical comparator cohort (the Delta wave corresponded to July 15, 2021, to ∼December 24, 2021, and the Omicron wave followed thereafter), we used a subgroup analysis evaluating only matched Omicron comparators to try to account for the differences in the average observed severity of infections caused by these variants [21].
Data were analyzed in R, version 2023.12.1 + 402, and Prism, version 10.2.0. All P values were 2-sided, and a P value <.05 was considered statistically significant. We used the STROBE cohort reporting guidelines [22]. This study was approved by the institutional review board at MGB, and the need for informed consent was waived.
RESULTS
A total of 1723 individuals with CKD or kidney failure prescribed nirmatrelvir-ritonavir for COVID-19 between March 16, 2022, and November 30, 2022, and 940 potential historical comparators with CKD or kidney failure diagnosed with COVID-19 via RT-qPCR between July 15, 2021, and March 15, 2022, met inclusion criteria (Figure 1). After propensity score matching, 1095 nirmatrelvir-ritonavir-treated patients were variably matched to 584 historical comparators (Figure 1). We achieved a good balance between the nirmatrelvir-ritonavir-treated patients and untreated comparators after propensity score matching (Table 1). Among matched patients, the mean age was 75 ± 12 years, and 908 (54%) were female. Comorbidities were common; 745 (44%) had diabetes, 1450 (86%) had hypertension, and 841 (50%) had coronary artery disease. Baseline CKD stages are shown in Table 1; 1578 (94%) patients had predialysis CKD, and 101 (6%) had kidney failure. The majority had been vaccinated against COVID-19 (n = 1537, 92%), and 37 (2%) had previously documented COVID-19 infection evidenced by a positive RT-qPCR test >90 days before the index infection in the study window. Patients were followed for a median (interquartile range) of 365 (320–365) days. Among matched patients, 62/1679 (3%) died within 365 days, and 480/1617 (30%) were lost to follow-up before 365 days. There was no statistical difference between the length of follow-up in the antiviral-treated cohort and matched comparators (P = .91).

Patient flow. Abbreviations: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; EUA, Emergency Use Authorization.
| . | Before Propensity Score Matching . | After Propensity Score Matching . | ||||
|---|---|---|---|---|---|---|
| Characteristic . | Antiviral-Treated (n = 1723) . | Untreated Historical Comparators (n = 940) . | Standard Mean Difference . | Antiviral-Treated (n = 1095) . | Untreated Historical Comparators (n = 584) . | Standard Mean Difference . |
| Female, No. (%) | 1001 (58) | 471 (50) | 0.16 | 600 (55) | 308 (53) | 0.05 |
| Baseline age, mean (SD), y | 76 (11) | 73 (13) | 0.27 | 76 (11) | 74 (13) | 0.08 |
| Comorbidities, No. (%) | ||||||
| Hypertension | 1553 (90) | 759 (81) | 0.24 | 954 (87) | 496 (85) | 0.02 |
| Diabetes | 683 (40) | 441 (47) | 0.15 | 467 (43) | 278 (48) | <0.01 |
| Coronary artery disease | 835 (48) | 462 (49) | 0.01 | 547 (50) | 294 (50) | 0.02 |
| Congestive heart failure | 118 (7) | 93 (10) | 0.10 | 86 (8) | 61 (10) | 0.05 |
| Chronic obstructive pulmonary disease | 256 (15) | 153 (16) | 0.04 | 168 (15) | 102 (17) | 0.03 |
| History of cancer | 874 (51) | 370 (39) | 0.16 | 519 (47) | 265 (45) | 0.02 |
| Chronic kidney disease group | ||||||
| eGFR 45–59.9 | 1341 (78) | 549 (58) | 0.39 | 807 (74) | 375 (64) | 0.08 |
| eGFR 30–44.9 | 312 (18) | 217 (23) | 0.12 | 221 (20) | 132 (23) | <0.01 |
| eGFR <30, not on dialysis | 15 (2) | 74 (8) | 0.26 | 15 (1) | 28 (5) | 0.09 |
| Kidney failure | 55 (3) | 100 (11) | 0.24 | 52 (5) | 49 (8) | 0.06 |
| Medications, No. (%) | ||||||
| Nonsteroidal anti-inflammatory drugs | 303 (18) | 175 (19) | 0.03 | 205 (19) | 117 (20) | 0.01 |
| Beta-blockers | 706 (41) | 381 (41) | <0.01 | 455 (42) | 256 (44) | 0.03 |
| Statins | 1088 (63) | 436 (46) | 0.34 | 641 (59) | 315 (54) | 0.02 |
| Angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers | 918 (53) | 359 (38) | 0.31 | 521 (48) | 262 (45) | 0.04 |
| Proton pump inhibitors | 509 (30) | 286 (30) | 0.02 | 356 (33) | 198 (34) | 0.02 |
| Diuretics | 694 (40) | 337 (36) | 0.09 | 417 (38) | 240 (41) | 0.06 |
| Immunosuppressants | 97 (6) | 47 (5) | 0.03 | 62 (6) | 29 (5) | 0.02 |
| Related health history, No. (%) | ||||||
| Prior COVID-19 vaccination doses | ||||||
| None | 63 (4) | 182 (19) | 0.40 | 63 (6) | 78 (13) | 0.07 |
| Primary series (1–2 doses) | 224 (13) | 460 (49) | 0.72 | 224 (20) | 227 (39) | 0.03 |
| 3+ doses | 1436 (83) | 298 (32) | 1.11 | 808 (74) | 279 (48) | 0.09 |
| Time since last COVID-19 vaccination dose, No. (%) | ||||||
| <180 d | 907 (53) | 407 (43) | 0.19 | 760 (69) | 299 (51) | 0.05 |
| ≥180 d | 816 (47) | 533 (57) | 0.19 | 335 (31) | 285 (49) | 0.05 |
| Prior infection detected by PCR, No. (%) | 26 (2) | 27 (3) | 0.08 | 22 (2) | 15 (3) | <0.01 |
| No. of serum creatinine measurements within 12 mo prior, No. (%) | ||||||
| 1 prior measurement | 513 (30) | 245 (26) | 0.09 | 286 (26) | 150 (26) | <0.01 |
| 2–4 prior measurements | 785 (46) | 350 (37) | 0.17 | 492 (45) | 226 (39) | 0.06 |
| 5+ prior measurements | 425 (25) | 345 (37) | 0.25 | 317 (29) | 208 (36) | 0.05 |
| Hospitalizations within 12 mo prior, No. (%) | ||||||
| 0 prior hospitalizations | 1505 (87) | 708 (75) | 0.28 | 922 (84) | 458 (78) | 0.05 |
| 1–2 prior hospitalizations | 146 (9) | 124 (13) | 0.14 | 115 (11) | 68 (12) | 0.02 |
| 3+ prior hospitalizations | 72 (4) | 108 (11) | 0.23 | 58 (5) | 58 (10) | 0.10 |
| . | Before Propensity Score Matching . | After Propensity Score Matching . | ||||
|---|---|---|---|---|---|---|
| Characteristic . | Antiviral-Treated (n = 1723) . | Untreated Historical Comparators (n = 940) . | Standard Mean Difference . | Antiviral-Treated (n = 1095) . | Untreated Historical Comparators (n = 584) . | Standard Mean Difference . |
| Female, No. (%) | 1001 (58) | 471 (50) | 0.16 | 600 (55) | 308 (53) | 0.05 |
| Baseline age, mean (SD), y | 76 (11) | 73 (13) | 0.27 | 76 (11) | 74 (13) | 0.08 |
| Comorbidities, No. (%) | ||||||
| Hypertension | 1553 (90) | 759 (81) | 0.24 | 954 (87) | 496 (85) | 0.02 |
| Diabetes | 683 (40) | 441 (47) | 0.15 | 467 (43) | 278 (48) | <0.01 |
| Coronary artery disease | 835 (48) | 462 (49) | 0.01 | 547 (50) | 294 (50) | 0.02 |
| Congestive heart failure | 118 (7) | 93 (10) | 0.10 | 86 (8) | 61 (10) | 0.05 |
| Chronic obstructive pulmonary disease | 256 (15) | 153 (16) | 0.04 | 168 (15) | 102 (17) | 0.03 |
| History of cancer | 874 (51) | 370 (39) | 0.16 | 519 (47) | 265 (45) | 0.02 |
| Chronic kidney disease group | ||||||
| eGFR 45–59.9 | 1341 (78) | 549 (58) | 0.39 | 807 (74) | 375 (64) | 0.08 |
| eGFR 30–44.9 | 312 (18) | 217 (23) | 0.12 | 221 (20) | 132 (23) | <0.01 |
| eGFR <30, not on dialysis | 15 (2) | 74 (8) | 0.26 | 15 (1) | 28 (5) | 0.09 |
| Kidney failure | 55 (3) | 100 (11) | 0.24 | 52 (5) | 49 (8) | 0.06 |
| Medications, No. (%) | ||||||
| Nonsteroidal anti-inflammatory drugs | 303 (18) | 175 (19) | 0.03 | 205 (19) | 117 (20) | 0.01 |
| Beta-blockers | 706 (41) | 381 (41) | <0.01 | 455 (42) | 256 (44) | 0.03 |
| Statins | 1088 (63) | 436 (46) | 0.34 | 641 (59) | 315 (54) | 0.02 |
| Angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers | 918 (53) | 359 (38) | 0.31 | 521 (48) | 262 (45) | 0.04 |
| Proton pump inhibitors | 509 (30) | 286 (30) | 0.02 | 356 (33) | 198 (34) | 0.02 |
| Diuretics | 694 (40) | 337 (36) | 0.09 | 417 (38) | 240 (41) | 0.06 |
| Immunosuppressants | 97 (6) | 47 (5) | 0.03 | 62 (6) | 29 (5) | 0.02 |
| Related health history, No. (%) | ||||||
| Prior COVID-19 vaccination doses | ||||||
| None | 63 (4) | 182 (19) | 0.40 | 63 (6) | 78 (13) | 0.07 |
| Primary series (1–2 doses) | 224 (13) | 460 (49) | 0.72 | 224 (20) | 227 (39) | 0.03 |
| 3+ doses | 1436 (83) | 298 (32) | 1.11 | 808 (74) | 279 (48) | 0.09 |
| Time since last COVID-19 vaccination dose, No. (%) | ||||||
| <180 d | 907 (53) | 407 (43) | 0.19 | 760 (69) | 299 (51) | 0.05 |
| ≥180 d | 816 (47) | 533 (57) | 0.19 | 335 (31) | 285 (49) | 0.05 |
| Prior infection detected by PCR, No. (%) | 26 (2) | 27 (3) | 0.08 | 22 (2) | 15 (3) | <0.01 |
| No. of serum creatinine measurements within 12 mo prior, No. (%) | ||||||
| 1 prior measurement | 513 (30) | 245 (26) | 0.09 | 286 (26) | 150 (26) | <0.01 |
| 2–4 prior measurements | 785 (46) | 350 (37) | 0.17 | 492 (45) | 226 (39) | 0.06 |
| 5+ prior measurements | 425 (25) | 345 (37) | 0.25 | 317 (29) | 208 (36) | 0.05 |
| Hospitalizations within 12 mo prior, No. (%) | ||||||
| 0 prior hospitalizations | 1505 (87) | 708 (75) | 0.28 | 922 (84) | 458 (78) | 0.05 |
| 1–2 prior hospitalizations | 146 (9) | 124 (13) | 0.14 | 115 (11) | 68 (12) | 0.02 |
| 3+ prior hospitalizations | 72 (4) | 108 (11) | 0.23 | 58 (5) | 58 (10) | 0.10 |
Among the matched nirmatrelvir-ritonavir-treated patients, 572 (52%) received nirmatrelvir 300 mg/ritonavir 100 mg twice daily for 5 days, and 523 (48%) received nirmatrelvir 150 mg/ritonavir 100 mg twice daily for 5 days. Among matched comparators, 189 (32%) were infected when the Delta variant was the most prevalent circulating variant (July 15, 2021–December 24, 2021) and 395 (68%) when Omicron was the most prevalent circulating variant (December 24, 2021–March 15, 2022).
Abbreviations: COVID-19, coronavirus disease 2019; eGFR, estimated glomerular filtration rate; PCR, polymerase chain reaction.
| . | Before Propensity Score Matching . | After Propensity Score Matching . | ||||
|---|---|---|---|---|---|---|
| Characteristic . | Antiviral-Treated (n = 1723) . | Untreated Historical Comparators (n = 940) . | Standard Mean Difference . | Antiviral-Treated (n = 1095) . | Untreated Historical Comparators (n = 584) . | Standard Mean Difference . |
| Female, No. (%) | 1001 (58) | 471 (50) | 0.16 | 600 (55) | 308 (53) | 0.05 |
| Baseline age, mean (SD), y | 76 (11) | 73 (13) | 0.27 | 76 (11) | 74 (13) | 0.08 |
| Comorbidities, No. (%) | ||||||
| Hypertension | 1553 (90) | 759 (81) | 0.24 | 954 (87) | 496 (85) | 0.02 |
| Diabetes | 683 (40) | 441 (47) | 0.15 | 467 (43) | 278 (48) | <0.01 |
| Coronary artery disease | 835 (48) | 462 (49) | 0.01 | 547 (50) | 294 (50) | 0.02 |
| Congestive heart failure | 118 (7) | 93 (10) | 0.10 | 86 (8) | 61 (10) | 0.05 |
| Chronic obstructive pulmonary disease | 256 (15) | 153 (16) | 0.04 | 168 (15) | 102 (17) | 0.03 |
| History of cancer | 874 (51) | 370 (39) | 0.16 | 519 (47) | 265 (45) | 0.02 |
| Chronic kidney disease group | ||||||
| eGFR 45–59.9 | 1341 (78) | 549 (58) | 0.39 | 807 (74) | 375 (64) | 0.08 |
| eGFR 30–44.9 | 312 (18) | 217 (23) | 0.12 | 221 (20) | 132 (23) | <0.01 |
| eGFR <30, not on dialysis | 15 (2) | 74 (8) | 0.26 | 15 (1) | 28 (5) | 0.09 |
| Kidney failure | 55 (3) | 100 (11) | 0.24 | 52 (5) | 49 (8) | 0.06 |
| Medications, No. (%) | ||||||
| Nonsteroidal anti-inflammatory drugs | 303 (18) | 175 (19) | 0.03 | 205 (19) | 117 (20) | 0.01 |
| Beta-blockers | 706 (41) | 381 (41) | <0.01 | 455 (42) | 256 (44) | 0.03 |
| Statins | 1088 (63) | 436 (46) | 0.34 | 641 (59) | 315 (54) | 0.02 |
| Angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers | 918 (53) | 359 (38) | 0.31 | 521 (48) | 262 (45) | 0.04 |
| Proton pump inhibitors | 509 (30) | 286 (30) | 0.02 | 356 (33) | 198 (34) | 0.02 |
| Diuretics | 694 (40) | 337 (36) | 0.09 | 417 (38) | 240 (41) | 0.06 |
| Immunosuppressants | 97 (6) | 47 (5) | 0.03 | 62 (6) | 29 (5) | 0.02 |
| Related health history, No. (%) | ||||||
| Prior COVID-19 vaccination doses | ||||||
| None | 63 (4) | 182 (19) | 0.40 | 63 (6) | 78 (13) | 0.07 |
| Primary series (1–2 doses) | 224 (13) | 460 (49) | 0.72 | 224 (20) | 227 (39) | 0.03 |
| 3+ doses | 1436 (83) | 298 (32) | 1.11 | 808 (74) | 279 (48) | 0.09 |
| Time since last COVID-19 vaccination dose, No. (%) | ||||||
| <180 d | 907 (53) | 407 (43) | 0.19 | 760 (69) | 299 (51) | 0.05 |
| ≥180 d | 816 (47) | 533 (57) | 0.19 | 335 (31) | 285 (49) | 0.05 |
| Prior infection detected by PCR, No. (%) | 26 (2) | 27 (3) | 0.08 | 22 (2) | 15 (3) | <0.01 |
| No. of serum creatinine measurements within 12 mo prior, No. (%) | ||||||
| 1 prior measurement | 513 (30) | 245 (26) | 0.09 | 286 (26) | 150 (26) | <0.01 |
| 2–4 prior measurements | 785 (46) | 350 (37) | 0.17 | 492 (45) | 226 (39) | 0.06 |
| 5+ prior measurements | 425 (25) | 345 (37) | 0.25 | 317 (29) | 208 (36) | 0.05 |
| Hospitalizations within 12 mo prior, No. (%) | ||||||
| 0 prior hospitalizations | 1505 (87) | 708 (75) | 0.28 | 922 (84) | 458 (78) | 0.05 |
| 1–2 prior hospitalizations | 146 (9) | 124 (13) | 0.14 | 115 (11) | 68 (12) | 0.02 |
| 3+ prior hospitalizations | 72 (4) | 108 (11) | 0.23 | 58 (5) | 58 (10) | 0.10 |
| . | Before Propensity Score Matching . | After Propensity Score Matching . | ||||
|---|---|---|---|---|---|---|
| Characteristic . | Antiviral-Treated (n = 1723) . | Untreated Historical Comparators (n = 940) . | Standard Mean Difference . | Antiviral-Treated (n = 1095) . | Untreated Historical Comparators (n = 584) . | Standard Mean Difference . |
| Female, No. (%) | 1001 (58) | 471 (50) | 0.16 | 600 (55) | 308 (53) | 0.05 |
| Baseline age, mean (SD), y | 76 (11) | 73 (13) | 0.27 | 76 (11) | 74 (13) | 0.08 |
| Comorbidities, No. (%) | ||||||
| Hypertension | 1553 (90) | 759 (81) | 0.24 | 954 (87) | 496 (85) | 0.02 |
| Diabetes | 683 (40) | 441 (47) | 0.15 | 467 (43) | 278 (48) | <0.01 |
| Coronary artery disease | 835 (48) | 462 (49) | 0.01 | 547 (50) | 294 (50) | 0.02 |
| Congestive heart failure | 118 (7) | 93 (10) | 0.10 | 86 (8) | 61 (10) | 0.05 |
| Chronic obstructive pulmonary disease | 256 (15) | 153 (16) | 0.04 | 168 (15) | 102 (17) | 0.03 |
| History of cancer | 874 (51) | 370 (39) | 0.16 | 519 (47) | 265 (45) | 0.02 |
| Chronic kidney disease group | ||||||
| eGFR 45–59.9 | 1341 (78) | 549 (58) | 0.39 | 807 (74) | 375 (64) | 0.08 |
| eGFR 30–44.9 | 312 (18) | 217 (23) | 0.12 | 221 (20) | 132 (23) | <0.01 |
| eGFR <30, not on dialysis | 15 (2) | 74 (8) | 0.26 | 15 (1) | 28 (5) | 0.09 |
| Kidney failure | 55 (3) | 100 (11) | 0.24 | 52 (5) | 49 (8) | 0.06 |
| Medications, No. (%) | ||||||
| Nonsteroidal anti-inflammatory drugs | 303 (18) | 175 (19) | 0.03 | 205 (19) | 117 (20) | 0.01 |
| Beta-blockers | 706 (41) | 381 (41) | <0.01 | 455 (42) | 256 (44) | 0.03 |
| Statins | 1088 (63) | 436 (46) | 0.34 | 641 (59) | 315 (54) | 0.02 |
| Angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers | 918 (53) | 359 (38) | 0.31 | 521 (48) | 262 (45) | 0.04 |
| Proton pump inhibitors | 509 (30) | 286 (30) | 0.02 | 356 (33) | 198 (34) | 0.02 |
| Diuretics | 694 (40) | 337 (36) | 0.09 | 417 (38) | 240 (41) | 0.06 |
| Immunosuppressants | 97 (6) | 47 (5) | 0.03 | 62 (6) | 29 (5) | 0.02 |
| Related health history, No. (%) | ||||||
| Prior COVID-19 vaccination doses | ||||||
| None | 63 (4) | 182 (19) | 0.40 | 63 (6) | 78 (13) | 0.07 |
| Primary series (1–2 doses) | 224 (13) | 460 (49) | 0.72 | 224 (20) | 227 (39) | 0.03 |
| 3+ doses | 1436 (83) | 298 (32) | 1.11 | 808 (74) | 279 (48) | 0.09 |
| Time since last COVID-19 vaccination dose, No. (%) | ||||||
| <180 d | 907 (53) | 407 (43) | 0.19 | 760 (69) | 299 (51) | 0.05 |
| ≥180 d | 816 (47) | 533 (57) | 0.19 | 335 (31) | 285 (49) | 0.05 |
| Prior infection detected by PCR, No. (%) | 26 (2) | 27 (3) | 0.08 | 22 (2) | 15 (3) | <0.01 |
| No. of serum creatinine measurements within 12 mo prior, No. (%) | ||||||
| 1 prior measurement | 513 (30) | 245 (26) | 0.09 | 286 (26) | 150 (26) | <0.01 |
| 2–4 prior measurements | 785 (46) | 350 (37) | 0.17 | 492 (45) | 226 (39) | 0.06 |
| 5+ prior measurements | 425 (25) | 345 (37) | 0.25 | 317 (29) | 208 (36) | 0.05 |
| Hospitalizations within 12 mo prior, No. (%) | ||||||
| 0 prior hospitalizations | 1505 (87) | 708 (75) | 0.28 | 922 (84) | 458 (78) | 0.05 |
| 1–2 prior hospitalizations | 146 (9) | 124 (13) | 0.14 | 115 (11) | 68 (12) | 0.02 |
| 3+ prior hospitalizations | 72 (4) | 108 (11) | 0.23 | 58 (5) | 58 (10) | 0.10 |
Among the matched nirmatrelvir-ritonavir-treated patients, 572 (52%) received nirmatrelvir 300 mg/ritonavir 100 mg twice daily for 5 days, and 523 (48%) received nirmatrelvir 150 mg/ritonavir 100 mg twice daily for 5 days. Among matched comparators, 189 (32%) were infected when the Delta variant was the most prevalent circulating variant (July 15, 2021–December 24, 2021) and 395 (68%) when Omicron was the most prevalent circulating variant (December 24, 2021–March 15, 2022).
Abbreviations: COVID-19, coronavirus disease 2019; eGFR, estimated glomerular filtration rate; PCR, polymerase chain reaction.
Patients who were prescribed nirmatrelvir-ritonavir were significantly less likely to be hospitalized within 30 days; 29 of 1095 (3%) nirmatrelvir-ritonavir-treated patients and 44 of 584 (8%) historical comparators were hospitalized (adjusted subdistribution hazard ratio [sHR], 0.44; 95% CI, 0.26–0.73; P < .01) (Figure 2A). Cumulative incidence of hospitalization within 30 days is shown in Figure 3. There were no deaths within 30 days in the nirmatrelvir-ritonavir-treated patients compared with 15 (3%) deaths within 30 days in historical comparators.
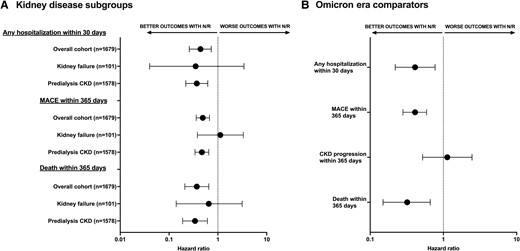
Sensitivity analyses. A, Forest plot showing both the adjusted subdistribution hazard ratios and adjusted hazard ratios for short- and long-term adverse outcomes among patients treated with nirmatrelvir-ritonavir vs historical comparators by kidney disease subgroups. Fine-Gray models were used for nondeath outcomes, while Cox proportional models were used for death outcomes. Outcomes by eGFR cutoffs among the patients with predialysis CKD (n = 1578) are shown Supplementary Table 3. Deaths within 30 days are not shown as there were 0 deaths in the antiviral-treated group and 15 deaths in the historical comparator group. B, Forest plot showing the adjusted hazard ratios for short- and long-term adverse outcomes among patients included in the sensitivity analysis restricted to Omicron era–only comparators (n = 395) and their antiviral-treated matches (n = 812). Error bars represent 95% CIs. Abbreviations: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; MACE, major adverse cardiovascular event; N/R, nirmatrelvir/ritonavir.
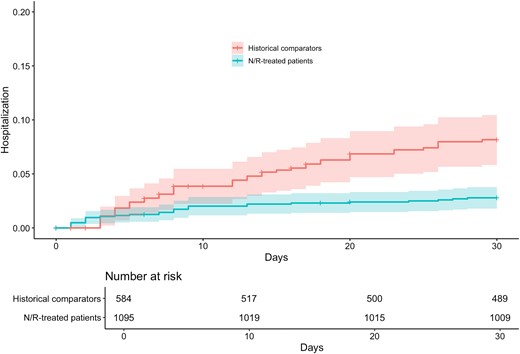
Hospitalization within 30 days. Cumulative incidence of hospitalization within 30 days of baseline in matched cohorts. The rate of any hospitalization within 30 d was significantly reduced in patients prescribed nirmatrelvir-ritonavir compared with propensity score–matched historical comparators diagnosed with COVID-19 by RT-qPCR in the period just before widespread use of oral antiviral therapies. Shaded areas represent 95% CIs. Abbreviations: COVID-19, coronavirus disease 2019; RT-qPCR, reverse transcriptase quantitative polymerase chain reaction.
Evaluation of adverse outcomes up to 1 year from infection showed that 86 (8%) nirmatrelvir-ritonavir-treated patients were hospitalized for MACE within 30 days of baseline, compared with 98 (17%) historical comparators, corresponding to a significant reduction in the risk of hospitalization for MACE among the nirmatrelvir-ritonavir-treated patients even after adjustment for covariates (adjusted sHR, 0.49; 95% CI, 0.36–0.67; P < .01) (Figure 2A). The cumulative incidence of hospitalization for MACE is shown in Figure 4A. A subgroup analysis demonstrated reduction in MACE in the predialysis CKD group, but not in patients with kidney failure (Figure 2A). CKD progression was assessed only among patients without kidney failure at baseline, which included 1043 nirmatrelvir-ritonavir-treated patients and 535 matched historical comparators. There was no significant difference in the rate of CKD progression within 1 year in nirmatrelvir-ritonavir-treated patients vs historical comparators (adjusted sHR, 0.85; 95% CI, 0.46–1.56; P = .59) (Figure 2A). The cumulative incidence of CKD progression is shown in Figure 4B. Similarly, there was no difference in chronic eGFR slope decline between groups (Supplementary Table 2).
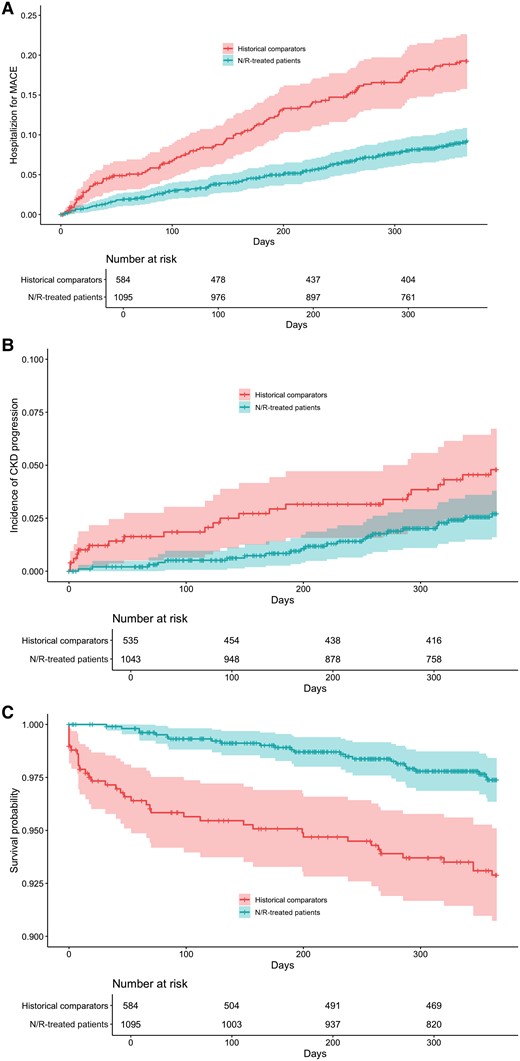
Adverse outcomes within 1 year of COVID-19. Cumulative incidence of hospitalization for MACE (A), CKD progression (B), and survival curve (C) in matched cohorts. Treatment with nirmatrelvir-ritonavir was associated with a decrease in the rate of hospitalization for MACE, but was not associated with a decrease in the risk of CKD progression, defined as doubling of creatinine or new diagnosis code for kidney failure within 365 days following baseline. Death within 1 year was significantly lower in patients who received nirmatrelvir-ritonavir. Shaded areas represent 95% CIs. Abbreviations: CKD, chronic kidney disease; COVID-19, coronavirus disease 2019; MACE, major adverse cardiovascular event.
Nirmatrelvir-ritonavir use was associated with a decreased risk of death within 1 year (adjusted hazard ratio [aHR], 0.37; 95% CI, 0.21–0.65; P < .01) (Figure 2A). One-year survival is shown in Figure 4C. The subgroup analysis demonstrated a significant reduction in the risk of death in patients with predialysis CKD and kidney failure (Figure 2A). However, beyond the acute phase, survival was not statistically significantly improved from day 31 to day 365 (aHR, 0.60; 95% CI, 0.31–1.13; P = .11). The survival curve from day 31 to day 365 is shown in Supplementary Figure 1.
The sensitivity analysis demonstrated a consistent association between nirmatrelvir-ritonavir and short- and long-term outcomes when restricted to Omicron era–only historical comparators. There were no deaths within 30 days in the nirmatrelvir-ritonavir-treated patients (n = 812) compared with 9 deaths (2%) within 30 days in the matched Omicron era comparators (n = 395). The risk of hospitalization within 30 days, MACE within 1 year, and risk of death within 1 year were all significantly reduced compared with Omicron era comparators; however, there was no association with CKD progression within 1 year (Figure 2B).
DISCUSSION
In this cohort study of 1679 patients with kidney disease who developed COVID-19, the majority (>90%) of whom had been vaccinated against COVID-19, we found that use of nirmatrelvir-ritonavir was associated with a decreased risk of both short- and long-term adverse health risks including hospitalization, MACE, and death when compared with propensity score–matched patients who tested positive for COVID-19 in the time period just before the EUA for nirmatrelvir-ritonavir.
Before widespread COVID-19 vaccination, rates of death among patients with kidney failure and COVID-19 were extremely high, ranging from 20% to 50% of hospitalized patients [23–26]. Rates of death have improved with the introduction of COVID-19 vaccines, but they remain high in patients with advanced kidney disease. Bell et al. found that the rate of death among fully ChAdOx1- or mRNA-vaccinated patients with kidney failure requiring kidney replacement therapy was 9.2%, and Torres et al. found that the rate of death among patients receiving chronic hemodialysis who had been fully vaccinated (BNT162b2) was 9.6% [27, 28]. Vaccine efficacy is limited by waning antibody responses and T-cell responses in patients with kidney failure [29, 30]. Anand et al. studied 4791 patients receiving hemodialysis and found that antibody responses to SARS-CoV-2 vaccination decreased rapidly in the months following vaccination; 1 in 5 patients had lost protective antibodies within 6 months of vaccination [31]. Furthermore, although primary vaccination rates among patients with kidney failure were high, current Centers for Disease Control and Prevention statistics show that <7% of patients receiving dialysis are currently up to date with COVID-19 vaccines [32]. This reinforces the need for antiviral therapies to decrease the residual risk of hospitalization and death in patients with CKD and kidney failure. Our study shows that among 584 matched, untreated historical comparators, 44 (8%) were hospitalized and 15 (3%) died within 30 days of infection.
The long-term adverse sequelae of COVID-19 are numerous, including effects on the neurologic, autonomic, reproductive, gastrointestinal, cardiovascular, and respiratory systems [33]. Xie et al. found that compared with control groups without COVID-19, patients who survived COVID-19 exhibited an increased burden of MACE when followed for 12 months [2]. Understanding whether treatments for COVID-19 reduce adverse long-term sequelae of COVID-19 is an important unmet need. In our study, 98 (17%) matched comparators were admitted for a MACE in the year following COVID-19 diagnosis, compared with 86 (8%) nirmatrelvir-ritonavir-treated patients, which corresponded to a fully adjusted 52% reduction in risk. This is in contrast to a recent analysis that did not detect a significant association between remdesivir use and MACE in patients with kidney disease who were hospitalized for COVID-19 [34]. We hypothesize that deploying outpatient antiviral strategies earlier in the disease course, before the development of severe COVID-19, may more effectively reduce the high rates of MACE. Our study is in line with a large study by Gu et al., who reported that nirmatrelvir-ritonavir reduced risk of both myocardial injury and MACE between 30 days and 16 months after COVID-19 diagnosis in 1083 patients hospitalized for COVID-19 during the Omicron wave vs 1452 propensity score–matched comparators; however, patients in this study had a mean baseline eGFR of 78 mL/min/1.73 m2; thus, the study did not include many patients with CKD [35].
In addition to MACE, adverse kidney outcomes are more common in patients who develop COVID-19. Various studies have suggested that the risk of acute kidney injury and CKD after COVID-19 exceeds the risk of other respiratory infections [36, 37]. Bowe et al. studied 1 726 683 veterans and demonstrated that survivors of COVID-19 exhibited higher risk of acute kidney injury, 50% eGFR decline, and kidney failure in the year following infection [3]. In addition, COVID-19 can trigger collapsing glomerulopathy in patients with a high-risk APOL1 genotype, which leads to extremely poor long-term kidney outcomes [38]. Disappointingly, we did not detect a protective effect of nirmatrevir-ritonavir use on the risk of CKD progression or slope of eGFR decline in the year following infection; however, our study does provide some reassurance that nirmatrelvir-ritonavir does not lead to detectable worsening of CKD progression or significant nephrotoxicity in patients with kidney disease.
Trials that led to the EUA for nirmatrelvir-ritonavir excluded patients with advanced kidney disease (eGFR < 30 mL/min per 1.73 m2 or kidney failure), so its use in this population has been restricted compared with the general population [5]. Early case reports suggested nirmatrelvir-ritonavir was safe and well tolerated in patients with advanced CKD and kidney failure [15–17]. Hiremath et al. studied 134 patients on dialysis who received nirmatrelvir-ritonavir and found that 6% were hospitalized for COVID-19, and none died within 30 days [18]. Cai et al. showed a marked reduction in all-cause death within 42 days of diagnosis (hazard ratio, 0.20; 95% CI, 0.06–0.65; P = .037) among hospitalized patients receiving nirmatrelvir-ritonavir compared with those who did not receive treatment; however, only 130 patients had an eGFR <60 mL/min per 1.73 m2 in this cohort [19]. In line with these studies, we demonstrated a 57% reduction in the risk of hospitalization within 30 days in patients who received nirmatrelvir-ritonavir compared with matched historical comparators; additionally, there were no deaths within 30 days in the matched patients who received nirmatrelvir-ritonavir. Taken together, the literature and our study suggest that nirmatrelvir-ritonavir may have a strong protective effect against hospitalization and death within the month following COVID-19 diagnosis among patients with CKD and kidney failure. Additionally, our work demonstrates the association between nirmatrelvir-ritonavir use and decreased adverse outcomes up to 1 year after COVID-19.
Our study has limitations. First, the historical comparator window represented both the Delta and Omicron waves, while the nirmatrelvir-ritonavir-treated patients were only exposed to the Omicron variant. This limitation was a byproduct of our choice to select historical comparators from the postvaccination era (July 2021–March 2022) before the approval of nirmatrelvir-ritonavir to avoid confounding by indication and minimize selection bias instead of using contemporary comparators who were not treated with nirmatrelvir-ritonavir. Importantly, our sensitivity analysis showed that the protective effect of nirmatrelvir-ritonavir was consistent when using only Omicron era comparators. There were no other significant changes to the standard of care management of COVID-19 during the study period. Second, though we matched and adjusted for vaccination status, time since most recent vaccination, and documented prior infections, the intervention group may still have had greater immunity at the time of the index infection due to more undocumented prior infections. This may cause some residual confounding of the observed reduction in severity in the intervention group. Third, historical comparators were identified via positive PCR testing, as we did not have access to the results of at-home antigen test results; patients who seek medical care and are diagnosed via RT-qPCR in a health care setting may be more ill than the general population testing at home via antigen testing, and we attempted to minimize this bias by matching for comorbidities and health care utilization and hospitalization in the year before infection. Fourth, patients may have been hospitalized outside our health care network in the year after infection, which would underestimate the risk of MACE; however, we matched patients based on the number of laboratory encounters and hospitalizations within in our health care network in the year before COVID-19 to minimize the risk that loss to follow-up was imbalanced between groups. Fifth, given the retrospective study design, we were not able to comprehensively ascertain adverse events or COVID-19 rebound, nor were we able to directly assess drug–drug interactions. Finally, because of the limitations of the retrospective study design and the fact that we were unable to reliably determine symptom onset even with manual chart review, we could not fully adopt the principles of target trial emulation, such as only including patients treated within 5 days of symptom onset. However, we did exclude comparators who were hospitalized within 2 days of diagnosis as they may have been too sick to obtain nirmatrelvir-ritonavir as an outpatient in order to minimize survival bias for treated patients.
In conclusion, use of nirmatrelvir-ritonavir in outpatients with CKD or kidney failure who were diagnosed with COVID-19 was associated with decreased risk of hospitalization and death within 30 days. The risk of hospitalization for MACE and death within 1 year was also decreased. As the role for nirmatrelvir-ritonavir in low-risk, vaccinated patients is clarified [13, 39], these results support its use in patients with CKD and kidney failure whose postvaccination risks of adverse events and death remain high, in order to decrease short- and long-term adverse health outcomes of COVID-19.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Data availability. Deidentified data are available upon reasonable request to Dr. Meghan Sise at [email protected] after execution of a data use agreement with Mass General Brigham.
Financial support. M.E.S. received funding from National Institutes of Health R03 DK128533.
References
Food and Drug Administration. FDA approves first oral antiviral for treatment of COVID-19 in adults 2023. Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-oral-antiviral-treatment-covid-19-adults#:~:text=Today%2C%20the%20 U.S.%20Food%20and,19%2C%20including%20hospitalization%20or%20death. Accessed April 24, 2024.
Author notes
Potential conflicts of interest. M.E.S. declares research funding from Angion, Otsuka, Gilead, Cabaletta, Novartis, EMD-Serono, Roche/Genetech, and Merck; has served on scientific advisory boards or had scientific consulting agreements with Vera, Travere, Calliditas, Mallinckrodt, Novartis, and Otsuka; and is a data safety monitoring committee member for Alpine Immune Sciences. S.Z. is the owner of Analytica Now. R.B. has received consulting fees from eMED, LLC (medical advisor, 2022–2023), and X-Biotix (scientific advisory board member, 2023–present). All other authors report no potential conflicts.





Comments