-
PDF
- Split View
-
Views
-
Cite
Cite
Andrea C Tello-Mercado, Bernardo A Martínez-Guerra, Carla M Román-Montes, Lisset Seoane-Hernandez, Andrea Rangel-Cordero, Rosa A Martínez-Gamboa, José Sifuentes-Osornio, Alfredo Ponce-de-León, María F González Lara, María D Niembro-Ortega, Histoplasmosis Beyond Human Immunodeficiency Virus (HIV): Clinical Characteristics and Outcomes in a Non-HIV Population, Open Forum Infectious Diseases, Volume 11, Issue 6, June 2024, ofae079, https://doi.org/10.1093/ofid/ofae079
Close - Share Icon Share
Abstract
Histoplasmosis is an endemic and invasive mycosis caused by Histoplasma capsulatum. We conducted a retrospective study comparing immunosuppressed patients without human immunodeficiency virus (HIV) with a historical cohort of people with HIV and histoplasmosis. We included 199 patients with proven or probable histoplasmosis, of which 25.1% were people without HIV. Diabetes mellitus, chronic kidney disease, hematologic neoplasms, rheumatologic diseases, and transplantations were more frequent among people without HIV (P < .01). Forty-four percent of immunocompromised patients without HIV died within the first 6-week period following their diagnosis. A high suspicion index for histoplasmosis should be kept in immunosuppressed patients.
Histoplasmosis, an invasive mycosis caused by Histoplasma capsulatum, is intensely endemic in the Ohio and Mississippi River valleys, the southeastern regions of Mexico, the Caribbean, and Central and South America. Increasing incidence in nonendemic zones has been described, and it is recently recognized as a highly prevalent AIDS-defining disease in Latin America, where it may surpass tuberculosis in some regions [1]. In Mexico, the annual incidence of 0.29/100 000 is considered a significant underestimation due to the lack of diagnostic tests and surveillance programs. Studies indicate that in the United States, the incidence rate is significantly higher, reaching 3.4/100 000 inhabitants [2]. As with other endemic diseases, climate change can contribute to histoplasmosis cases in extreme latitudes, and exposure history may be difficult to identify in nonendemic regions [3].
Historically, the data and research have been of relevance in people with HIV/AIDS (PWH), who usually present with disseminated infection, resulting in poor outcomes. However, with increasing incidence in other groups susceptible for disseminated disease, such as transplant recipients, people with hematological neoplasms or rheumatic diseases, or steroid users, the true burden is unknown [4–6]. Few studies compare clinical presentation according to immune status [6–8].
We aimed to outline the clinical characteristics and outcomes of histoplasmosis among the non-HIV-infected population at a tertiary center in Mexico City.
METHODS
Study Population
We performed a retrospective and transverse study including all consecutive non-HIV-infected immunosuppressed adult hospitalized patients with proven or probable histoplasmosis from 1 January 2000 to 31 December 2021. Our center is a tertiary care center in Mexico City, serving as a referral center for patients with chronic diseases, patients with autoimmune disease or cancer, and transplant recipients. It has 227 hospital beds, offering 5166 in-hospital consultations and >240 000 outpatient visits during 2022.
Other immunocompromised (OIC) patients without HIV were defined by the presence of any of the following: rheumatic disease, diabetes, chronic lung disease, chronic kidney disease, cirrhosis, active cancer (solid/hematologic), chemotherapy, solid organ or stem-cell transplant, or immunosuppressive medications.
Inclusion Criteria
Patients in the non-HIV cohort who were 18 years or older were selected if they were diagnosed with histoplasmosis based on any of the following criteria: microbiologic isolation of H capsulatum from any source, histopathologic identification of H capsulatum, or positive Histoplasma antigen from urine or serum. Data such as comorbidities, type of immunosuppression, clinical manifestations, laboratory results, imaging findings, and overall mortality after diagnosis were recovered from the medical records. None were excluded. For comparison, data on PWH were obtained from the database of a prospective multicenter diagnostic test study conducted in our center between December 2015 and April 2018. In brief, Martínez-Gamboa et al included PWH with suspected progressive disseminated histoplasmosis (PDH) defined as 3 or more of the following criteria: fever, weight loss, lymphadenopathy, hepatomegaly, splenomegaly, mucous or skin lesions, bicytopenia, or increased aspartate aminotransferase, lactate dehydrogenase, and/or ferritin. Among 415 eligible patients, 149 were included in this study: 108 had proven PDH, and 41 had probable PDH. The rest, who did not meet the definition of proven or probable PDH or had another opportunistic infection, were excluded.
Proven histoplasmosis was considered when compatible histopathologic findings or direct microscopy of specimens from an affected site showed characteristic yeasts, or after recovery by culture of H capsulatum of clinical specimens from affected sites. Probable histoplasmosis was considered when there was a compatible clinical presentation with a positive Histoplasma urine antigen test [9].
Localized pulmonary histoplasmosis was considered when isolated lung involvement was found. Disseminated histoplasmosis was defined as extrapulmonary involvement such as blood, bone marrow, skin, liver, adrenal gland, extrapulmonary lymph nodes, or central nervous system. The outcome was defined all-cause mortality at 6 weeks.
Ethical Considerations
The design of the work was approved by the local human ethics and research committees (INF-3691-21-22-1).
Patient Consent
Need for informed consent was waived due to the retrospective nature of the study upon approval by the local human ethics and research committees. For every PWH, written informed consent was obtained.
Statistical Analysis
Our primary outcome was to compare the clinical, diagnostic, and outcome differences in OIC patients without HIV compared to PWH. We used descriptive statistics for patient demographic and clinical variables. For comparison between groups, Student t, Mann-Whitney U, Pearson χ2, or Fisher exact tests were used. Statistical analysis was performed using Stata 14/MP software (StataCorp LLC, College Station, Texas).
RESULTS
From January 2000 to December 2021, 199 histoplasmosis cases were identified; 50 (25%) were OIC and 149 (75%) were PWH. Figure 1 shows the geographic distribution according to the place of residency at the time of diagnosis. Patients with histoplasmosis were originally from central and southern Mexico. The median age was 39.5 (interquartile range [IQR], 27–54) years in the OIC group and 34 (IQR, 27–40) years in the PWH group (P = .03). PWH were more often men (85% vs 48%, P < .01; Table 1).

Geographic distribution of 199 histoplasmosis cases considering place of residence at diagnosis.
Comparison of Baseline Characteristics Between People With Human Immunodeficiency Virus (HIV) and Non-HIV Patients With Histoplasmosis
| Baseline Characteristic . | Overall (N = 199) . | HIV (n = 149) . | Non-HIV (n = 50) . | P Value . |
|---|---|---|---|---|
| Age, y, median (IQR) | 34 (27–42) | 34 (27–40) | 39.5 (27–54) | .03 |
| Male sex | 151 (76) | 127 (85) | 24 (48) | <.01a |
| Comorbidities | ||||
| Diabetes | 8 (4) | 1 (0.7) | 7 (14) | <.01a |
| Chronic lung disease | 1 (0.5) | 0 | 1 (2) | .08a |
| Chronic kidney disease | 13 (7) | 1 (0.7) | 12 (24) | <.01a |
| Cirrhosis | 2 (1) | 0 | 2 (4) | .01a |
| Solid malignant neoplasm | 4 (2) | 2 (1) | 2 (4) | .26a |
| Hematologic neoplasm | 9 (5) | 1 (0.7) | 8 (16) | <.01a |
| Rheumatic disease | 22 (11) | 0 | 22 (44) | <.01a |
| Kidney transplant | 10 (5) | 0 | 10 (20) | <.01a |
| Immunosuppressorsb | 52 (26) | 10 (7) | 42 (84) | <.01a |
| Corticosteroids | 43 (22) | 8 (5) | 35 (70) | <.01a |
| Azathioprine | 9 (4.5) | 0 | 9 (18) | <.01a |
| Hydroxychloroquine | 6 (3) | 0 | 6 (12) | <.01a |
| Calcineurin inhibitor | 10 (5) | 0 | 10 (20) | <.01a |
| Mycophenolate mofetil | 13 (6.5) | 0 | 13 (26) | <.01a |
| Methotrexate | 7 (3.5) | 0 | 7 (14) | <.01a |
| Chemotherapy | 10 (5) | 2 (1) | 8 (16) | <.01a |
| Baseline Characteristic . | Overall (N = 199) . | HIV (n = 149) . | Non-HIV (n = 50) . | P Value . |
|---|---|---|---|---|
| Age, y, median (IQR) | 34 (27–42) | 34 (27–40) | 39.5 (27–54) | .03 |
| Male sex | 151 (76) | 127 (85) | 24 (48) | <.01a |
| Comorbidities | ||||
| Diabetes | 8 (4) | 1 (0.7) | 7 (14) | <.01a |
| Chronic lung disease | 1 (0.5) | 0 | 1 (2) | .08a |
| Chronic kidney disease | 13 (7) | 1 (0.7) | 12 (24) | <.01a |
| Cirrhosis | 2 (1) | 0 | 2 (4) | .01a |
| Solid malignant neoplasm | 4 (2) | 2 (1) | 2 (4) | .26a |
| Hematologic neoplasm | 9 (5) | 1 (0.7) | 8 (16) | <.01a |
| Rheumatic disease | 22 (11) | 0 | 22 (44) | <.01a |
| Kidney transplant | 10 (5) | 0 | 10 (20) | <.01a |
| Immunosuppressorsb | 52 (26) | 10 (7) | 42 (84) | <.01a |
| Corticosteroids | 43 (22) | 8 (5) | 35 (70) | <.01a |
| Azathioprine | 9 (4.5) | 0 | 9 (18) | <.01a |
| Hydroxychloroquine | 6 (3) | 0 | 6 (12) | <.01a |
| Calcineurin inhibitor | 10 (5) | 0 | 10 (20) | <.01a |
| Mycophenolate mofetil | 13 (6.5) | 0 | 13 (26) | <.01a |
| Methotrexate | 7 (3.5) | 0 | 7 (14) | <.01a |
| Chemotherapy | 10 (5) | 2 (1) | 8 (16) | <.01a |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range.
aP values for categorical variables were calculated with Fisher exact test (comparison of HIV vs non-HIV).
bImmunosuppressive regimens may include >1 drug.
Comparison of Baseline Characteristics Between People With Human Immunodeficiency Virus (HIV) and Non-HIV Patients With Histoplasmosis
| Baseline Characteristic . | Overall (N = 199) . | HIV (n = 149) . | Non-HIV (n = 50) . | P Value . |
|---|---|---|---|---|
| Age, y, median (IQR) | 34 (27–42) | 34 (27–40) | 39.5 (27–54) | .03 |
| Male sex | 151 (76) | 127 (85) | 24 (48) | <.01a |
| Comorbidities | ||||
| Diabetes | 8 (4) | 1 (0.7) | 7 (14) | <.01a |
| Chronic lung disease | 1 (0.5) | 0 | 1 (2) | .08a |
| Chronic kidney disease | 13 (7) | 1 (0.7) | 12 (24) | <.01a |
| Cirrhosis | 2 (1) | 0 | 2 (4) | .01a |
| Solid malignant neoplasm | 4 (2) | 2 (1) | 2 (4) | .26a |
| Hematologic neoplasm | 9 (5) | 1 (0.7) | 8 (16) | <.01a |
| Rheumatic disease | 22 (11) | 0 | 22 (44) | <.01a |
| Kidney transplant | 10 (5) | 0 | 10 (20) | <.01a |
| Immunosuppressorsb | 52 (26) | 10 (7) | 42 (84) | <.01a |
| Corticosteroids | 43 (22) | 8 (5) | 35 (70) | <.01a |
| Azathioprine | 9 (4.5) | 0 | 9 (18) | <.01a |
| Hydroxychloroquine | 6 (3) | 0 | 6 (12) | <.01a |
| Calcineurin inhibitor | 10 (5) | 0 | 10 (20) | <.01a |
| Mycophenolate mofetil | 13 (6.5) | 0 | 13 (26) | <.01a |
| Methotrexate | 7 (3.5) | 0 | 7 (14) | <.01a |
| Chemotherapy | 10 (5) | 2 (1) | 8 (16) | <.01a |
| Baseline Characteristic . | Overall (N = 199) . | HIV (n = 149) . | Non-HIV (n = 50) . | P Value . |
|---|---|---|---|---|
| Age, y, median (IQR) | 34 (27–42) | 34 (27–40) | 39.5 (27–54) | .03 |
| Male sex | 151 (76) | 127 (85) | 24 (48) | <.01a |
| Comorbidities | ||||
| Diabetes | 8 (4) | 1 (0.7) | 7 (14) | <.01a |
| Chronic lung disease | 1 (0.5) | 0 | 1 (2) | .08a |
| Chronic kidney disease | 13 (7) | 1 (0.7) | 12 (24) | <.01a |
| Cirrhosis | 2 (1) | 0 | 2 (4) | .01a |
| Solid malignant neoplasm | 4 (2) | 2 (1) | 2 (4) | .26a |
| Hematologic neoplasm | 9 (5) | 1 (0.7) | 8 (16) | <.01a |
| Rheumatic disease | 22 (11) | 0 | 22 (44) | <.01a |
| Kidney transplant | 10 (5) | 0 | 10 (20) | <.01a |
| Immunosuppressorsb | 52 (26) | 10 (7) | 42 (84) | <.01a |
| Corticosteroids | 43 (22) | 8 (5) | 35 (70) | <.01a |
| Azathioprine | 9 (4.5) | 0 | 9 (18) | <.01a |
| Hydroxychloroquine | 6 (3) | 0 | 6 (12) | <.01a |
| Calcineurin inhibitor | 10 (5) | 0 | 10 (20) | <.01a |
| Mycophenolate mofetil | 13 (6.5) | 0 | 13 (26) | <.01a |
| Methotrexate | 7 (3.5) | 0 | 7 (14) | <.01a |
| Chemotherapy | 10 (5) | 2 (1) | 8 (16) | <.01a |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range.
aP values for categorical variables were calculated with Fisher exact test (comparison of HIV vs non-HIV).
bImmunosuppressive regimens may include >1 drug.
In the OIC group, at diagnosis, 42 of 50 patients (84%) were on immunosuppressors, of which 70% were corticosteroids, 26% (13/50) mycophenolate mofetil, 18% (9/50) azathioprine, 16% (8/50) cancer chemotherapy, 14% (7/50) methotrexate, and 20% (10/50) calcineurin inhibitors. The most frequent comorbidities were autoimmune diseases in 22 (44%), of which 14 had systemic erythematous lupus; chronic kidney disease in 12 (24%); and kidney transplant recipients in 10 (20%), of which acute rejection occurred in 8 of 10 (80%) prior to histoplasmosis and the median time from transplant was 81.1 (IQR, 16–189) months.
Among PWH, 57% of patients (85/149) were naive to antiretroviral treatment. The median CD4 count was 31.2 (IQR, 3–69) cells/μL, and 66% of patients had CD4 count <50 cells/μL.
Clinical Presentation and Imaging
Fever (99% vs 76%), weight loss (92% vs 28%), diarrhea (53% vs 32%), adenopathy (56% vs 32%), and dyspnea (56% vs 32%) were more frequent in PWH (P < .05), whereas splenomegaly was more frequent in OIC (54% vs 32%, P < .01). Pancytopenia was found in 29 of 199 (15%) overall. In PWH, aspartate aminotransferase (54% vs 27%, P < .01) and lactate dehydrogenase (64% vs 25%, P < .01) were more frequently elevated. Pulmonary imaging abnormalities (consolidation, nodules, ground glass opacities, pleural effusion, and mediastinal adenopathy) were more frequent in the OIC group (Figure 2).
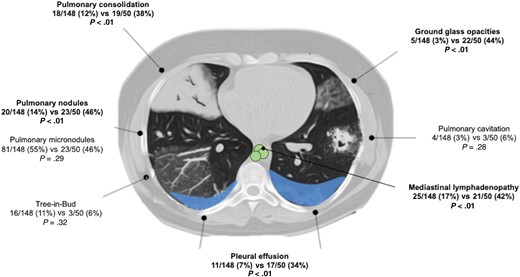
Pulmonary imaging findings comparing patients living with HIV vs OIC patients without HIV.
In the OIC group, 49 of 50 (98%) had proven histoplasmosis and 1 had a probable case; 46 of 50 (92%) had disseminated histoplasmosis. In the PWH group, 108 of 149 (72%) cases were proven and 41 of 149 (28%) were probable; all had disseminated histoplasmosis.
Forty-nine patients (96%) in the OIC group had a positive culture with H capsulatum compared to 64% of PWH (P < .01). The most frequent isolation site was blood (14/49), followed by bone marrow (13/49) and lung (10/49). Only 10% of patients (4/49) had a positive skin culture. Histopathologic confirmation (32% vs 73%, P ≤ .01) was more common in PWH. Urinary antigen positivity was found in 121 of 145 (83%) PWH and 6 of 7 (86%) in the OIC group (Table 2).
Comparison of Laboratory and Diagnostic Tests Between People With Human Immunodeficiency Virus (HIV) and Non-HIV Patients With Histoplasmosis
| Baseline Characteristic . | Overall (N = 199) . | HIV (n = 149) . | Non-HIV (n = 50) . | P Value . |
|---|---|---|---|---|
| Laboratory tests | ||||
| Hemoglobin, mg/dLa | 9.4 (7.8–11.2) | 9.4 (7.5–11.1) | 9.8 (7.9–12.1) | .08b |
| Leukocyte count, ×103/µLa | 3.6 (2.2–6.0) | 3.4 (2.2–5.7) | 4.5 (2.4–6.7) | .38b |
| Platelets, ×103/µLa | 101 (54–213) | 96 (59–204) | 130 (47–248) | .67b |
| ALT, U/La | 44 (26–67), n = 188 | 44 (25–68), n = 139 | 43 (28–63), n = 49 | .79b |
| AST, U/La | 71 (41–175), n = 191 | 89 (49–208), n = 142 | 43 (29–80), n = 49 | <.01b |
| Total bilirubin, mg/dLa | 0.7 (0.5–1.3), n = 187 | 0.7 (0.5–1.3), n = 138 | 0.74 (0.54–1.3), n = 49 | .49b |
| ALP, median, U/La | 171 (92–353), n = 183 | 194 (97–374), n = 134 | 121 (84–291), n = 49 | .03b |
| Albumin, g/dLa | 2.4 (1.8–2.9), n = 176 | 2.3 (1.7–2.7), n = 127 | 2.9 (2.0–3.6), n = 49 | <.01b |
| GGT, U/La | 129 (57–253), n = 87) | 126 (51–253), n = 76 | 215 (96–312), n = 11 | .20b |
| ESR, mm/ha | 27 (12–66), n = 39 | 28 (14–64), n = 21 | 25 (11–66), n = 18 | .77b |
| CRP, mg/dLa | 15.1 (6.7–33), n = 55 | 25.3 (12.2–112.0), n = 34 | 9.2 (3.8–12.8), n = 21 | <.01b |
| LDH, U/L | 629 (320–1465), n = 169 | 780 (381–1860), n = 130 | 332 (210–606), n = 39 | <.01b |
| Ferritin, ng/mL | 1501 (455–3673), n = 40 | 1759 (977–2092), n = 21 | 863 (166–14 479), n = 19 | .38b |
| Diagnostic tests | ||||
| Positive UAg | 127/152 (86) | 121/145 (83) | 6/7 (86) | .087c |
| Blood culture | 87/173 (50) | 73/141 (52) | 14/32 (44) | .41c |
| Bone marrow culture | 162/198 (82) | 142/149 (95.3) | 20/49 (41) | <.01c |
| Histopathology | 53/99 (53.5) | 42/68 (62) | 11/31 (35.5) | .02c |
| Baseline Characteristic . | Overall (N = 199) . | HIV (n = 149) . | Non-HIV (n = 50) . | P Value . |
|---|---|---|---|---|
| Laboratory tests | ||||
| Hemoglobin, mg/dLa | 9.4 (7.8–11.2) | 9.4 (7.5–11.1) | 9.8 (7.9–12.1) | .08b |
| Leukocyte count, ×103/µLa | 3.6 (2.2–6.0) | 3.4 (2.2–5.7) | 4.5 (2.4–6.7) | .38b |
| Platelets, ×103/µLa | 101 (54–213) | 96 (59–204) | 130 (47–248) | .67b |
| ALT, U/La | 44 (26–67), n = 188 | 44 (25–68), n = 139 | 43 (28–63), n = 49 | .79b |
| AST, U/La | 71 (41–175), n = 191 | 89 (49–208), n = 142 | 43 (29–80), n = 49 | <.01b |
| Total bilirubin, mg/dLa | 0.7 (0.5–1.3), n = 187 | 0.7 (0.5–1.3), n = 138 | 0.74 (0.54–1.3), n = 49 | .49b |
| ALP, median, U/La | 171 (92–353), n = 183 | 194 (97–374), n = 134 | 121 (84–291), n = 49 | .03b |
| Albumin, g/dLa | 2.4 (1.8–2.9), n = 176 | 2.3 (1.7–2.7), n = 127 | 2.9 (2.0–3.6), n = 49 | <.01b |
| GGT, U/La | 129 (57–253), n = 87) | 126 (51–253), n = 76 | 215 (96–312), n = 11 | .20b |
| ESR, mm/ha | 27 (12–66), n = 39 | 28 (14–64), n = 21 | 25 (11–66), n = 18 | .77b |
| CRP, mg/dLa | 15.1 (6.7–33), n = 55 | 25.3 (12.2–112.0), n = 34 | 9.2 (3.8–12.8), n = 21 | <.01b |
| LDH, U/L | 629 (320–1465), n = 169 | 780 (381–1860), n = 130 | 332 (210–606), n = 39 | <.01b |
| Ferritin, ng/mL | 1501 (455–3673), n = 40 | 1759 (977–2092), n = 21 | 863 (166–14 479), n = 19 | .38b |
| Diagnostic tests | ||||
| Positive UAg | 127/152 (86) | 121/145 (83) | 6/7 (86) | .087c |
| Blood culture | 87/173 (50) | 73/141 (52) | 14/32 (44) | .41c |
| Bone marrow culture | 162/198 (82) | 142/149 (95.3) | 20/49 (41) | <.01c |
| Histopathology | 53/99 (53.5) | 42/68 (62) | 11/31 (35.5) | .02c |
Abbreviations: ALT, alanine aminotransferase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; GGT, γ-glutamyl transferase; HIV, human immunodeficiency virus; LDH, lactate dehydrogenase; UAg, urine antigen.
aAll laboratory data are presented as median with interquartile range.
bP values for quantitative variables were calculated using the Mann-Whitney U test.
cP values for categorical variables were calculated with χ2 test (comparison of HIV vs non-HIV).
Comparison of Laboratory and Diagnostic Tests Between People With Human Immunodeficiency Virus (HIV) and Non-HIV Patients With Histoplasmosis
| Baseline Characteristic . | Overall (N = 199) . | HIV (n = 149) . | Non-HIV (n = 50) . | P Value . |
|---|---|---|---|---|
| Laboratory tests | ||||
| Hemoglobin, mg/dLa | 9.4 (7.8–11.2) | 9.4 (7.5–11.1) | 9.8 (7.9–12.1) | .08b |
| Leukocyte count, ×103/µLa | 3.6 (2.2–6.0) | 3.4 (2.2–5.7) | 4.5 (2.4–6.7) | .38b |
| Platelets, ×103/µLa | 101 (54–213) | 96 (59–204) | 130 (47–248) | .67b |
| ALT, U/La | 44 (26–67), n = 188 | 44 (25–68), n = 139 | 43 (28–63), n = 49 | .79b |
| AST, U/La | 71 (41–175), n = 191 | 89 (49–208), n = 142 | 43 (29–80), n = 49 | <.01b |
| Total bilirubin, mg/dLa | 0.7 (0.5–1.3), n = 187 | 0.7 (0.5–1.3), n = 138 | 0.74 (0.54–1.3), n = 49 | .49b |
| ALP, median, U/La | 171 (92–353), n = 183 | 194 (97–374), n = 134 | 121 (84–291), n = 49 | .03b |
| Albumin, g/dLa | 2.4 (1.8–2.9), n = 176 | 2.3 (1.7–2.7), n = 127 | 2.9 (2.0–3.6), n = 49 | <.01b |
| GGT, U/La | 129 (57–253), n = 87) | 126 (51–253), n = 76 | 215 (96–312), n = 11 | .20b |
| ESR, mm/ha | 27 (12–66), n = 39 | 28 (14–64), n = 21 | 25 (11–66), n = 18 | .77b |
| CRP, mg/dLa | 15.1 (6.7–33), n = 55 | 25.3 (12.2–112.0), n = 34 | 9.2 (3.8–12.8), n = 21 | <.01b |
| LDH, U/L | 629 (320–1465), n = 169 | 780 (381–1860), n = 130 | 332 (210–606), n = 39 | <.01b |
| Ferritin, ng/mL | 1501 (455–3673), n = 40 | 1759 (977–2092), n = 21 | 863 (166–14 479), n = 19 | .38b |
| Diagnostic tests | ||||
| Positive UAg | 127/152 (86) | 121/145 (83) | 6/7 (86) | .087c |
| Blood culture | 87/173 (50) | 73/141 (52) | 14/32 (44) | .41c |
| Bone marrow culture | 162/198 (82) | 142/149 (95.3) | 20/49 (41) | <.01c |
| Histopathology | 53/99 (53.5) | 42/68 (62) | 11/31 (35.5) | .02c |
| Baseline Characteristic . | Overall (N = 199) . | HIV (n = 149) . | Non-HIV (n = 50) . | P Value . |
|---|---|---|---|---|
| Laboratory tests | ||||
| Hemoglobin, mg/dLa | 9.4 (7.8–11.2) | 9.4 (7.5–11.1) | 9.8 (7.9–12.1) | .08b |
| Leukocyte count, ×103/µLa | 3.6 (2.2–6.0) | 3.4 (2.2–5.7) | 4.5 (2.4–6.7) | .38b |
| Platelets, ×103/µLa | 101 (54–213) | 96 (59–204) | 130 (47–248) | .67b |
| ALT, U/La | 44 (26–67), n = 188 | 44 (25–68), n = 139 | 43 (28–63), n = 49 | .79b |
| AST, U/La | 71 (41–175), n = 191 | 89 (49–208), n = 142 | 43 (29–80), n = 49 | <.01b |
| Total bilirubin, mg/dLa | 0.7 (0.5–1.3), n = 187 | 0.7 (0.5–1.3), n = 138 | 0.74 (0.54–1.3), n = 49 | .49b |
| ALP, median, U/La | 171 (92–353), n = 183 | 194 (97–374), n = 134 | 121 (84–291), n = 49 | .03b |
| Albumin, g/dLa | 2.4 (1.8–2.9), n = 176 | 2.3 (1.7–2.7), n = 127 | 2.9 (2.0–3.6), n = 49 | <.01b |
| GGT, U/La | 129 (57–253), n = 87) | 126 (51–253), n = 76 | 215 (96–312), n = 11 | .20b |
| ESR, mm/ha | 27 (12–66), n = 39 | 28 (14–64), n = 21 | 25 (11–66), n = 18 | .77b |
| CRP, mg/dLa | 15.1 (6.7–33), n = 55 | 25.3 (12.2–112.0), n = 34 | 9.2 (3.8–12.8), n = 21 | <.01b |
| LDH, U/L | 629 (320–1465), n = 169 | 780 (381–1860), n = 130 | 332 (210–606), n = 39 | <.01b |
| Ferritin, ng/mL | 1501 (455–3673), n = 40 | 1759 (977–2092), n = 21 | 863 (166–14 479), n = 19 | .38b |
| Diagnostic tests | ||||
| Positive UAg | 127/152 (86) | 121/145 (83) | 6/7 (86) | .087c |
| Blood culture | 87/173 (50) | 73/141 (52) | 14/32 (44) | .41c |
| Bone marrow culture | 162/198 (82) | 142/149 (95.3) | 20/49 (41) | <.01c |
| Histopathology | 53/99 (53.5) | 42/68 (62) | 11/31 (35.5) | .02c |
Abbreviations: ALT, alanine aminotransferase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; GGT, γ-glutamyl transferase; HIV, human immunodeficiency virus; LDH, lactate dehydrogenase; UAg, urine antigen.
aAll laboratory data are presented as median with interquartile range.
bP values for quantitative variables were calculated using the Mann-Whitney U test.
cP values for categorical variables were calculated with χ2 test (comparison of HIV vs non-HIV).
Disseminated disease was present in all PWH and 92% of the OIC patients. Among OIC patients, 41 (82%) received antifungal treatment—24 with deoxycholate amphotericin B, 16 with itraconazole, and 1 with liposomal amphoterin B and voriconazole. Among untreated patients, 8 died before diagnosis and 1 received an echinocandin for suspected invasive fungal infection. The median treatment duration was 12 (IQR, 1–12) months. Among the OIC, 22 of 50 (44%) died at 6 weeks. Two had localized pulmonary histoplasmosis, while the rest had disseminated disease.
DISCUSSION
We describe a large cohort of patients with histoplasmosis and compare the main differences regarding presentation, diagnosis, and outcome among PWH and patients with OIC. Histoplasmosis in immunocompromised people without HIV presented with fewer clinical manifestations and abnormal laboratory findings. HIV status did not affect the outcome in patients with histoplasmosis.
The most frequent cause for immunosuppression in the OIC patients was pharmacological, mostly corticosteroids followed by various drugs, reflecting the diversity of comorbidities in this group. Contemporary studies of histoplasmosis in the United States describe a transition with decreasing histoplasmosis-related hospitalizations in PWH with increasing frequency of transplantation, conditions treated with biologic agents, and diabetes [7, 10].
Although the Transplant-Associated Infection Surveillance Network (TRANSNET) multicenter prospective study found an overall reduced incidence of endemic mycoses in solid organ transplant recipients, compared to other invasive fungal infections, the bimodal distribution with 34% of cases occurring >2 years post transplantation should raise awareness for early diagnosis and treatment [11]. In this study, most kidney transplant recipients were treated for acute rejection prior to histoplasmosis, which has not been consistently found [11, 12].
Previous cohorts have described the same percentage of patients on steroids. However, few of those studies have included rheumatologic patients [6–8]. Our findings are consistent with the literature, where autoimmune diseases nowadays represent the most common underlying disease in patients with histoplasmosis [10–13]. Of note, our center is a reference for this group of patients.
Regarding PWH, the majority had a CD4 cell count <50 cells/μL and a median CD4 count of 31.2 cells/μL. Previous studies comparing different immune status included patients with advanced HIV infection who had median CD4 counts of 15–53 cells/μL [14–16]. Our HIV population was from the study by Martínez-Gamboa et al, where they included patients with suspected PDH, which may be a bias in our comparison group [17]. In contrast to studies from the United States where HIV-associated histoplasmosis may be declining, a recent modeling study estimated that the burden of histoplasmosis may be surpassing tuberculosis in hotspots of Central and South America. A high frequency of late HIV diagnosis, in addition to the lack of diagnostic laboratory capacity for mycological diagnosis, contributes to increased burden in this region [18].
The clinical picture of disseminated disease often consists of nonspecific symptoms such as fever, fatigue, night sweats, and weight loss. Despite the fact that most of the included patients had disseminated histoplasmosis, we found an increased burden of clinical manifestations and laboratory abnormalities among PWH compared to patients with OIC, as previously described [7]. Systemic corticosteroids may blunt the systemic inflammatory response syndrome. Other studies did not find differences according to HIV status [15, 16, 19].
Widespread urine antigen testing has resulted in improved diagnosis with the potential for earlier diagnosis and improved outcome. However, its introduction has lagged in Latin America, where until 2019, 43% countries in the region had access to antigen testing. The majority still depend on culture-based diagnosis, probably resulting in late diagnosis [20, 21]. In this study, the comparator group of PWH who had access to urine antigen testing resulted in 83% positivity, compared to the standard local practice at our center during the study period for the OIC group, in whom a single case had a positive result. Of note, several studies have shown high sensitivity for disseminated disease in various immunocompromised patients [7].
We observed an increased mortality (44%) among OIC patients compared to other series. In those series, immunocompromised people without HIV exhibited a mortality of 23% and 26% with disseminated and localized disease, respectively [7, 8].
Histoplasma is usually found in tropical and subtropical regions in areas with high soil humidity, especially where temperatures are 22°C–29°C and annual rainfall is 35–50 inches [22]. Mexico experiences significant regional variations in climate due to its diverse topography and geographic positioning. Temperatures range from 15°C–20°C in the central uplands to 23°C–27°C in the coastal lowlands, with minimal seasonal temperature fluctuations in the south. Rainfall patterns differ across the southern regions and central highlands, featuring distinct wet seasons during specific months of the year. Different environmental conditions, such as soil acidity, land cover, and distance from water, may influence where Histoplasma is found.
Although the endemic zone for histoplasmosis in Mexico is not fully described, because it does not require obligatory reporting to health authorities, most reported cases and outbreaks occur in the central and southeast regions [23]. In this study, many patients were originally from states in the central and western regions, particularly from the state of Jalisco, which has not been previously considered an endemic zone. Although data on occupational exposure or travel are lacking, this study contributes to increasing awareness about histoplasmosis within the Mexican territory. It is likely that reporting bias and reduced access to mycological diagnosis preclude estimating the true burden of the disease. Given the changing epidemiology and the increasing number of histoplasmosis cases in nonendemic areas, the information collected and the map presented in the present study may be of particular interest to clinicians working with immunosuppressed HIV-negative patients.
We recognize several limitations due to the retrospective nature of the study. A major limitation is the missing data on mortality, which precluded comparison between groups. This is because data on PWH were obtained from the database of a prospective diagnostic study as previously described, which did not systematically assess mortality within a specific timeframe. Therefore, it was possible only to compare the mortality of OIC patients in this study with published reports. Also, we included 2 cohorts within distinct timeframes. OIC patients were included from 2000 to 2021, whereas PWH were included from 2015 to 2018; during this timeframe, local practices may have evolved in our institution, such as widespread availability of liposomal amphotericin B since 2018 (previously, amphotericin deoxycholate was the only available formulation). The clarus Histoplasma GM immune assay (IMMY, Norman, Oklahoma) for urine antigen detection was locally available since 2019. Previously, diagnosis was limited to culture confirmation and compatible histopathologic results, which may have led to delayed diagnosis, antifungal treatment, and worse prognosis. Also, the introduction of novel immunotherapies for cancer chemotherapies results in heterogeneity among the OIC group.
Moreover, heterogeneous subpopulations of OIC preclude comparison between groups. Also, the lack of detailed information regarding mortality and antifungal treatment in the PWH group limited further analysis. During most of the study period, urinary Histoplasma antigen was not available for routine evaluation.
Despite limitations, this is a large study comparing the clinical presentation according to HIV status. Recently, the mortality and incidence rates of histoplasmosis in Latin America are equivalent or even surpass tuberculosis in certain hotspots [24]. Along with the expanding population of immunocompromised individuals and the neglected status of the disease, our findings are relevant to increase awareness and emphasize the need for urine antigen accessibility in our region.
CONCLUSIONS
Immunosuppressed patients with disseminated histoplasmosis presented with fewer clinical and laboratory abnormalities compared to PWH. Pharmacological immunosuppression and rheumatic diseases were frequent. These unspecific findings may delay diagnosis in this patient population and increase mortality. Clinicians should be highly suspicious of histoplasmosis and strive for an accurate and rapid diagnosis.
Notes
Acknowledgments. We appreciate and acknowledge all microbiology laboratory personnel who support us in the processing and analysis for diagnosis and all the doctors involved in caring for patients with fungal infections.
Author contributions. M.D.N.O., M.F.G.L., B.A.M.G., A.C.T.M., C.M.R.M., and A.P.D.L. conceived the research question and study design. M.D.N.O., A.C.T.M., and L.S.H. curated the data and developed the study. A.R.C. and R.A.M.G. performed and reviewed all laboratory studies. L.S.H., A.C.T.M., and M.F.G.L. performed the analysis. J.S.O. and A.P.D.L. reviewed and supervised the study. All authors contributed to and revised the final manuscript.
Data availability. The data used for this study are from a healthcare system database maintained by local research and can be obtained by writing to the corresponding authors.
Financial support. A local institutional resource from the Infectious Diseases Department at INCMNSZ provided support for this work.
References
Author notes
Potential conflicts of interest. All authors: No reported conflicts.





Comments