-
PDF
- Split View
-
Views
-
Cite
Cite
Ryan P Moenster, Ashleigh Wallace-Lacey, Hannah Western, Seth Tiefenaur, Anosha Abdulbasir, Justin Alberts, Jonathan Doty, Hartley Abner, Danielle Skouby, Michael Lorenz, Rebecca Fong, Jyoti Arora, Travis W Linneman, Oritavancin vs Standard of Care for Treatment of Nonendovascular Gram-Positive Bloodstream Infections, Open Forum Infectious Diseases, Volume 10, Issue 11, November 2023, ofad411, https://doi.org/10.1093/ofid/ofad411
Close - Share Icon Share
Abstract
Data is limited comparing oritavancin (ORT) to the standard-of-care (SOC) for the treatment gram-positive blood stream infections (BSI).
This was a retrospective study of all patients in the Veteran's Affairs Health Care System treated with at least 1 dose of oritavancin or at least 5 days of vancomycin, daptomycin, ceftaroline, ampicillin, ampicillin-sulbactam, nafcillin, oxacillin, or cefazolin for a documented gram-positive BSI from 1 January 2015 to 30 June 2021. Patients with polymicrobial blood cultures or positive cultures from other sites were included if the organisms were sensitive to the incident antimicrobial; no concomitant antimicrobials could be used once the incident agent was started. Individuals were also excluded if they were diagnosed with endocarditis, had a neutrophil count 96-hours of treatment before the incident antimicrobial was started.
The primary composite outcome was clinical failure, defined as all-cause mortality within 30-days from the end of therapy, or blood cultures positive for the incident organisms ≥72 hours after administration of the first dose and ≤30 days after the administration of the final dose of the study antimicrobial, or any drug or line-related readmissions within 30-days of hospital discharge.
Two hundred-forty patients were identified for screening with 96 meeting criteria (27 in ORT and 69 in SOC groups). Baseline characteristics were generally balanced between groups except more patients in the ORT group received >96-hours of treatment before the incident antimicrobial was started (70.3% (19/27) vs 13.04% 9/69); P < .001). The pathogen most prevalent was methicillin susceptible Staphylococcus aureus (MSSA) (ORT 33.3% (9/27) vs SOC 46.4% (32/69)). Clinical failure occurred in 7.4% (2/27) in the ORT group and 17.4% (12/69) in SOC (P = .34). No components of the primary outcome were significantly different between groups, but AKI did occur more commonly in the SOC group (27.5% (19/69) vs 3.7% (1/27); P = .01).
ORT appears to be a safe and effective option when directly compared to the SOC for non-endocarditis BSIs.
Gram-positive bloodstream infections (BSIs) remain a significant cause of morbidity and mortality in the United States; published mortality rates vary greatly by geography and comorbidities but are generally between 12% and 34% [1]. For some gram-positive pathogens, such as Staphylococcus aureus, current recommendations are to treat for ≥4 weeks in cases of complicated non–line-associated BSIs [2].
This length of treatment can be problematic for many patients. Outpatient parenteral antimicrobial therapy (OPAT) has revolutionized the management of many infectious diseases, including BSIs, and allowed patients to safely complete treatment at home; however, many patients do not have the support system in place to safely administer these therapies outside of a directly observed setting. Additionally, long-term intravenous (IV) access is associated with complications. In one study observing patients at the Cleveland Clinic receiving OPAT, 9% (176/1950) of the OPAT courses experienced a vascular access complication; occlusion was the most common event [3]. In a study conducted in the United Kingdom, line-related complications among patients receiving OPAT were significantly more common than drug-related adverse effects at 5.9% vs 2.4% [4]. Drug-related adverse effects are still a significant issue associated with OPAT. Vancomycin remains one of the most prescribed OPAT agents and is associated with acute kidney injury (AKI) at rates of 5% to 43% [5]. There are clear advantages to avoiding line placement and longer treatment durations with traditional antimicrobials for BSIs when these agents demonstrate in vitro activity and are appropriately dosed.
Oritavancin (ORT), a long-acting lipoglycopeptide, is an attractive option for the treatment of gram-positive BSIs. Its long half-life, rapidly bactericidal activity, and beneficial safety profile could allow for this agent to be administered to patients with gram-positive BSIs as single or multiple doses and avoid the need for chronic line placement and OPAT [6]. The current data available for ORT use in gram-positive BSIs are limited to noncomparative studies, clinical registries, and small cohort analyses [7, 8].
We conducted a retrospective cohort study of patients in the Veterans Affairs (VA) Health Care System who have been treated with at least 1 dose of IV ORT for a gram-positive BSI and compared those patients with individuals receiving the standard-of-care (SOC) treatment for gram-positive BSIs.
METHODS
The present study was a retrospective evaluation of all patients treated within the VA Health Care System from 1 January 2015 to 30 June 2021 for at least 1 positive blood culture with any of the following organisms:
Methicillin-resistant S aureus (MRSA) or methicillin-sensitive S aureus (MSSA)
Staphylococcus epidermidis, S lugdunensis, S cohnii, S hominis, S haemolyticus, S simulans, or S warnerii
Enterococcus faecalis or E faecium
Streptococcus pyogenes, S agalactiae, S dysgalactiae, S sanguinis, S anginosus, S gallolyticus, S constellatus, S oralis, S salivarius, or S mutans
These patients must have also received at least 1 inpatient dose of ORT or the SOC, defined as at least 5 inpatient days of therapy with 1 of the following agents: vancomycin, daptomycin, ceftaroline, IV ampicillin, ampicillin-sulbactam, cefazolin, nafcillin, or oxacillin. Additionally, patients could not receive any concomitant antibiotics after the studied antibiotic was initiated. Patients were excluded from evaluation if any of the following applied: a diagnosis of infective endocarditis, either a neutrophil count <500 cells/mm [3] or a diagnosis of HIV at the time that the blood culture was drawn, another organism in blood culture that was not susceptible to the studied antibiotic, or a culture from another sterile site growing an organism that was not susceptible to the studied antibiotic.
Patients were first identified by querying the VA Informatics and Computing Infrastructure data for blood culture positivity and administration of the appropriate number of days of an antibiotic of interest. Once a cohort of patients was identified from the initial query, further inclusion/exclusion criteria were applied by accessing the patients’ chart information through the VA's Compensation and Pension Record Interchange (CAPRI) and Joint Legacy Viewer (JLV). Patients who met all inclusion criteria and none of the exclusion criteria were deemed to be a part of the final full cohort, and the rest of the study data was collected by the study team from CAPRI and JLV.
Because a history of IV drug use (IVDU) and medication noncompliance are often factors that can influence whether a patient is assessed as a good candidate to receive OPAT and therefore if they received ORT, data on IVDU and medication noncompliance were also collected. Patients were considered to have a history of IVDU (ie, not an active IVDU) if (1) this was mentioned in one of the chart notes for the incident hospitalization and (2) they did not have a positive result upon urine drug screen within the previous year. Patients were assessed as being noncompliant with medications if, upon JLV review, there was a documented gap in filling any medications for chronic disease states (hypertension, gout, diabetes, etc) ≥30 days in the previous year.
Approval and Patient Consent
This study was approved by the Institutional Review Board at the VA St Louis Health Care System. Because of the retrospective nature of the study, it would not have been possible to contact all patients involved, and a waiver of informed consent was granted by the board.
Outcomes
The primary outcome was clinical failure, which was a composite of (1) all-cause mortality within 30 days of the end of therapy, (2) positive blood culture result with the incident organism ≥72 hours after administration of the first dose of the studied drug and ≤30 days from the administration of the final dose, or (3) any drug- or line-related readmissions within 30 days of hospital discharge. Secondary outcomes included individual components of the primary outcome, hospital length of stay, time to blood culture clearance, all-cause 30-day readmission rate, and rates of AKI. A drug-related readmission was defined as any readmission within the VA system in the 30 days after discharge that was deemed by the admitting team, based on CAPRI review, to be due to a drug-related adverse effect (eg, rashes, AKI, other laboratory abnormalities related to the primary antibiotic therapy). A line-related readmission was any readmission in the 30 days after discharge to correct an issue with the patient's long-term IV access (eg, line-related infection, line occlusion, line dislodgement) as documented in the CAPRI system. One dose of ORT therapy was considered equivalent to 14 days of treatment, given the long half-life of the drug and how it was studied in the SOLO I and II trials [9, 10]. Patients were evaluated in 3 cohorts: the full cohort (all those meeting inclusion/exclusion criteria), one based on an inverse probability of treatment weighting (IPTW), and patients being treated for S aureus BSI (MSSA and MRSA).
Statistical Analysis
Considering that (1) 30-day mortality rates for S aureus BSIs remain close to 20%, (2) composite clinical outcomes in other studies reveal broadly ranging success rates in treating S aureus BSIs (50%–75%), and (3) internal VA data demonstrated that approximately 3500 patients had received a lipoglycopeptide for some indication during the study period, we assumed that 25% of patients in ORT group and 40% in the SOC group would have a negative outcome [11]. Given these parameters and assumptions to achieve a 2-sided α of .05 and a β of .2, an overall 165 patients were needed per group. Continuous variables were assessed with the Student t test and categorical variables with the chi-square test.
To correct for selection bias in the patients who were assigned treatment with ORT, an IPTW method of propensity score matching was employed for 1 cohort. Factors included in propensity score calculation were history of IVDU, previous OPAT within the last 2 years, history of medication noncompliance, Pitt bacteremia score >2, any cultures in the previous 2 years growing MRSA or vancomycin-resistant enterococci, current MRSA nasal swab positivity, creatinine clearance ≤59 mL/min at treatment initiation, planned treatment duration >2 weeks, and charted allergy to β-lactams. Weighting was calculated as follows:
Patients treated with ORT: 1/propensity score
SOC group: 1/(1 – propensity score)
In an attempt to identify independent risk factors associated with the composite outcome of clinical failure, a nested case-control analysis was also performed. To achieve this, a univariate analysis and multivariate logistic regression (for variables in the univariate analysis with a P ≤ .2) were performed on the full cohort, the S aureus cohort, and the IPTW cohort. Variables included in the analysis were receipt of ORT, receipt of >1 dose of ORT, MRSA isolated from the blood, Enterococcus isolated from the blood, daptomycin therapy, vancomycin therapy, and obesity (body mass index ≥30).
RESULTS
Initial query of the VA Informatics and Computing Infrastructure database according to the parameters of time frame, blood culture of interest, and antibiotic of interest identified 236 patients: 106 receiving ORT and 130 treated with SOC. After review of CAPRI and JLV and application of other inclusion/exclusion criteria by the study team, 96 patients met criteria for inclusion into the full cohort: 27 receiving ORT and 69 treated with SOC. Most patients were excluded for not having a positive blood culture result (ORT group, n = 60) and not receiving the correct duration of antibiotics (SOC group, n = 17).
Baseline characteristics were well balanced between groups; however, in the ORT group, more patients received >96 hours of other antibiotic treatment before starting ORT, and more patients had a history of medication noncompliance. The most common source of infection was skin and skin structure (46.8%, 45/96), and MSSA was the most common organism identified (42.7%, 41/96). Only 1 patient in the study was female and most patients were White (74%, 71/96). The most common antibiotics used in the SOC group were cefazolin (n = 26) and vancomycin (n = 23). Most patients (70.4%, 19/27) received a single dose of ORT. See Table 1 for complete baseline characteristics.
| . | Group, No. (%) or Median [IQR] . | . | |
|---|---|---|---|
| Characteristic . | Oritavancin (n = 27) . | Standard of Care (n = 69) . | P Value . |
| Age, y | 62 [15] | 69 [9] | .05 |
| Sex: female | 1 (3.7) | 0 | .281 |
| Ethnicity: white | 18 (69.2) | 53 (81.5) | .419 |
| Weight, kg | 90.9 [19.3] | 93.5 [28.3] | .997 |
| BMI, kg/m2 | 27.6 [5.3] | 30 [10] | .625 |
| Median SCr 1 y prior to admission, mg/dL | 1.08 [0.23] | 1.03 [0.55] | .776 |
| CrCl, mL/min | 103.5 [60.5] | 88.5 [51.6] | .079 |
| CrCl ≤59 mL/min at treatment initiation | 16 (23.2) | 3 (11.1) | .257 |
| History | |||
| IVDU | 5 (18.5) | 4 (5.8) | .111 |
| OPAT use within 2 y | 1 (3.7) | 3 (4.4) | >.99 |
| Medication noncompliance | 9 (33.3) | 10 (14.5) | .735 |
| MRSA in previous 2 y | 4 (14.8) | 8 (11.6) | .735 |
| VRE in previous 2 y | 0 | 0 | — |
| Current MRSA nasal swab positivity | 5 (18.5) | 16 (23.2) | .619 |
| Listed β-lactam allergy | 5 (18.5) | 11 (15.9) | .761 |
| Temperature at time of blood culture, °F | 98.4 [2.6] | 98.4 [1.1] | .689 |
| Pitt bacteremia score | 0 [0] | 0 [0] | .782 |
| >96 h of other antibiotics before incident antibiotic | 19 (70.4) | 9 (13) | <.001 |
| Organisms cultured | |||
| MSSA | 9 (33.3) | 32 (46.4) | … |
| MRSA | 6 (22.2) | 17 (24.6) | … |
| S epidermidis | 5 (18.5) | 7 (10.1) | … |
| E faecalis | 0 | 6 (8.7) | … |
| E faecium | 1 (3.7) | 0 | … |
| S hominis | 0 | 2 (2.9) | … |
| S lugdunensis | 1 (3.7) | 0 | … |
| S oralis | 1 (3.7) | 0 | … |
| S simulans | 0 | 1 (1.45) | … |
| S warnerii | 0 | 1 (1.45) | … |
| S haemolyticus | 1 (3.7) | 0 | … |
| S dysgalactiae | 0 | 1 (1.45) | … |
| S agalactiae | 0 | 1 (1.45) | … |
| S anginosus | 0 | 1 (1.45) | … |
| Other GP not listed | 3 (11.1) | 0 | … |
| Likely source of infection | … | ||
| SSTI | 17 (62.9) | 28 (40.6) | … |
| Central line | 2 (7.4) | 4 (5.8) | … |
| Urinary | 1 (3.7) | 5 (7.3) | … |
| Pneumonia | 0 | 3 (4.4) | … |
| Other | 6 (22.2) | 13 (18.8) | … |
| Unknown | 1 (3.7) | 16 (23.2) | … |
| Other cultures positive in addition to blood cultures | 6 (22.2) | 25 (36.2) | .187 |
| SCr, mg/dL | … | ||
| Initiation | 0.91 [0.24] | 1.05 [0.67] | .146 |
| Nadir | 0.87 [0.3] | 0.9 [0.53] | .118 |
| Peak | 1.07 [0.23] | 1.17 [0.84] | .093 |
| WBC, cells/mm3 | … | ||
| Initiation | 9.3 [5.8] | 6 [6.8] | .796 |
| Nadir | 8.5 [4.3] | 6.2 [3.9] | .087 |
| Peak | 9.7 [5.6] | 10.9 [6] | .504 |
| Vancomycin troughs, mcg/mL | … | … | |
| 1st | 12.8 [9.8] | … | |
| 2nd | 18.05 [9] | … | |
| 3rd | 19 [4.2] | … | |
| 4th | 17.8 [8.2] | … | |
| 5th | 18.1 [5.7] | … | |
| Antimicrobials used | … | … | |
| Ampicillin-sulbactam | 2 (2.9) | … | |
| Ampicillin | 4 (5.8) | … | |
| Ceftaroline | 3 (4.3) | … | |
| Daptomycin | 2 (2.9) | … | |
| Nafcillin | 5 (7.2) | … | |
| Oxacillin | 4 (5.8) | … | |
| Vancomycin | 23 (33.3) | … | |
| Cefazolin | 26 (37.7) | … | |
| Length of, d | … | ||
| Hospital stay | 12 [9] | 5 [3] | <.001 |
| Treatmenta | 14 [14] | 14.5 [17] | .986 |
| Time between, d | … | ||
| Initial culture and start of incident antimicrobial | 6 [3] | 2 [2] | <.001 |
| Oritavancin administration and hospital discharge | 0 [1] | … | … |
| Doses of oritavancin administered | … | … | |
| 1 | 19 (70.4) | … | |
| 2 | 4 (14.8) | … | |
| 3 | 3 (11.1) | … | |
| 4 | 1 (3.7) | … | |
| . | Group, No. (%) or Median [IQR] . | . | |
|---|---|---|---|
| Characteristic . | Oritavancin (n = 27) . | Standard of Care (n = 69) . | P Value . |
| Age, y | 62 [15] | 69 [9] | .05 |
| Sex: female | 1 (3.7) | 0 | .281 |
| Ethnicity: white | 18 (69.2) | 53 (81.5) | .419 |
| Weight, kg | 90.9 [19.3] | 93.5 [28.3] | .997 |
| BMI, kg/m2 | 27.6 [5.3] | 30 [10] | .625 |
| Median SCr 1 y prior to admission, mg/dL | 1.08 [0.23] | 1.03 [0.55] | .776 |
| CrCl, mL/min | 103.5 [60.5] | 88.5 [51.6] | .079 |
| CrCl ≤59 mL/min at treatment initiation | 16 (23.2) | 3 (11.1) | .257 |
| History | |||
| IVDU | 5 (18.5) | 4 (5.8) | .111 |
| OPAT use within 2 y | 1 (3.7) | 3 (4.4) | >.99 |
| Medication noncompliance | 9 (33.3) | 10 (14.5) | .735 |
| MRSA in previous 2 y | 4 (14.8) | 8 (11.6) | .735 |
| VRE in previous 2 y | 0 | 0 | — |
| Current MRSA nasal swab positivity | 5 (18.5) | 16 (23.2) | .619 |
| Listed β-lactam allergy | 5 (18.5) | 11 (15.9) | .761 |
| Temperature at time of blood culture, °F | 98.4 [2.6] | 98.4 [1.1] | .689 |
| Pitt bacteremia score | 0 [0] | 0 [0] | .782 |
| >96 h of other antibiotics before incident antibiotic | 19 (70.4) | 9 (13) | <.001 |
| Organisms cultured | |||
| MSSA | 9 (33.3) | 32 (46.4) | … |
| MRSA | 6 (22.2) | 17 (24.6) | … |
| S epidermidis | 5 (18.5) | 7 (10.1) | … |
| E faecalis | 0 | 6 (8.7) | … |
| E faecium | 1 (3.7) | 0 | … |
| S hominis | 0 | 2 (2.9) | … |
| S lugdunensis | 1 (3.7) | 0 | … |
| S oralis | 1 (3.7) | 0 | … |
| S simulans | 0 | 1 (1.45) | … |
| S warnerii | 0 | 1 (1.45) | … |
| S haemolyticus | 1 (3.7) | 0 | … |
| S dysgalactiae | 0 | 1 (1.45) | … |
| S agalactiae | 0 | 1 (1.45) | … |
| S anginosus | 0 | 1 (1.45) | … |
| Other GP not listed | 3 (11.1) | 0 | … |
| Likely source of infection | … | ||
| SSTI | 17 (62.9) | 28 (40.6) | … |
| Central line | 2 (7.4) | 4 (5.8) | … |
| Urinary | 1 (3.7) | 5 (7.3) | … |
| Pneumonia | 0 | 3 (4.4) | … |
| Other | 6 (22.2) | 13 (18.8) | … |
| Unknown | 1 (3.7) | 16 (23.2) | … |
| Other cultures positive in addition to blood cultures | 6 (22.2) | 25 (36.2) | .187 |
| SCr, mg/dL | … | ||
| Initiation | 0.91 [0.24] | 1.05 [0.67] | .146 |
| Nadir | 0.87 [0.3] | 0.9 [0.53] | .118 |
| Peak | 1.07 [0.23] | 1.17 [0.84] | .093 |
| WBC, cells/mm3 | … | ||
| Initiation | 9.3 [5.8] | 6 [6.8] | .796 |
| Nadir | 8.5 [4.3] | 6.2 [3.9] | .087 |
| Peak | 9.7 [5.6] | 10.9 [6] | .504 |
| Vancomycin troughs, mcg/mL | … | … | |
| 1st | 12.8 [9.8] | … | |
| 2nd | 18.05 [9] | … | |
| 3rd | 19 [4.2] | … | |
| 4th | 17.8 [8.2] | … | |
| 5th | 18.1 [5.7] | … | |
| Antimicrobials used | … | … | |
| Ampicillin-sulbactam | 2 (2.9) | … | |
| Ampicillin | 4 (5.8) | … | |
| Ceftaroline | 3 (4.3) | … | |
| Daptomycin | 2 (2.9) | … | |
| Nafcillin | 5 (7.2) | … | |
| Oxacillin | 4 (5.8) | … | |
| Vancomycin | 23 (33.3) | … | |
| Cefazolin | 26 (37.7) | … | |
| Length of, d | … | ||
| Hospital stay | 12 [9] | 5 [3] | <.001 |
| Treatmenta | 14 [14] | 14.5 [17] | .986 |
| Time between, d | … | ||
| Initial culture and start of incident antimicrobial | 6 [3] | 2 [2] | <.001 |
| Oritavancin administration and hospital discharge | 0 [1] | … | … |
| Doses of oritavancin administered | … | … | |
| 1 | 19 (70.4) | … | |
| 2 | 4 (14.8) | … | |
| 3 | 3 (11.1) | … | |
| 4 | 1 (3.7) | … | |
Abbreviations: BMI, body mass index; CrCl, creatinine clearance; GP, gram positive; IVDU, intravenous drug use; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; OPAT, outpatient parenteral antibiotic therapy; SCr, serum creatinine; SSTI, skin and skin structure infection; VRE, vancomycin-resistant enterococci; WBC, white blood cell.
aThe treatment duration for oritavancin therapy was calculated as follows: each dose of oritavancin = 14 days of treatment.
| . | Group, No. (%) or Median [IQR] . | . | |
|---|---|---|---|
| Characteristic . | Oritavancin (n = 27) . | Standard of Care (n = 69) . | P Value . |
| Age, y | 62 [15] | 69 [9] | .05 |
| Sex: female | 1 (3.7) | 0 | .281 |
| Ethnicity: white | 18 (69.2) | 53 (81.5) | .419 |
| Weight, kg | 90.9 [19.3] | 93.5 [28.3] | .997 |
| BMI, kg/m2 | 27.6 [5.3] | 30 [10] | .625 |
| Median SCr 1 y prior to admission, mg/dL | 1.08 [0.23] | 1.03 [0.55] | .776 |
| CrCl, mL/min | 103.5 [60.5] | 88.5 [51.6] | .079 |
| CrCl ≤59 mL/min at treatment initiation | 16 (23.2) | 3 (11.1) | .257 |
| History | |||
| IVDU | 5 (18.5) | 4 (5.8) | .111 |
| OPAT use within 2 y | 1 (3.7) | 3 (4.4) | >.99 |
| Medication noncompliance | 9 (33.3) | 10 (14.5) | .735 |
| MRSA in previous 2 y | 4 (14.8) | 8 (11.6) | .735 |
| VRE in previous 2 y | 0 | 0 | — |
| Current MRSA nasal swab positivity | 5 (18.5) | 16 (23.2) | .619 |
| Listed β-lactam allergy | 5 (18.5) | 11 (15.9) | .761 |
| Temperature at time of blood culture, °F | 98.4 [2.6] | 98.4 [1.1] | .689 |
| Pitt bacteremia score | 0 [0] | 0 [0] | .782 |
| >96 h of other antibiotics before incident antibiotic | 19 (70.4) | 9 (13) | <.001 |
| Organisms cultured | |||
| MSSA | 9 (33.3) | 32 (46.4) | … |
| MRSA | 6 (22.2) | 17 (24.6) | … |
| S epidermidis | 5 (18.5) | 7 (10.1) | … |
| E faecalis | 0 | 6 (8.7) | … |
| E faecium | 1 (3.7) | 0 | … |
| S hominis | 0 | 2 (2.9) | … |
| S lugdunensis | 1 (3.7) | 0 | … |
| S oralis | 1 (3.7) | 0 | … |
| S simulans | 0 | 1 (1.45) | … |
| S warnerii | 0 | 1 (1.45) | … |
| S haemolyticus | 1 (3.7) | 0 | … |
| S dysgalactiae | 0 | 1 (1.45) | … |
| S agalactiae | 0 | 1 (1.45) | … |
| S anginosus | 0 | 1 (1.45) | … |
| Other GP not listed | 3 (11.1) | 0 | … |
| Likely source of infection | … | ||
| SSTI | 17 (62.9) | 28 (40.6) | … |
| Central line | 2 (7.4) | 4 (5.8) | … |
| Urinary | 1 (3.7) | 5 (7.3) | … |
| Pneumonia | 0 | 3 (4.4) | … |
| Other | 6 (22.2) | 13 (18.8) | … |
| Unknown | 1 (3.7) | 16 (23.2) | … |
| Other cultures positive in addition to blood cultures | 6 (22.2) | 25 (36.2) | .187 |
| SCr, mg/dL | … | ||
| Initiation | 0.91 [0.24] | 1.05 [0.67] | .146 |
| Nadir | 0.87 [0.3] | 0.9 [0.53] | .118 |
| Peak | 1.07 [0.23] | 1.17 [0.84] | .093 |
| WBC, cells/mm3 | … | ||
| Initiation | 9.3 [5.8] | 6 [6.8] | .796 |
| Nadir | 8.5 [4.3] | 6.2 [3.9] | .087 |
| Peak | 9.7 [5.6] | 10.9 [6] | .504 |
| Vancomycin troughs, mcg/mL | … | … | |
| 1st | 12.8 [9.8] | … | |
| 2nd | 18.05 [9] | … | |
| 3rd | 19 [4.2] | … | |
| 4th | 17.8 [8.2] | … | |
| 5th | 18.1 [5.7] | … | |
| Antimicrobials used | … | … | |
| Ampicillin-sulbactam | 2 (2.9) | … | |
| Ampicillin | 4 (5.8) | … | |
| Ceftaroline | 3 (4.3) | … | |
| Daptomycin | 2 (2.9) | … | |
| Nafcillin | 5 (7.2) | … | |
| Oxacillin | 4 (5.8) | … | |
| Vancomycin | 23 (33.3) | … | |
| Cefazolin | 26 (37.7) | … | |
| Length of, d | … | ||
| Hospital stay | 12 [9] | 5 [3] | <.001 |
| Treatmenta | 14 [14] | 14.5 [17] | .986 |
| Time between, d | … | ||
| Initial culture and start of incident antimicrobial | 6 [3] | 2 [2] | <.001 |
| Oritavancin administration and hospital discharge | 0 [1] | … | … |
| Doses of oritavancin administered | … | … | |
| 1 | 19 (70.4) | … | |
| 2 | 4 (14.8) | … | |
| 3 | 3 (11.1) | … | |
| 4 | 1 (3.7) | … | |
| . | Group, No. (%) or Median [IQR] . | . | |
|---|---|---|---|
| Characteristic . | Oritavancin (n = 27) . | Standard of Care (n = 69) . | P Value . |
| Age, y | 62 [15] | 69 [9] | .05 |
| Sex: female | 1 (3.7) | 0 | .281 |
| Ethnicity: white | 18 (69.2) | 53 (81.5) | .419 |
| Weight, kg | 90.9 [19.3] | 93.5 [28.3] | .997 |
| BMI, kg/m2 | 27.6 [5.3] | 30 [10] | .625 |
| Median SCr 1 y prior to admission, mg/dL | 1.08 [0.23] | 1.03 [0.55] | .776 |
| CrCl, mL/min | 103.5 [60.5] | 88.5 [51.6] | .079 |
| CrCl ≤59 mL/min at treatment initiation | 16 (23.2) | 3 (11.1) | .257 |
| History | |||
| IVDU | 5 (18.5) | 4 (5.8) | .111 |
| OPAT use within 2 y | 1 (3.7) | 3 (4.4) | >.99 |
| Medication noncompliance | 9 (33.3) | 10 (14.5) | .735 |
| MRSA in previous 2 y | 4 (14.8) | 8 (11.6) | .735 |
| VRE in previous 2 y | 0 | 0 | — |
| Current MRSA nasal swab positivity | 5 (18.5) | 16 (23.2) | .619 |
| Listed β-lactam allergy | 5 (18.5) | 11 (15.9) | .761 |
| Temperature at time of blood culture, °F | 98.4 [2.6] | 98.4 [1.1] | .689 |
| Pitt bacteremia score | 0 [0] | 0 [0] | .782 |
| >96 h of other antibiotics before incident antibiotic | 19 (70.4) | 9 (13) | <.001 |
| Organisms cultured | |||
| MSSA | 9 (33.3) | 32 (46.4) | … |
| MRSA | 6 (22.2) | 17 (24.6) | … |
| S epidermidis | 5 (18.5) | 7 (10.1) | … |
| E faecalis | 0 | 6 (8.7) | … |
| E faecium | 1 (3.7) | 0 | … |
| S hominis | 0 | 2 (2.9) | … |
| S lugdunensis | 1 (3.7) | 0 | … |
| S oralis | 1 (3.7) | 0 | … |
| S simulans | 0 | 1 (1.45) | … |
| S warnerii | 0 | 1 (1.45) | … |
| S haemolyticus | 1 (3.7) | 0 | … |
| S dysgalactiae | 0 | 1 (1.45) | … |
| S agalactiae | 0 | 1 (1.45) | … |
| S anginosus | 0 | 1 (1.45) | … |
| Other GP not listed | 3 (11.1) | 0 | … |
| Likely source of infection | … | ||
| SSTI | 17 (62.9) | 28 (40.6) | … |
| Central line | 2 (7.4) | 4 (5.8) | … |
| Urinary | 1 (3.7) | 5 (7.3) | … |
| Pneumonia | 0 | 3 (4.4) | … |
| Other | 6 (22.2) | 13 (18.8) | … |
| Unknown | 1 (3.7) | 16 (23.2) | … |
| Other cultures positive in addition to blood cultures | 6 (22.2) | 25 (36.2) | .187 |
| SCr, mg/dL | … | ||
| Initiation | 0.91 [0.24] | 1.05 [0.67] | .146 |
| Nadir | 0.87 [0.3] | 0.9 [0.53] | .118 |
| Peak | 1.07 [0.23] | 1.17 [0.84] | .093 |
| WBC, cells/mm3 | … | ||
| Initiation | 9.3 [5.8] | 6 [6.8] | .796 |
| Nadir | 8.5 [4.3] | 6.2 [3.9] | .087 |
| Peak | 9.7 [5.6] | 10.9 [6] | .504 |
| Vancomycin troughs, mcg/mL | … | … | |
| 1st | 12.8 [9.8] | … | |
| 2nd | 18.05 [9] | … | |
| 3rd | 19 [4.2] | … | |
| 4th | 17.8 [8.2] | … | |
| 5th | 18.1 [5.7] | … | |
| Antimicrobials used | … | … | |
| Ampicillin-sulbactam | 2 (2.9) | … | |
| Ampicillin | 4 (5.8) | … | |
| Ceftaroline | 3 (4.3) | … | |
| Daptomycin | 2 (2.9) | … | |
| Nafcillin | 5 (7.2) | … | |
| Oxacillin | 4 (5.8) | … | |
| Vancomycin | 23 (33.3) | … | |
| Cefazolin | 26 (37.7) | … | |
| Length of, d | … | ||
| Hospital stay | 12 [9] | 5 [3] | <.001 |
| Treatmenta | 14 [14] | 14.5 [17] | .986 |
| Time between, d | … | ||
| Initial culture and start of incident antimicrobial | 6 [3] | 2 [2] | <.001 |
| Oritavancin administration and hospital discharge | 0 [1] | … | … |
| Doses of oritavancin administered | … | … | |
| 1 | 19 (70.4) | … | |
| 2 | 4 (14.8) | … | |
| 3 | 3 (11.1) | … | |
| 4 | 1 (3.7) | … | |
Abbreviations: BMI, body mass index; CrCl, creatinine clearance; GP, gram positive; IVDU, intravenous drug use; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; OPAT, outpatient parenteral antibiotic therapy; SCr, serum creatinine; SSTI, skin and skin structure infection; VRE, vancomycin-resistant enterococci; WBC, white blood cell.
aThe treatment duration for oritavancin therapy was calculated as follows: each dose of oritavancin = 14 days of treatment.
Full Cohort
Overall 15.6% (15/96) of patients developed the composite outcome of clinical failure: 9 with all-cause mortality (9.4%), 1 with a subsequent positive blood culture result, and 5 (5.2%) with readmittance within 30 days of hospital discharge for a drug- or line-related complication.
In the full cohort of patients 7.4% (2/27) in the ORT group and 18.8% (13/69) in the SOC group had the primary outcome of clinical failure (P = .336). There were no significant differences in any of the components of the primary outcome (Figure 1). Hospital length of stay was a median 12 days in the ORT group and 5 days in the SOC group (P < .001); all-cause 30-day readmission was not significantly different between groups. AKI occurred in 3.7% (1/27) of the ORT group and 27.5% (19/69) of the SOC group (P = .011) (Figure 2). In this cohort, only the variables of MRSA isolated in blood culture, body mass index ≥30, and >96 hours of prior antibiotics met criteria for inclusion in the multivariate regression model (Table 2). In the multivariate regression, only MRSA isolated in blood culture was significantly associated with clinical failure (odds ratio, 3.52; 95% CI, 1.06–11.76; P = .04). Full multivariate results are available in Table 3.
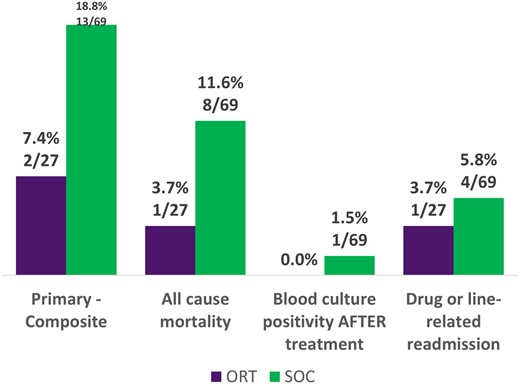
Primary outcome: full cohort. ORT, oritavancin; SOC, standard of care.
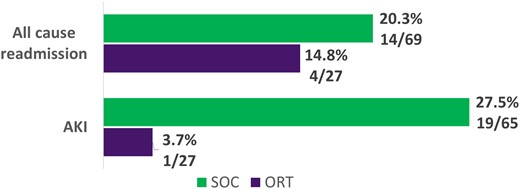
Secondary outcomes: full cohort. AKI, acute kidney injury; ORT, oritavancin; SOC, standard of care.
| Covariate . | Clinical Failure . | No Clinical Failure . | P Value . |
|---|---|---|---|
| Received oritavancin therapy | 2 | 25 | .227 |
| Received >1 dose of oritavancin | 2 | 6 | .392 |
| MRSA isolated from the blood | 7 | 16 | .019 |
| Enterococcus isolated from the blood | 0 | 7 | .976 |
| Received daptomycin | 2 | 0 | .976 |
| Received vancomycin | 5 | 18 | .271 |
| BMI ≥30 | 4 | 39 | .195 |
| Received >96 h of prior antibiotics | 2 | 26 | .200 |
| Other cultures positive | 3 | 28 | .353 |
| Covariate . | Clinical Failure . | No Clinical Failure . | P Value . |
|---|---|---|---|
| Received oritavancin therapy | 2 | 25 | .227 |
| Received >1 dose of oritavancin | 2 | 6 | .392 |
| MRSA isolated from the blood | 7 | 16 | .019 |
| Enterococcus isolated from the blood | 0 | 7 | .976 |
| Received daptomycin | 2 | 0 | .976 |
| Received vancomycin | 5 | 18 | .271 |
| BMI ≥30 | 4 | 39 | .195 |
| Received >96 h of prior antibiotics | 2 | 26 | .200 |
| Other cultures positive | 3 | 28 | .353 |
Bold indicates P ≤ .2.
Abbreviations: BMI, body mass index; MRSA, methicillin-resistant Staphylococcus aureus.
| Covariate . | Clinical Failure . | No Clinical Failure . | P Value . |
|---|---|---|---|
| Received oritavancin therapy | 2 | 25 | .227 |
| Received >1 dose of oritavancin | 2 | 6 | .392 |
| MRSA isolated from the blood | 7 | 16 | .019 |
| Enterococcus isolated from the blood | 0 | 7 | .976 |
| Received daptomycin | 2 | 0 | .976 |
| Received vancomycin | 5 | 18 | .271 |
| BMI ≥30 | 4 | 39 | .195 |
| Received >96 h of prior antibiotics | 2 | 26 | .200 |
| Other cultures positive | 3 | 28 | .353 |
| Covariate . | Clinical Failure . | No Clinical Failure . | P Value . |
|---|---|---|---|
| Received oritavancin therapy | 2 | 25 | .227 |
| Received >1 dose of oritavancin | 2 | 6 | .392 |
| MRSA isolated from the blood | 7 | 16 | .019 |
| Enterococcus isolated from the blood | 0 | 7 | .976 |
| Received daptomycin | 2 | 0 | .976 |
| Received vancomycin | 5 | 18 | .271 |
| BMI ≥30 | 4 | 39 | .195 |
| Received >96 h of prior antibiotics | 2 | 26 | .200 |
| Other cultures positive | 3 | 28 | .353 |
Bold indicates P ≤ .2.
Abbreviations: BMI, body mass index; MRSA, methicillin-resistant Staphylococcus aureus.
| Covariate . | Odds Ratio (95% CI) . | P Value . |
|---|---|---|
| MRSA isolated from blood | 3.52 (1.06–11.76) | .04 |
| BMI ≥30 | .44 (.12–1.61) | .217 |
| Received >96 h of prior antibiotics | .38 (.07–1.92) | .241 |
| Covariate . | Odds Ratio (95% CI) . | P Value . |
|---|---|---|
| MRSA isolated from blood | 3.52 (1.06–11.76) | .04 |
| BMI ≥30 | .44 (.12–1.61) | .217 |
| Received >96 h of prior antibiotics | .38 (.07–1.92) | .241 |
Bold indicates P < .05.
Abbreviations: BMI, body mass index; MRSA, methicillin-resistant Staphylococcus aureus.
| Covariate . | Odds Ratio (95% CI) . | P Value . |
|---|---|---|
| MRSA isolated from blood | 3.52 (1.06–11.76) | .04 |
| BMI ≥30 | .44 (.12–1.61) | .217 |
| Received >96 h of prior antibiotics | .38 (.07–1.92) | .241 |
| Covariate . | Odds Ratio (95% CI) . | P Value . |
|---|---|---|
| MRSA isolated from blood | 3.52 (1.06–11.76) | .04 |
| BMI ≥30 | .44 (.12–1.61) | .217 |
| Received >96 h of prior antibiotics | .38 (.07–1.92) | .241 |
Bold indicates P < .05.
Abbreviations: BMI, body mass index; MRSA, methicillin-resistant Staphylococcus aureus.
S aureus Cohort
In the cohort of patients being treated for S aureus (n = 64), 13.3% (2/15) in the ORT group vs 20.4% (10/49) in the SOC group had clinical failure; all-cause mortality was 6.7% (1/15) for ORT and 12.2% (6/49) for SOC (Figure 3). AKI occurred in 6.7% (1/15) of the ORT group and 24.5% (12/49) of the SOC group (Figure 4). Only MRSA isolated in blood culture was included in the multivariate regression model (Table 4.), but this was not significantly associated with clinical failure (odds ratio, 2.74; 95% CI, .73–10.2; P = .134).
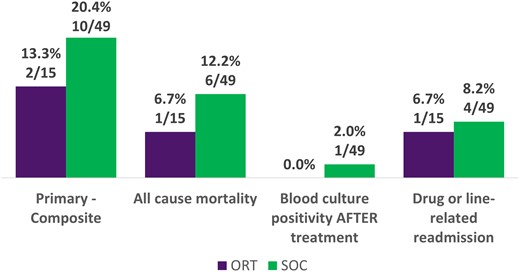
Primary outcomes: cohort treated for Staphylococcus aureus. ORT, oritavancin; SOC, standard of care.

Secondary outcomes: cohort treated for Staphylococcus aureus. AKI, acute kidney injury; ORT, oritavancin; SOC, standard of care.
| Covariate . | Clinical Failure . | No Clinical Failure . | P Value . |
|---|---|---|---|
| Received oritavancin therapy | 2 | 13 | .542 |
| Received >1 dose of oritavancin | 2 | 6 | .348 |
| MRSA isolated from the blood | 7 | 16 | .081 |
| Enterococcus isolated from the blood | — | — | — |
| Received daptomycin | 2 | 0 | .974 |
| Received vancomycin | 4 | 10 | .293 |
| BMI ≥30 | 4 | 26 | .303 |
| Received >96 h of prior antibiotics | 2 | 16 | .337 |
| Other cultures positive | 2 | 20 | .167 |
| Covariate . | Clinical Failure . | No Clinical Failure . | P Value . |
|---|---|---|---|
| Received oritavancin therapy | 2 | 13 | .542 |
| Received >1 dose of oritavancin | 2 | 6 | .348 |
| MRSA isolated from the blood | 7 | 16 | .081 |
| Enterococcus isolated from the blood | — | — | — |
| Received daptomycin | 2 | 0 | .974 |
| Received vancomycin | 4 | 10 | .293 |
| BMI ≥30 | 4 | 26 | .303 |
| Received >96 h of prior antibiotics | 2 | 16 | .337 |
| Other cultures positive | 2 | 20 | .167 |
Bold indicates P ≤ .2.
Abbreviations: BMI, body mass index; MRSA, methicillin-resistant Staphylococcus aureus.
| Covariate . | Clinical Failure . | No Clinical Failure . | P Value . |
|---|---|---|---|
| Received oritavancin therapy | 2 | 13 | .542 |
| Received >1 dose of oritavancin | 2 | 6 | .348 |
| MRSA isolated from the blood | 7 | 16 | .081 |
| Enterococcus isolated from the blood | — | — | — |
| Received daptomycin | 2 | 0 | .974 |
| Received vancomycin | 4 | 10 | .293 |
| BMI ≥30 | 4 | 26 | .303 |
| Received >96 h of prior antibiotics | 2 | 16 | .337 |
| Other cultures positive | 2 | 20 | .167 |
| Covariate . | Clinical Failure . | No Clinical Failure . | P Value . |
|---|---|---|---|
| Received oritavancin therapy | 2 | 13 | .542 |
| Received >1 dose of oritavancin | 2 | 6 | .348 |
| MRSA isolated from the blood | 7 | 16 | .081 |
| Enterococcus isolated from the blood | — | — | — |
| Received daptomycin | 2 | 0 | .974 |
| Received vancomycin | 4 | 10 | .293 |
| BMI ≥30 | 4 | 26 | .303 |
| Received >96 h of prior antibiotics | 2 | 16 | .337 |
| Other cultures positive | 2 | 20 | .167 |
Bold indicates P ≤ .2.
Abbreviations: BMI, body mass index; MRSA, methicillin-resistant Staphylococcus aureus.
IPTW Cohort
IPTW was applied to both groups (ORT and SOC), and there was no significant difference in the primary outcome as compared with the full cohort. The univariate analysis in the IPTW cohort yielded significance among the variables of MRSA isolated from the blood (P = .0038) and receipt of >1 dose of ORT therapy (P = .057). In the multivariate regression model, absence of MRSA isolation was significantly protective of clinical failure (odds ratio, 0.12; 95% CI, .03–.52).
It was decided post hoc to evaluate the cohort of patients receiving >96 hours of antibiotics prior to the initiation of the study medication; however, this group was small (n = 28). In this cohort, 10.5% (2/19) treated with ORT had clinical failure, as opposed to no patients in the SOC group; the failures in the ORT group occurred from all-cause mortality (n = 1) and a drug- or line-related readmission (n = 1). This cohort was deemed too small for univariate or multivariate regression.
DISCUSSION
In this national cohort of veterans being treated for gram-positive BSIs with either ORT or SOC, there was no difference with respect to the composite outcome of clinical failure between groups in any of the cohorts evaluated. Numerically more patients in the SOC group experienced all-cause 30-day mortality, and significantly more treated with the SOC developed AKI. Length of stay was significantly greater in the ORT group, and only the isolation of MRSA from the incident blood culture was identified as a significant predictor of clinical failure in the full and IPTW cohorts.
The low number of drug- or line-related readmissions occurring in the SOC group was surprising, but this could be related to the exclusion of many deeper-seated infections and shorter overall durations of therapy (median duration for SOC group, 14 days). It was not as surprising that length of stay was significantly greater for patients receiving ORT; this likely reflects that these patients were not OPAT candidates and were being held inpatient until all options for treatment had been considered. ORT therapy did appear safer, as there were significantly fewer cases of AKI in this group. This difference may be related to the fact that the SOC antibiotics that patients received required more frequent monitoring and there were more laboratories available to identify AKI in the SOC group than the ORT group.
Since their development, there has been interest in using long-acting lipoglycopeptides such as ORT for the treatment of BSIs. ORT is particularly attractive given that its elimination half-life is ∼245 hours [6].
Peak plasma concentration has been found to be close to 140 mcg/mL; however, <10% of the peak concentration remains in the serum 24 hours postinfusion [6, 12, 13]. Despite this fact, in a pharmacokinetic evaluation of patients involved in the SOLO I and II trials with available pharmacokinetic data, serum levels remained >1 mcg/mL for ∼504 hours. This is particularly informative given that the Food and Drug Administration–approved minimum inhibitory concentration (MIC) breakpoint for ORT to S aureus is ≤0.12 mcg/mL; after a single 1200-mg dose, serum ORT concentrations should remain above the MIC breakpoint for S aureus for >40 days [6, 13]. Additionally, ORT is among the most rapidly bactericidal antibiotics available against S aureus, noted to achieve 3-log reduction in colony-forming units per milliliter in 0.5 hours [14].
Unfortunately, clinical evidence with ORT for the treatment of BSIs is mostly limited to registries and small case series or cohort studies [7, 8, 15, 16]. One cohort study from 2017 examined 5 patients who received treatment for MSSA BSI. All patients were treated with only a single dose and 3 of 5 achieved cure [15]. In another study, 3 patients were treated with ORT to facilitate transition to hospice care. Two of these patients died within the 15 days of ORT treatment, and 1 lived for 5 weeks [16]. In 2018 Schulz et al [7] reported the outcomes of 17 patients who were treated for complex gram-positive infections with multiple doses of ORT, all of whom achieved clinical success. Two of these patients were treated for BSIs or an endovascular infection; 1 patient received an initial 1200-mg dose, then 800 mg weekly for 3 doses; the other received an initial 1200-mg dose, followed by 800 mg weekly for 11 doses and then a subsequent treatment course for an additional 5 weeks [7].
Perhaps the most robust picture of ORT for the treatment of BSIs comes from the CHROME registry [8], a retrospective observational program describing patients treated with ORT from 2014 to 2017 in the United States for a variety of infections. In this registry, 7 patients were treated with ORT for BSIs: 5 for primary infection and 2 secondary to skin and skin structure infection, with 3 of 7 isolates being S aureus. All 7 patients received single-dose therapy with 1200 mg, and all achieved clinical cure [8].
Dosing of ORT for BSI treatment remains somewhat controversial in the available literature. Two patients discussed earlier received initial loading doses of 1200 mg, followed by 800 mg weekly for varying durations. These patients had complex infections—one with vancomycin-resistant enterococci and the other an endovascular infection caused by S lugdunensis [8]. There remains a similar paucity of data with ORT for the treatment of osteomyelitis: another complex typically gram-positive–driven infection that can occasionally be caused by or lead to secondary BSIs. In the available literature describing ORT in the treatment of osteomyelitis, there remains a similar lack of standardization in dosing, particularly with regard to frequency and total number of doses; however, nearly all cases are managed with multiple doses [8, 17, 18]. In the present study, endocarditis was a criterion for exclusion, and there were few patients who had osteomyelitis (classified as skin and skin structure infection—primary source). Most patients here were treated with single-dose therapy, and this was likely appropriate because most BSIs were not secondary to deeper-seated infections. All patients, regardless of how many doses they received, were administered 1200 mg of ORT. Additionally, patients in the present study were not severely ill, as demonstrated by their median Pitt bacteremia score of 0. Single-dose therapy for less complex BSIs also appears to be supported by pharmacokinetic/pharmacodynamic data: ORT is a concentration-dependent bactericidal agent, and one of the pharmacokinetic indices most associated with antibacterial activity is the ratio of peak serum concentration to MIC [9, 19, 20]. Based on these data, the sustained plasma concentrations, and the low MICs to gram-positive pathogens, single-dose therapy for non–deep-seated BSIs appears justifiable.
The present study is not without limitations. The retrospective nonrandomized nature creates several issues, not the least of which is the lack of standardization of ORT dosing or the timing when doses occurred; furthermore, the frequency of ORT dosing was not collected or evaluated, only the total number of doses that the patient received. Additionally, significantly more patients in the ORT group received >96 hours of other antibiotics before being treated with ORT (70.4%), possibly confounding results. Finally, the analysis was underpowered, as not enough patients nationally within the VA Health Care System receiving ORT for a gram-positive BSI met inclusion criteria. Because power was not met, the possibility of a type II error within the study does exist.
An IPTW method of propensity score matching was implemented to mitigate selection bias, and this cohort showed no difference in outcomes from the full unmatched cohort. While more patients treated with ORT initially received >96 hours of other antibiotics and the time from initial culture to ORT administration was greater than the time to administration of SOC therapy in the other group (median, 6 vs 2 days), we believe that this study provides an accurate picture of how clinicians are approaching ORT in the treatment of BSIs, at least within the VA Health Care System—it is an option of last resort in patients who are likely not OPAT candidates, and the medication is not being administered until immediately prior to discharge (median time between ORT administration and hospital discharge, 0 days). Of note, all patients who received ORT had cleared their blood cultures by the time when ORT was administered.
This study, to date, is the only evaluation comparing ORT vs SOC for the treatment of gram-positive BSIs. An additional strength is the significant number of S aureus BSIs present. S aureus BSIs are typically associated with high morbidity and mortality and treated for a duration ≥4 weeks in many cases; this evaluation demonstrated similar outcomes between patients treated with ORT and those who received SOC. While the study did not meet power, it remains the largest study of a population of patients across the United States receiving ORT for a BSI and informs how this antibiotic is currently being used for BSIs in the health care system.
CONCLUSIONS
Patients treated with ORT within the VA Health Care System for some of the most common non–deep-seated gram-positive BSIs had outcomes similar to patients treated with SOC. This first comparative study suggests that ORT is a potentially safe and effective option for the management of gram-positive BSIs, but prospective studies with standardized dosing regimens and initiation times are needed to better define optimal courses of treatment.
Notes
Financial support. This work was supported by an investigator-initiated trial grant from Melinta Therapeutics. Only the authors managed and analyzed data; the role of the funding source was merely supportive. The authors alone had final say over the contents of this manuscript.
References
Author notes
Potential conflicts of interest. R. P. M. reports receiving honoraria as a member of the Melinta Therapeutics speaker's bureau. All other authors report no potential conflicts. While this work was supported by an investigator-initiated trial grant from Melinta Therapeutics, and R.P.M. has received honoraria in the past as a member of the Melinta Therapeutics spearkers bureau, only the authors collected, reviewed, and analyzed data. Additionally, only the listed author's developed conclusions and prepared the manuscript.
- vancomycin
- cefazolin
- daptomycin
- gram-positive bacteria
- gram-positive bacterial infections
- gram-positive cocci
- gram-positive rods
- patient discharge
- patient readmission
- mortality
- antimicrobials
- blood culture
- health care systems
- oritavancin
- bloodstream infections
- methicillin-susceptible staphylococcus aureus
- standard of care
- primary outcome measure
- composite outcomes





Comments