-
PDF
- Split View
-
Views
-
Cite
Cite
Charlotte Wigston, Melanie Lavender, Rebecca Long, Dipen Sankhesara, David Ching, Graham Weaire-Buchanan, Shakeel Mowlaboccus, Geoffrey W Coombs, Kaitlyn Lam, Jeremy Wrobel, Meow Cheong Yaw, Michael Musk, Peter Boan, Mycoplasma and Ureaplasma Donor-Derived Infection and Hyperammonemia Syndrome in 4 Solid Organ Transplant Recipients From a Single Donor, Open Forum Infectious Diseases, Volume 10, Issue 6, June 2023, ofad263, https://doi.org/10.1093/ofid/ofad263
Close - Share Icon Share
Abstract
Hyperammonemia syndrome (HS) is a life-threatening condition occurring in solid organ transplant patients, affecting primarily lung recipients, and is associated with Mycoplasma hominis and/or Ureaplasma spp infection. The organ donor was a young man who died of hypoxic brain injury and had urethral discharge antemortem. The donor and 4 solid organ transplant recipients had infection with M hominis and/or Ureaplasma spp. The lung and heart recipients both developed altered conscious state and HS associated with M hominis and Ureaplasma spp infections. Despite treatment with antibiotics and ammonia scavengers, both the lung and heart recipients died at day +102 and day +254, respectively. After diagnosis in the thoracic recipients, screening samples from the liver recipient and 1 kidney recipient were culture positive for M hominis with or without Ureaplasma spp. Neither the liver nor kidney recipients developed HS. Our case series demonstrates the unique finding of M hominis and Ureaplasma spp dissemination from an immunocompetent donor across 4 different organ recipients. Phylogenetic whole genome sequencing analysis demonstrated that M hominis samples from recipients and donor were closely related, suggesting donor-derived infection. Screening of lung donors and/or recipients for Mycoplasma and Ureaplasma spp is recommended, as well as prompt treatment with antimicrobials to prevent morbidity.
Hyperammonemia syndrome (HS) is a life-threatening condition after solid organ transplant (SOT) occurring primarily in lung recipients, associated with Mycoplasma hominis and/or Ureaplasma spp infection [1]. Key features are elevated serum ammonia, altered conscious state, and cerebral edema [2]. We report M hominis and/or Ureaplasma spp infection in 4 SOT recipients derived from the same donor, leading to HS in lung and cardiac recipients.
CASE SERIES
The organ donor was a young man who died of hypoxic ischemic brain injury secondary to suicide by hanging. Unusually, he had urethral discharge noted at donor assessment; however, sexual history and symptomatology were not known. Penile swab culture was negative for Neisseria gonorrhoea, Candida, and other pathogenic organisms (not specifically tested by culture or polymerase chain reaction [PCR] for M hominis or Ureaplasma spp). Chest radiograph was clear at presentation and macroaspiration was not noted in the clinical assessment; however, lower zone airspace infiltrates were present at the time of donation 4 days after admission. Predonation bronchial washings retrospectively tested by PCR validated to be species specific were positive for M hominis and Ureaplasma urealyticum (Ureaplasma parvum PCR negative; culture could not be performed). Retrospective ethylenediaminetetraacetic acid blood PCR for M hominis and Ureaplasma spp was negative.
All SOT recipients followed standard induction immunosuppression and perioperative antibiotic prophylactic protocols (Table 1) [3].
Clinical and Microbiological Details of 4 Solid Organ Transplant Recipients With Mycoplasma hominis and Ureaplasma Species Infection Derived From a Single Donor, Complicated by Hyperammonemia Syndrome (Lung and Cardiac Recipients)
| Patient . | Organ Transplanted . | Sex and Age, y . | Indication for Transplant . | Initial Immunosuppression . | Positive Recipient M hominis/Ureaplasma spp Culture or PCR . | Treatment . | Clinical Correlation . |
|---|---|---|---|---|---|---|---|
| A | Bilateral sequential lung | M, 65 | Emphysema | Basiliximab (D+0, D+4) Methylprednisolone (D+0) MMF (D+1) Ciclosporin (D+1) | D+4 BAL culture and PCR M hominis, U parvum, U urealyticum D+7 blood culture and PCR M hominis, U parvum D+9, D+14, D+19 BAL PCR M hominis, U parvum, U urealyticum (culture neg) D+18 pleural fluid PCR M hominis, U parvum, U urealyticum (culture neg) D+67 BAL culture and PCR M hominis | D0–D+7 cefepime (prophylaxis) D+7–D+45 moxifloxacin and azithromycin D+7 sodium benxoate, arginine, levocarnitine, rifaximin, metronidazole, lactulose, CRRT D+71–D+102 (death) second treatment course moxifloxacin and minocycline | D+6 reduced conscious level, serum ammonia 177 μmol/L D+12 conscious state improved D+69 relapse M hominis and HS and diagnosis of PTLD Remained ventilator and dialysis dependent Refractory GI bleeding D+102 died |
| B | Cardiac | M, 63 | Sarcoid cardiomyopathy | Basiliximab (D0, D+4, D+10) Methylprednisolone (D+0) MMF (D+1) Tacrolimus (D+6) | D+8, D+10 blood culture and PCR M hominis D+10 sputum PCR M hominis, U parvum, U urealyticum (culture neg) D+10 pleural fluid PCR M hominis, U parvum, U urealyticum (culture neg) D+12 sternal tissue culture M hominis, PCR M hominis, U parvum, U urealyticum D+12 pericardial tissue PCR M hominis, U parvum, U urealyticum (culture neg) | D0 cefazolin (prophylaxis) D+8–D+10 piperacillin/tazobactam (empiric) D+9–D+12 vancomycin D+10–D+12 meropenem D+11 moxifloxacin and minocycline for 6 wk D+11 sodium benzoate, arginine, levocarnitine, rifaximin, metronidazole, lactulose, CRRT | D+8 altered mental state D+10 serum ammonia level 549 μmol/L, reintubated D+15 conscious state improved D+24 MRI brain changes of HS D+76 transient serum ammonia level rise to 313 μmol/L associated increased inotropic requirement for 48 h D+140 transferred to rehabilitation unit, dialysis dependent with critical illness myopathy, cognitive deficits, refractory sepsis D+254 died |
| C | Liver | F, 48 | Mesenchymal hepatic hematoma | Basiliximab (D0, D4) Methylprednisolone (D0) MMF (D0) Tacrolimus (D+1) | D+11 abdominal drain fluid culture and PCR M hominis | Pretransplant (–D0) norfloxacin (SBP prophylaxis) D0a D10–D+13 piperacillin/tazobactam (empiric) D+12 doxycycline for 2 wk | D+12 transient fever Normal serum ammonia level D+500 clinically well, good graft function |
| D | Renal | F, 26 | Chronic glomerulonephritis | Basiliximab (D+0, D+4) Methylprednisolone (D+0) MMF (D+0) Tacrolimus (D+1) | D+12 blood culture and PCR M hominis D+12 operative wound swab culture and PCR M hominis D+12 urine culture and PCR-positive M hominis, U parvum | D0 cefazolin (prophylaxis) D+12 doxycycline and ciprofloxacin for 2 wk | D+3 transient fever Normal serum ammonia level D+500 clinically well, good graft function |
| Patient . | Organ Transplanted . | Sex and Age, y . | Indication for Transplant . | Initial Immunosuppression . | Positive Recipient M hominis/Ureaplasma spp Culture or PCR . | Treatment . | Clinical Correlation . |
|---|---|---|---|---|---|---|---|
| A | Bilateral sequential lung | M, 65 | Emphysema | Basiliximab (D+0, D+4) Methylprednisolone (D+0) MMF (D+1) Ciclosporin (D+1) | D+4 BAL culture and PCR M hominis, U parvum, U urealyticum D+7 blood culture and PCR M hominis, U parvum D+9, D+14, D+19 BAL PCR M hominis, U parvum, U urealyticum (culture neg) D+18 pleural fluid PCR M hominis, U parvum, U urealyticum (culture neg) D+67 BAL culture and PCR M hominis | D0–D+7 cefepime (prophylaxis) D+7–D+45 moxifloxacin and azithromycin D+7 sodium benxoate, arginine, levocarnitine, rifaximin, metronidazole, lactulose, CRRT D+71–D+102 (death) second treatment course moxifloxacin and minocycline | D+6 reduced conscious level, serum ammonia 177 μmol/L D+12 conscious state improved D+69 relapse M hominis and HS and diagnosis of PTLD Remained ventilator and dialysis dependent Refractory GI bleeding D+102 died |
| B | Cardiac | M, 63 | Sarcoid cardiomyopathy | Basiliximab (D0, D+4, D+10) Methylprednisolone (D+0) MMF (D+1) Tacrolimus (D+6) | D+8, D+10 blood culture and PCR M hominis D+10 sputum PCR M hominis, U parvum, U urealyticum (culture neg) D+10 pleural fluid PCR M hominis, U parvum, U urealyticum (culture neg) D+12 sternal tissue culture M hominis, PCR M hominis, U parvum, U urealyticum D+12 pericardial tissue PCR M hominis, U parvum, U urealyticum (culture neg) | D0 cefazolin (prophylaxis) D+8–D+10 piperacillin/tazobactam (empiric) D+9–D+12 vancomycin D+10–D+12 meropenem D+11 moxifloxacin and minocycline for 6 wk D+11 sodium benzoate, arginine, levocarnitine, rifaximin, metronidazole, lactulose, CRRT | D+8 altered mental state D+10 serum ammonia level 549 μmol/L, reintubated D+15 conscious state improved D+24 MRI brain changes of HS D+76 transient serum ammonia level rise to 313 μmol/L associated increased inotropic requirement for 48 h D+140 transferred to rehabilitation unit, dialysis dependent with critical illness myopathy, cognitive deficits, refractory sepsis D+254 died |
| C | Liver | F, 48 | Mesenchymal hepatic hematoma | Basiliximab (D0, D4) Methylprednisolone (D0) MMF (D0) Tacrolimus (D+1) | D+11 abdominal drain fluid culture and PCR M hominis | Pretransplant (–D0) norfloxacin (SBP prophylaxis) D0a D10–D+13 piperacillin/tazobactam (empiric) D+12 doxycycline for 2 wk | D+12 transient fever Normal serum ammonia level D+500 clinically well, good graft function |
| D | Renal | F, 26 | Chronic glomerulonephritis | Basiliximab (D+0, D+4) Methylprednisolone (D+0) MMF (D+0) Tacrolimus (D+1) | D+12 blood culture and PCR M hominis D+12 operative wound swab culture and PCR M hominis D+12 urine culture and PCR-positive M hominis, U parvum | D0 cefazolin (prophylaxis) D+12 doxycycline and ciprofloxacin for 2 wk | D+3 transient fever Normal serum ammonia level D+500 clinically well, good graft function |
Abbreviations: BAL, bronchoalveolar lavage; CRRT, continuous renal replacement therapy; D+, day posttransplant; F, female; GI, gastrointestinal; HS, hyperammonemia syndrome; MRI, magnetic resonance imaging; M, male; M hominis, Mycoplasma hominis; MMF, mycophenolate mofetil; neg, negative; PCR, polymerase chain reaction; PTLD, posttransplant lymphoproliferative disease; SBP, spontaneous bacterial peritonitis; U parvum, Ureaplasma parvum; U urealyticum, Ureaplasma urealyticum.
Perioperative prophylaxis unknown.
Clinical and Microbiological Details of 4 Solid Organ Transplant Recipients With Mycoplasma hominis and Ureaplasma Species Infection Derived From a Single Donor, Complicated by Hyperammonemia Syndrome (Lung and Cardiac Recipients)
| Patient . | Organ Transplanted . | Sex and Age, y . | Indication for Transplant . | Initial Immunosuppression . | Positive Recipient M hominis/Ureaplasma spp Culture or PCR . | Treatment . | Clinical Correlation . |
|---|---|---|---|---|---|---|---|
| A | Bilateral sequential lung | M, 65 | Emphysema | Basiliximab (D+0, D+4) Methylprednisolone (D+0) MMF (D+1) Ciclosporin (D+1) | D+4 BAL culture and PCR M hominis, U parvum, U urealyticum D+7 blood culture and PCR M hominis, U parvum D+9, D+14, D+19 BAL PCR M hominis, U parvum, U urealyticum (culture neg) D+18 pleural fluid PCR M hominis, U parvum, U urealyticum (culture neg) D+67 BAL culture and PCR M hominis | D0–D+7 cefepime (prophylaxis) D+7–D+45 moxifloxacin and azithromycin D+7 sodium benxoate, arginine, levocarnitine, rifaximin, metronidazole, lactulose, CRRT D+71–D+102 (death) second treatment course moxifloxacin and minocycline | D+6 reduced conscious level, serum ammonia 177 μmol/L D+12 conscious state improved D+69 relapse M hominis and HS and diagnosis of PTLD Remained ventilator and dialysis dependent Refractory GI bleeding D+102 died |
| B | Cardiac | M, 63 | Sarcoid cardiomyopathy | Basiliximab (D0, D+4, D+10) Methylprednisolone (D+0) MMF (D+1) Tacrolimus (D+6) | D+8, D+10 blood culture and PCR M hominis D+10 sputum PCR M hominis, U parvum, U urealyticum (culture neg) D+10 pleural fluid PCR M hominis, U parvum, U urealyticum (culture neg) D+12 sternal tissue culture M hominis, PCR M hominis, U parvum, U urealyticum D+12 pericardial tissue PCR M hominis, U parvum, U urealyticum (culture neg) | D0 cefazolin (prophylaxis) D+8–D+10 piperacillin/tazobactam (empiric) D+9–D+12 vancomycin D+10–D+12 meropenem D+11 moxifloxacin and minocycline for 6 wk D+11 sodium benzoate, arginine, levocarnitine, rifaximin, metronidazole, lactulose, CRRT | D+8 altered mental state D+10 serum ammonia level 549 μmol/L, reintubated D+15 conscious state improved D+24 MRI brain changes of HS D+76 transient serum ammonia level rise to 313 μmol/L associated increased inotropic requirement for 48 h D+140 transferred to rehabilitation unit, dialysis dependent with critical illness myopathy, cognitive deficits, refractory sepsis D+254 died |
| C | Liver | F, 48 | Mesenchymal hepatic hematoma | Basiliximab (D0, D4) Methylprednisolone (D0) MMF (D0) Tacrolimus (D+1) | D+11 abdominal drain fluid culture and PCR M hominis | Pretransplant (–D0) norfloxacin (SBP prophylaxis) D0a D10–D+13 piperacillin/tazobactam (empiric) D+12 doxycycline for 2 wk | D+12 transient fever Normal serum ammonia level D+500 clinically well, good graft function |
| D | Renal | F, 26 | Chronic glomerulonephritis | Basiliximab (D+0, D+4) Methylprednisolone (D+0) MMF (D+0) Tacrolimus (D+1) | D+12 blood culture and PCR M hominis D+12 operative wound swab culture and PCR M hominis D+12 urine culture and PCR-positive M hominis, U parvum | D0 cefazolin (prophylaxis) D+12 doxycycline and ciprofloxacin for 2 wk | D+3 transient fever Normal serum ammonia level D+500 clinically well, good graft function |
| Patient . | Organ Transplanted . | Sex and Age, y . | Indication for Transplant . | Initial Immunosuppression . | Positive Recipient M hominis/Ureaplasma spp Culture or PCR . | Treatment . | Clinical Correlation . |
|---|---|---|---|---|---|---|---|
| A | Bilateral sequential lung | M, 65 | Emphysema | Basiliximab (D+0, D+4) Methylprednisolone (D+0) MMF (D+1) Ciclosporin (D+1) | D+4 BAL culture and PCR M hominis, U parvum, U urealyticum D+7 blood culture and PCR M hominis, U parvum D+9, D+14, D+19 BAL PCR M hominis, U parvum, U urealyticum (culture neg) D+18 pleural fluid PCR M hominis, U parvum, U urealyticum (culture neg) D+67 BAL culture and PCR M hominis | D0–D+7 cefepime (prophylaxis) D+7–D+45 moxifloxacin and azithromycin D+7 sodium benxoate, arginine, levocarnitine, rifaximin, metronidazole, lactulose, CRRT D+71–D+102 (death) second treatment course moxifloxacin and minocycline | D+6 reduced conscious level, serum ammonia 177 μmol/L D+12 conscious state improved D+69 relapse M hominis and HS and diagnosis of PTLD Remained ventilator and dialysis dependent Refractory GI bleeding D+102 died |
| B | Cardiac | M, 63 | Sarcoid cardiomyopathy | Basiliximab (D0, D+4, D+10) Methylprednisolone (D+0) MMF (D+1) Tacrolimus (D+6) | D+8, D+10 blood culture and PCR M hominis D+10 sputum PCR M hominis, U parvum, U urealyticum (culture neg) D+10 pleural fluid PCR M hominis, U parvum, U urealyticum (culture neg) D+12 sternal tissue culture M hominis, PCR M hominis, U parvum, U urealyticum D+12 pericardial tissue PCR M hominis, U parvum, U urealyticum (culture neg) | D0 cefazolin (prophylaxis) D+8–D+10 piperacillin/tazobactam (empiric) D+9–D+12 vancomycin D+10–D+12 meropenem D+11 moxifloxacin and minocycline for 6 wk D+11 sodium benzoate, arginine, levocarnitine, rifaximin, metronidazole, lactulose, CRRT | D+8 altered mental state D+10 serum ammonia level 549 μmol/L, reintubated D+15 conscious state improved D+24 MRI brain changes of HS D+76 transient serum ammonia level rise to 313 μmol/L associated increased inotropic requirement for 48 h D+140 transferred to rehabilitation unit, dialysis dependent with critical illness myopathy, cognitive deficits, refractory sepsis D+254 died |
| C | Liver | F, 48 | Mesenchymal hepatic hematoma | Basiliximab (D0, D4) Methylprednisolone (D0) MMF (D0) Tacrolimus (D+1) | D+11 abdominal drain fluid culture and PCR M hominis | Pretransplant (–D0) norfloxacin (SBP prophylaxis) D0a D10–D+13 piperacillin/tazobactam (empiric) D+12 doxycycline for 2 wk | D+12 transient fever Normal serum ammonia level D+500 clinically well, good graft function |
| D | Renal | F, 26 | Chronic glomerulonephritis | Basiliximab (D+0, D+4) Methylprednisolone (D+0) MMF (D+0) Tacrolimus (D+1) | D+12 blood culture and PCR M hominis D+12 operative wound swab culture and PCR M hominis D+12 urine culture and PCR-positive M hominis, U parvum | D0 cefazolin (prophylaxis) D+12 doxycycline and ciprofloxacin for 2 wk | D+3 transient fever Normal serum ammonia level D+500 clinically well, good graft function |
Abbreviations: BAL, bronchoalveolar lavage; CRRT, continuous renal replacement therapy; D+, day posttransplant; F, female; GI, gastrointestinal; HS, hyperammonemia syndrome; MRI, magnetic resonance imaging; M, male; M hominis, Mycoplasma hominis; MMF, mycophenolate mofetil; neg, negative; PCR, polymerase chain reaction; PTLD, posttransplant lymphoproliferative disease; SBP, spontaneous bacterial peritonitis; U parvum, Ureaplasma parvum; U urealyticum, Ureaplasma urealyticum.
Perioperative prophylaxis unknown.
Patient A
The bilateral lung transplant recipient became drowsy, with unilateral weakness at day 6 posttransplant (day +6). He remained intubated and ventilated due to poor lung compliance; sedation had been weaned. Computed tomography (CT) of the brain showed no acute changes. Serum ammonia peaked day +6 at 177 µmol/L (normal <60 µmol/L). Culture and PCR were positive for M hominis and U parvum from blood, bronchoalveolar lavage fluid (BALF), and pleural fluid (Table 1). PCR was positive for U urealyticum (culture negative) from BALF. He was treated with ammonia scavengers and was given an empirical antibiotic duration (to day +45) of moxifloxacin with azithromycin. Continuous renal replacement therapy (CRRT) was commenced for ammonia clearance and posttransplant acute kidney injury. This treatment protocol adapted from Chen et al has been used in this center for the management of hyperammonemia after lung transplant [4].
The patient’s conscious state improved by day +12. Infection relapsed on day +67 when BALF cultured M hominis associated with ammonia elevation to 150 µmol/L and reduced conscious state, prompting treatment with moxifloxacin and minocycline. He developed dense pulmonary nodules on chest CT and recurrent gastrointestinal bleeding from ileal ulceration. Ileal and transbronchial biopsies did not show any abnormalities; however, on the basis of elevated peripheral blood Epstein-Barr virus (EBV) viral load (994 IU/mL at day +69, rising to 13 352 IU/mL at day +87), a presumptive diagnosis of posttransplant lymphoproliferative disorder (PTLD) was made. A single 1-g dose of rituximab was administered, and mycophenolate was withheld. He remained ventilator and dialysis dependent with poor conscious state and progressive global weakness. He suffered severe refractory gastrointestinal bleeding, received palliative care, and died on day +102. Limited postmortem examination of lung and intestinal tissue demonstrated features consistent with EBV-driven PTLD in a lymphomatoid granulomatosis pattern.
Patient B
The cardiac transplant recipient developed a reduced conscious state, hallucinations, and worsening renal function at day +8. He was treated with empiric antibiotics for sepsis (Table 1). Serum ammonia peaked at day +10 at 549 µmol/L. At this time, the diagnosis of HS in the heart and lung recipients prompted communication to the donor procurement agency to screen other organ recipients for Mycoplasma/Ureaplasma spp infection and HS. Magnetic resonance imaging (MRI) of the brain at day +10 and cerebrospinal fluid analysis were unremarkable; however, brain MRI at day +24 showed findings consistent with changes found in hyperammonemic encephalopathy (Figure 1) [5, 6]. Culture and PCR were positive for M hominis and U parvum from blood, sputum, pleural fluid, sternal tissue, and pericardium. PCR was positive (culture negative) for U urealyticum from sputum, pleural fluid, and sternal and pericardial tissue. The patient received a 6-week course of moxifloxacin with minocycline, ammonia scavenger agents, and CRRT. Conscious state improved by day +15. Scedosporium apiospermum complex was identified in pleural fluid, sternal tissue, and pericardial tissue on day +10 and persisted in pleural and pericardial fluid on day +127 despite 2 sternal washouts and antifungal therapy. Scedosporium infection was not diagnosed in the donor or other recipients. He remained dialysis dependent with persistent critical illness myopathy and cognitive deficits. He suffered recurrent episodes of sepsis, received palliative care, and died on day +254.
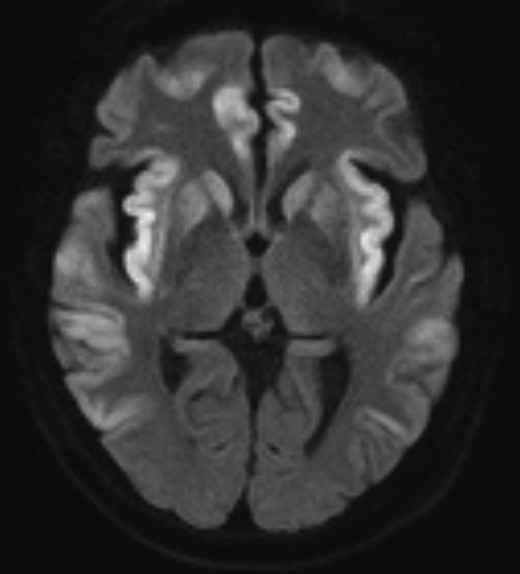
Magnetic resonance diffusion-weighted imaging of the brain of the cardiac recipient at day 24 posttransplant demonstrating changes of hyperammonemic encephalopathy: symmetrical cortical edema and diffusion restriction in the basal ganglia, insula cortex, cingulate cortex, temporal lobes, and frontal lobes, and sparing of the occipital lobes.
Patient C
The liver recipient developed fever, positive culture, and PCR for M hominis from abdominal drain fluid at day +11. Blood culture and PCR were negative at day +13 and day +19. Serum ammonia was consistently <9 µmol/L. A 2-week duration of doxycycline was given from day +12 (Table 1).
Patient D
One kidney recipient had transient fever on day +3, and serous operative wound discharge. Mycoplasma hominis was detected by culture and PCR in blood, urine, and wound swab. Urine was also culture and PCR positive for U parvum. Serum ammonia on day +12 was 14 µmol/L. The patient was given a 2-week duration of ciprofloxacin with doxycycline starting day +12. There was resolution of operative wound discharge (Table 1).
Patient E
The other kidney recipient was managed in another jurisdiction. They did not develop hyperammonemia or clinical signs of infection, and Mycoplasma/Ureaplasma spp testing was negative. They received antimicrobial therapy against these species after identification of infection in other recipients, though specific choice and duration are unknown.
Phylogenetic Analysis
Positive PCR samples could not be effectively enriched for adequate sequencing coverage, and Ureaplasma could not be subcultured to enable sequencing. The genomic DNA of 7 M hominis culture isolates from lung, cardiac, and kidney recipients were extracted with the QIAGEN DNeasy Blood and Tissue Kit (Qiagen, 69504). DNA libraries were prepared using the Nextera XT library preparation kit (Illumina) and sequenced on the NextSeq 500 Illumina platform. Raw sequence reads were examined for quality and trimmed using Trimmomatic version 0.4. Single-nucleotide polymorphisms (SNPs) were identified by mapping the quality-trimmed paired-end reads to the M hominis ATCC 23114 (accession number NC_013511) reference genome using NASP [7]. SNPs were called with minimum 10× depth and base frequency 0.9. A maximum likelihood tree was inferred in IQTree with the best-fit substitution model automatically selected during the run, and 1000 ultrafast bootstrap replicates.
Recipient M hominis genomes mapped to 93.2%–93.5% of the reference genome with an average depth 200× coverage. The M hominis genomes were closely clustered with only 9–33 SNPs (over a 665 445 bp alignment) between recipient isolates and 19–33 SNPs within recipient isolates compared to 7608–10 879 SNPs to unrelated isolates, consistent with donor origin of infection (Figure 2).
![Maximum likelihood phylogenetic tree of Mycoplasma hominis isolates. The tree includes 7 M hominis isolates from this study, 3 isolates from Hinić et al [8], 14 isolates from Smibert et al [9], and the reference isolate ATCC 23114. The scale bar represents the number of nucleotide substitutions per site. B927004049 (cardiac, blood, day +10), B929002719 (kidney, urine, day +11), B927005959 (cardiac, blood, day +10), B921005224 (lung, bronchoalveolar lavage, day +4), B929002693 (kidney, blood, day +12), B929012377 (cardiac, sternum, day +12), B925002262 (cardiac, blood, day +8).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ofid/10/6/10.1093_ofid_ofad263/1/m_ofad263f2.jpeg?Expires=1750189345&Signature=u4iAapfKIgweXHEcdvQKmKKqhgw4Ywfv6Ilrh3vQ5y3cvaavGUdCc53f-mfmQRdOoTfsqkMrKXjWHxnRM4kO4jfKePEOm0cdNxElO1cBZAigPxZ0O8C3cmijvA1E4KE63g44ClFAvZ-ASTFFWcgEEgQaJ7~rNr-88EDgoXt1Vi2z38yutWsT47Y9dsQjV2RBlHzCD1xa0x0jj~IIWs78-SUFgTJaqrmBaeQCZtH0GaZ6Hy7x8eqYU43AqUWMQq~yGMrFg7a5Z2XCdW6VnJgoT8XRKr30hgI1kH3h~Y~VoimoCynt97Ho5CL5ARLFfRclUN~StLsR-RBhuOy2G-QE6g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Maximum likelihood phylogenetic tree of Mycoplasma hominis isolates. The tree includes 7 M hominis isolates from this study, 3 isolates from Hinić et al [8], 14 isolates from Smibert et al [9], and the reference isolate ATCC 23114. The scale bar represents the number of nucleotide substitutions per site. B927004049 (cardiac, blood, day +10), B929002719 (kidney, urine, day +11), B927005959 (cardiac, blood, day +10), B921005224 (lung, bronchoalveolar lavage, day +4), B929002693 (kidney, blood, day +12), B929012377 (cardiac, sternum, day +12), B925002262 (cardiac, blood, day +8).
DISCUSSION
We describe donor-derived M hominis and Ureaplasma spp infection with transmission to 4 of 5 SOT recipients, and the development of HS in the thoracic recipients. HS early after SOT is increasingly attributed to infection with these organisms, in particular Ureaplasma spp infection in lung transplant recipients [2, 10–12].
Our series demonstrates that not all infected SOT recipients infected with M hominis/Ureaplasma spp develop HS, as our kidney and liver recipients had normal ammonia levels. In a lung transplant case series, only 10 of 24 Ureaplasma spp–positive recipients developed HS [1]. While not described in this meta-analysis, this may have been influenced by Ureaplasma-active peritransplant antimicrobials, which seemed to prevent HS in another study [10]. Apart from norfloxacin use prior to the liver transplant, none of the recipients in our case series had Mycoplasma/Ureaplasma-active antibiotics empirically directly after transplant. Lung recipients are particularly susceptible to donor-derived M hominis/Ureaplasma spp infection and HS as these species colonize the respiratory tract (26% of post–lung transplant BALF samples were positive in 1 study [2]).
Our case series demonstrates the unique finding of M hominis and Ureaplasma spp dissemination from an immunocompetent donor across 4 different organ recipients, with genomic data supporting this hypothesis for donor-derived M hominis infection. Bloodstream dissemination to donor organs may have been precipitated by indwelling catheter insertion in the context of urethritis and critical illness, though aspiration remains an alternative primary source of bacteremia. We postulate that HS development in the cardiac and lung recipients and not liver or kidney recipients was related to the increased physiological stress of cardiothoracic surgery, which may induce a catabolic state, uncovering a urea cycle or glutamine synthetase deficit [11]. The physiological stress of surgery, combined with higher immunosuppression doses in thoracic recipients, increases the risk of sepsis and associated organ dysfunction [11, 12]. Acute kidney injury (AKI) reduces the urinary excretion of ammonia, which is important for keeping serum ammonia at steady state [13]. AKI occurs in in 60%–70% of lung recipients and 10%–15% of cardiac recipients [14, 15], and may have been a contributing factor for HS noting that only the thoracic recipients developed AKI in the early posttransplant period.
Mycoplasma hominis/Ureaplasma spp donor-derived infection may go undetected because clinical manifestations may be mild or absent, as demonstrated in our liver and kidney recipients despite bacteremia. Subsequent testing for these organisms was prompted only by illness in the thoracic recipients. Although administered late, antimicrobial therapy to liver and kidney recipients may have averted clinical disease, and this highlights the importance of timely reporting and communication between transplant teams and donation agencies. Additionally, laboratory diagnosis requires specialized culture or PCR and will not be detected by routine microbiological cultures. In our case, nonblood culture specimens were cultured onto A7 agar in anaerobic conditions for 48 hours, and BACTEC blood culture bottles (regardless of positivity) were blind-subcultured similarly after 48 and 120 hours of incubation. These factors may explain why we could only find 1 other report of donor transmission to >1 SOT recipient, and only 2 other reports in cardiac recipients [8, 11, 16].
HS after transplant has a high mortality rate, reported to be approximately 75% of cases in lung transplant recipients [10, 17–19]. In the lung transplant population, a higher mortality was noted in Ureaplasma spp–associated HS compared to HS from other causes (21% vs 2.8%) [1]. In case studies, neurological deficits were present in 15 of 25 patients who survived (18/43 died) [1]. Infection may have other consequences, as we consider whether M hominis/Ureaplasma spp infection contributed to EBV reactivation and PTLD in the lung recipient, which has been demonstrated with other persisting infections [20, 21]. In lung transplant recipients, Ureaplasma spp infection (10.2%) and hyperammonemia (6.8%) are common, and preemptive M hominis/Ureaplasma spp antibiotic treatment may prevent the development of HS, leading some units to routinely test lung donor and/or recipient respiratory tract for M hominis/Ureaplasma spp and to administer preemptive antimicrobials to lung recipients if results will be delayed [22, 23].
We did not have the required media to perform susceptibility testing; however, in a recent multinational study using standardized susceptibility testing, levofloxacin and moxifloxacin were routinely active against M hominis/Ureaplasma spp [24]. Tetracycline susceptibility was found in 97% of Ureaplasma spp and 78% of M hominis. All M hominis were clindamycin susceptible (Ureaplasma spp are intrinsically resistant) and all Ureaplasma spp were macrolide susceptible (M hominis are intrinsically resistant) [24]. Due to concern for resistance, combination therapy has been suggested [23]. A quinolone with/without a tetracycline is expected to be effective therapy for M hominis/Ureaplasma spp or a combination including a quinolone, a tetracycline, and azithromycin if Ureaplasma spp are predominantly targeted.
CONCLUSIONS
Donor-derived M hominis/Ureaplasma spp infection occurred in 4 SOT recipients from a single donor, leading to HS and significant consequences in the thoracic recipients. Some units perform routine testing of lung donor and/or recipient respiratory tract for M hominis/Ureaplasma spp, with preemptive administration of active antimicrobials to lung recipients if screening results are delayed. In all SOT recipients, altered conscious state or culture-negative infection early after transplantation should prompt M hominis/Ureaplasma spp and serum ammonia testing.
Notes
Author contributions. All authors designed the study. C. W., M. L., R. L., D. S., D. C., G. W.-B., S. M., and P. B. performed the analysis. C. W., M. L., R. L., D. S., and P. B. drafted the manuscript. All authors revised the manuscript.
Patient consent. Written consent was obtained from all patients prior to transplant for research and publication.
Data availability. The raw sequencing data were uploaded to the National Center for Biotechnology Information Sequence Read Archive under BioProject PRJNA924736.
References
Author notes
Potential conflicts of interest. All authors: No reported conflicts.





Comments