-
PDF
- Split View
-
Views
-
Cite
Cite
Lucinda J Van Anglen, Claudia P Schroeder, Kimberly A Couch, A Real-world Multicenter Outpatient Experience of Ceftolozane/Tazobactam, Open Forum Infectious Diseases, Volume 10, Issue 5, May 2023, ofad173, https://doi.org/10.1093/ofid/ofad173
Close - Share Icon Share
Abstract
Ceftolozane/tazobactam (C/T) is indicated for the treatment of complicated intra-abdominal infection (IAI), complicated urinary tract infection (UTI), and hospital-acquired/ventilator-associated bacterial pneumonia caused by susceptible bacteria. As real-world data are limited, we report utilization and associated outcomes of C/T use in the outpatient setting.
This is a multicenter, retrospective study of patients who received C/T between May 2015 and December 2020. Demographics, infection types, C/T utilization characteristics, microbiology, and health care resource utilization were collected. Clinical success was defined as complete or partial symptom resolution at completion of C/T. Persistent infection and discontinuation of C/T were deemed nonsuccess. Logistic regression analysis was used to identify predictors associated with clinical outcomes.
A total of 126 patients (median age, 59 years; 59% male; median Charlson index, 5) from 33 office infusion centers were identified. Infection types included 27% bone and joint infection (BJI), 23% UTI, 18% respiratory tract infection (RTI), 16% IAI, 13% complicated skin and soft tissue infection (cSSTI), and 3% bacteremia. The median daily dose of C/T was 4.5 g, primarily administered via elastomeric pumps as intermittent infusion. The most common gram-negative pathogen was P. aeruginosa (63%), 66% of which was multidrug-resistant and 45% carbapenem-resistant. Enterobacterales was identified in 26% of isolates, of which 44% were extended-spectrum beta-lactamase producers. The overall clinical success rate of C/T was 84.7%. Nonsuccessful outcomes were due to persistent infections (9.7%) and drug discontinuations (5.6%).
C/T was successfully used in the outpatient setting to treat a variety of serious infections with a high prevalence of resistant pathogens.
Ceftolozane/tazobactam (C/T) is indicated to treat adults with complicated intra-abdominal infection (IAI), complicated urinary tract infection (UTI) including pyelonephritis, hospital-acquired bacterial pneumonia (HABP), and ventilator-associated bacterial pneumonia (VABP) caused by susceptible microorganisms [1–4]. It was the first cephalosporin/beta-lactamase inhibitor combination antibiotic approved by the US Food and Drug Administration [5]. In addition to randomized clinical trials, various real-world observational studies have demonstrated safe and effective use of C/T for the treatment of IAI, UTI, and respiratory tract infection (RTI) [6–13]. Other postmarketing studies have reported clinical outcomes of C/T in bacteremia [8–14], complicated skin and soft tissue infection (cSSTI) [8–10, 12, 15], and bone and joint infection (BJI) including osteomyelitis [8–11, 13–18], with most of them conducted in acute care settings.
C/T has demonstrated an extended spectrum of activity against Pseudomonas aeruginosa including difficult-to-treat and multidrug-resistant (MDR) strains and common gram-negative bacteria including extended-spectrum beta-lactamase (ESBL)–producing Enterobacterales [19]. “Difficult to treat” is defined as P. aeruginosa that exhibits nonsusceptitiblity to all of the following: piperacillin-tazobactam, ceftazidime, cefepime, aztreonam, meropenem, imipenem-cilastatin, ciprofloxacin, and levofloxacin [20]. In addition, C/T exhibits activity against gram-negative anaerobes mostly limited to Bacteroides fragilis and some Streptococcus spp. [21]. However, while C/T demonstrates in vitro activity against gram-positive, aerobic, and anaerobic bacteria, this activity is clinically limited and may require addition of more highly active agents [5]. The increasing resistance of gram-negative pathogens to standard antimicrobial agents has become a major concern in health care facilities and the community setting [20]. Before C/T, there were only a few treatment options for MDR Pseudomonas aeruginosa. Most of the treatments, including colistin and polymyxin B, precluded transition to outpatient therapy due to potential adverse events, especially nephrotoxicity [22–24]. In addition, real-world experience of C/T utilization and associated outcomes in the outpatient setting is limited to case studies [6, 11, 14, 16, 18, 25]. Previous studies have reported successful outcomes following continuous infusion of C/T for P. aeruginosa infections in both inpatient and outpatient settings [11, 14]. Other case reports have demonstrated successful outcomes with C/T in the community including 1 patient with MDR P. aeruginosa UTI [6], 1 patient with carbapenem-resistant (CR) P. aeruginosa lung abscess [25], and 2 cases with resistant P. aeruginosa osteomyelitis [16, 18].
Outpatient parenteral antimicrobial therapy (OPAT) offers safe and effective care to patients with serious infections outside the hospital [26]. Provision of OPAT through an office infusion center (OIC) with infusion pharmacy services has demonstrated high rates of success for the treatment of moderate to severe infections [27, 28]. The infusion centers are staffed with highly trained clinical pharmacists and nurses, who adhere to standardized protocols and documentation under the supervision of infectious disease (ID) physicians. C/T is well suited for intravenous administration through OICs and similar outpatient settings given its stability for up to 24 hours at room temperature and up to 7 days at refrigerated storage conditions [29–31].
The aim of this real-world experience study was to evaluate utilization characteristics and clinical outcomes of C/T for the treatment of complicated infections in the outpatient setting and to analyze variables associated with nonsuccess.
METHODS
Study Design and Data Collection
We conducted a multicenter, retrospective review of patients (≥18 years) who received C/T for ≥72 hours through ID office infusion centers between May 2015 and December 2020. Data from 33 geographically diverse OICs in the United States were included. Approval was obtained by an independent institutional review board (Advarra/IntegReview IRB, Austin, TX, USA).
Data collection from electronic health care records included demographics (age, gender), body mass index (BMI), patient location before outpatient treatment, comorbid conditions, infection type, and intravenous antibiotic therapies received within 72 hours before C/T. Comorbidity burden was assessed using the Charlson comorbidity index. Baseline creatinine clearance (CrCl) was determined using the Cockcroft-Gault equation and serum creatinine level (SCr). Utilization characteristics of C/T included OPAT duration (days), dosage (g) per day, frequency of infusion, and method of administration. Concomitant use of intravenous antibiotics was recorded. C/T (1 g/0.5 g) was compounded and dispensed by sterile products pharmacies under controlled aseptic conditions, allowing stability for up to 24 hours at room temperature and up to 7 days at refrigerated temperature at 2°C–8°C [31]. C/T was delivered in either elastomeric infusion pumps for self-administration at home or polyvinyl chloride (PVC) bags for use with ambulatory or stationary infusion pumps. Clinical outcomes of C/T were assessed at the end of outpatient therapy by the prescribing ID physician at each site.
Study Definitions
Compromised immunity was defined as use of immunosuppressive medication (steroids, methotrexate, biologics, chemotherapy) and/or presence of an underlying disease (immune deficiency, organ transplant, chronic kidney disease). Antimicrobial susceptibilities and ESBL testing of gram-negative pathogens were collected from laboratory reports obtained at baseline. MDR was defined as nonsusceptibility to at least 1 agent in 3 or more classes of antimicrobials [32]. Carbapenem-resistant (CR) P. aeruginosa was defined as any isolate that tested intermediate or resistant to imipenem and/or meropenem [33]. Outcomes were assessed at the completion of therapy. Clinical success was defined as complete or partial resolution of signs and symptoms of infection without the need for escalation of intravenous antibiotics; switch to oral antibiotics was allowed. Persistent or recurrent infection, development of new signs and symptoms of infection, and the need for discontinuation of C/T were deemed nonsuccess. An indeterminant outcome was reported as nonevaluable. Health care resource utilization during outpatient therapy with C/T was recorded and included hospitalizations and emergency department visits.
Statistical Analysis
Baseline characteristics were evaluated using descriptive statistics and expressed as absolute frequency (%) for categorical variables and mean ± SD or median and interquartile range (IQR) for continuous variables. Bivariate comparison of baseline variables between patients with success and nonsuccess at the end of OPAT was performed using the chi-square test or Fisher exact test for categorical variables and Wilcoxon rank test for continuous variables, with P values of <.05 considered statistically significant. Nonevaluable outcomes were reported but not included in the analysis. Statistical data analyses were conducted using SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Patient Characteristics
A total of 126 patients received C/T during the study period. Baseline demographics and clinical characteristics of the cohort are shown in Table 1. The median age (IQR) was 59 (49–69) years, 38% were ≥65 years, 59% were male, and 43% had a BMI ≥30 kg/m2. At baseline, 70 patients (56%) had a CrCl >90 mL/min, 39 (31%) between 51 and 90 mL/min, 11 (9%) between 30 and 50 mL/min, and 4 (3%) had <30 mL/min, with 1 patient receiving intermittent hemodialysis. Before initiation of C/T in the OIC, 56% of patients (n = 71) were directly treated in the community without prior hospitalization. Consequently, 44% of patients (n = 55) were discharged from a hospital to initiate OPAT in the outpatient setting, following a mean length of inpatient stay of 7 ± 5 days. The median Charlson comorbidity index score (IQR) was 5 (3–7). The most common comorbid conditions were cardiovascular disease (n = 85, 67%), diabetes mellitus (n = 49, 39%), immunocompromised state (n = 49, 39%), asthma/chronic obstructive pulmonary disease (n = 29, 23%), current malignancy (n = 28, 22%), chronic kidney disease (n = 15, 12%), and liver disease (n = 8, 6%). Infection types treated with C/T were 27% bone and joint (n = 34), 23% urinary tract (n = 29), 18% respiratory tract (n = 23), 16% IAI (n = 20), 13% cSSTI (n = 17), and 3% bacteremia (n = 3), with endocarditis being the source in 2 patients. For the BJI cohort, 50% of patients (n = 17) had osteomyelitis secondary to a diabetic foot infection. Concurrent bacteremia occurred in 12 patients (10%) including 5 BJI, 2 cSSTI, 2 UTI, 2 RTI, and 1 IAI. Half of the patients (n = 63) received other intravenous antibiotic therapies for GN pathogens before C/T, most commonly carbapenems (n = 23).
| Characteristics . | Results (n = 126), No. (%) . |
|---|---|
| Age, median (IQR), y | 59 (49–69) |
| ≥65 y | 48 (38) |
| Male gender | 74 (59) |
| BMI, median (IQR), kg/m2 | 28 (24–34) |
| BMI ≥30 kg/m2 | 54 (43) |
| Baseline CrCl | 94 (67–134) |
| >90 mL/min | 70 (56) |
| 51–90 mL/min | 39 (31) |
| 30–50 mL/min | 10 (9) |
| <30 mL/min | 4 (3) |
| Location before C/T | |
| Community | 71 (56) |
| Hospital | 55 (44) |
| Charlson comorbidity index, median (IQR) | 5 (3–7) |
| Common comorbid conditions | |
| Cardiovascular disease | 85 (67) |
| Diabetes mellitus | 49 (39) |
| Immunocompromised | 49 (39) |
| Asthma/COPD | 29 (23) |
| Malignancy (current) | 28 (22) |
| Chronic kidney disease | 15 (12) |
| Liver disease | 8 (6) |
| Infection type | |
| Bone and joint | 34 (27) |
| Osteomyelitis | 29 |
| Spinal infection | 3 |
| Prosthetic joint infection | 1 |
| Septic arthritis | 1 |
| Urinary tract | 29 (23) |
| Complicated urinary tract infection | 25 |
| Pyelonephritis | 4 |
| Respiratory tract | 23 (18) |
| Pneumonia | 16 |
| Bronchitis | 3 |
| Pansinusitis | 2 |
| Empyema | 1 |
| Otitis media | 1 |
| Intra-abdominal | 20 (16) |
| Complicated intra-abdominal abscess | 13 |
| Diverticulitis | 4 |
| Appendicitis | 2 |
| Peritonitis | 1 |
| Complicated skin and soft tissue | 17 (13) |
| Abscess | 9 |
| Cellulitis | 8 |
| Bacteremia | 3 (3) |
| Endocarditis | 2 |
| CLABSI | 1 |
| Concurrent bacteremia | 12 (10) |
| Intravenous antibiotic therapy before C/T | 63 (50) |
| Characteristics . | Results (n = 126), No. (%) . |
|---|---|
| Age, median (IQR), y | 59 (49–69) |
| ≥65 y | 48 (38) |
| Male gender | 74 (59) |
| BMI, median (IQR), kg/m2 | 28 (24–34) |
| BMI ≥30 kg/m2 | 54 (43) |
| Baseline CrCl | 94 (67–134) |
| >90 mL/min | 70 (56) |
| 51–90 mL/min | 39 (31) |
| 30–50 mL/min | 10 (9) |
| <30 mL/min | 4 (3) |
| Location before C/T | |
| Community | 71 (56) |
| Hospital | 55 (44) |
| Charlson comorbidity index, median (IQR) | 5 (3–7) |
| Common comorbid conditions | |
| Cardiovascular disease | 85 (67) |
| Diabetes mellitus | 49 (39) |
| Immunocompromised | 49 (39) |
| Asthma/COPD | 29 (23) |
| Malignancy (current) | 28 (22) |
| Chronic kidney disease | 15 (12) |
| Liver disease | 8 (6) |
| Infection type | |
| Bone and joint | 34 (27) |
| Osteomyelitis | 29 |
| Spinal infection | 3 |
| Prosthetic joint infection | 1 |
| Septic arthritis | 1 |
| Urinary tract | 29 (23) |
| Complicated urinary tract infection | 25 |
| Pyelonephritis | 4 |
| Respiratory tract | 23 (18) |
| Pneumonia | 16 |
| Bronchitis | 3 |
| Pansinusitis | 2 |
| Empyema | 1 |
| Otitis media | 1 |
| Intra-abdominal | 20 (16) |
| Complicated intra-abdominal abscess | 13 |
| Diverticulitis | 4 |
| Appendicitis | 2 |
| Peritonitis | 1 |
| Complicated skin and soft tissue | 17 (13) |
| Abscess | 9 |
| Cellulitis | 8 |
| Bacteremia | 3 (3) |
| Endocarditis | 2 |
| CLABSI | 1 |
| Concurrent bacteremia | 12 (10) |
| Intravenous antibiotic therapy before C/T | 63 (50) |
Abbreviations: BMI, body mass index; CLABSI, central line bloodstream infection; COPD, chronic obstructive pulmonary disease; CrCl, creatinine clearance; C/T, ceftolozane/tazobactam.
All values represent % of total population unless otherwise indicated.
Estimated using Cockroft-Gault equation, available for 124 of 126 patients.
Including 1 patient on intermittent dialysis.
Due to immunosuppressive medication (chemotherapy, steroids, biologics) or underlying immune deficiency (cancer, AIDS, genetic disorder, autoimmune disease, organ transplant, chronic kidney disease).
Within 72 hours before initiation of C/T for gram-negative pathogens: meropenem (n = 16), imipenem/cilastatin (n = 5), ertapenem (n = 2), cefepime (n = 13), ceftazidime (n = 2), cefotaxime (n = 1), piperacillin/tazobactam (n = 14).
| Characteristics . | Results (n = 126), No. (%) . |
|---|---|
| Age, median (IQR), y | 59 (49–69) |
| ≥65 y | 48 (38) |
| Male gender | 74 (59) |
| BMI, median (IQR), kg/m2 | 28 (24–34) |
| BMI ≥30 kg/m2 | 54 (43) |
| Baseline CrCl | 94 (67–134) |
| >90 mL/min | 70 (56) |
| 51–90 mL/min | 39 (31) |
| 30–50 mL/min | 10 (9) |
| <30 mL/min | 4 (3) |
| Location before C/T | |
| Community | 71 (56) |
| Hospital | 55 (44) |
| Charlson comorbidity index, median (IQR) | 5 (3–7) |
| Common comorbid conditions | |
| Cardiovascular disease | 85 (67) |
| Diabetes mellitus | 49 (39) |
| Immunocompromised | 49 (39) |
| Asthma/COPD | 29 (23) |
| Malignancy (current) | 28 (22) |
| Chronic kidney disease | 15 (12) |
| Liver disease | 8 (6) |
| Infection type | |
| Bone and joint | 34 (27) |
| Osteomyelitis | 29 |
| Spinal infection | 3 |
| Prosthetic joint infection | 1 |
| Septic arthritis | 1 |
| Urinary tract | 29 (23) |
| Complicated urinary tract infection | 25 |
| Pyelonephritis | 4 |
| Respiratory tract | 23 (18) |
| Pneumonia | 16 |
| Bronchitis | 3 |
| Pansinusitis | 2 |
| Empyema | 1 |
| Otitis media | 1 |
| Intra-abdominal | 20 (16) |
| Complicated intra-abdominal abscess | 13 |
| Diverticulitis | 4 |
| Appendicitis | 2 |
| Peritonitis | 1 |
| Complicated skin and soft tissue | 17 (13) |
| Abscess | 9 |
| Cellulitis | 8 |
| Bacteremia | 3 (3) |
| Endocarditis | 2 |
| CLABSI | 1 |
| Concurrent bacteremia | 12 (10) |
| Intravenous antibiotic therapy before C/T | 63 (50) |
| Characteristics . | Results (n = 126), No. (%) . |
|---|---|
| Age, median (IQR), y | 59 (49–69) |
| ≥65 y | 48 (38) |
| Male gender | 74 (59) |
| BMI, median (IQR), kg/m2 | 28 (24–34) |
| BMI ≥30 kg/m2 | 54 (43) |
| Baseline CrCl | 94 (67–134) |
| >90 mL/min | 70 (56) |
| 51–90 mL/min | 39 (31) |
| 30–50 mL/min | 10 (9) |
| <30 mL/min | 4 (3) |
| Location before C/T | |
| Community | 71 (56) |
| Hospital | 55 (44) |
| Charlson comorbidity index, median (IQR) | 5 (3–7) |
| Common comorbid conditions | |
| Cardiovascular disease | 85 (67) |
| Diabetes mellitus | 49 (39) |
| Immunocompromised | 49 (39) |
| Asthma/COPD | 29 (23) |
| Malignancy (current) | 28 (22) |
| Chronic kidney disease | 15 (12) |
| Liver disease | 8 (6) |
| Infection type | |
| Bone and joint | 34 (27) |
| Osteomyelitis | 29 |
| Spinal infection | 3 |
| Prosthetic joint infection | 1 |
| Septic arthritis | 1 |
| Urinary tract | 29 (23) |
| Complicated urinary tract infection | 25 |
| Pyelonephritis | 4 |
| Respiratory tract | 23 (18) |
| Pneumonia | 16 |
| Bronchitis | 3 |
| Pansinusitis | 2 |
| Empyema | 1 |
| Otitis media | 1 |
| Intra-abdominal | 20 (16) |
| Complicated intra-abdominal abscess | 13 |
| Diverticulitis | 4 |
| Appendicitis | 2 |
| Peritonitis | 1 |
| Complicated skin and soft tissue | 17 (13) |
| Abscess | 9 |
| Cellulitis | 8 |
| Bacteremia | 3 (3) |
| Endocarditis | 2 |
| CLABSI | 1 |
| Concurrent bacteremia | 12 (10) |
| Intravenous antibiotic therapy before C/T | 63 (50) |
Abbreviations: BMI, body mass index; CLABSI, central line bloodstream infection; COPD, chronic obstructive pulmonary disease; CrCl, creatinine clearance; C/T, ceftolozane/tazobactam.
All values represent % of total population unless otherwise indicated.
Estimated using Cockroft-Gault equation, available for 124 of 126 patients.
Including 1 patient on intermittent dialysis.
Due to immunosuppressive medication (chemotherapy, steroids, biologics) or underlying immune deficiency (cancer, AIDS, genetic disorder, autoimmune disease, organ transplant, chronic kidney disease).
Within 72 hours before initiation of C/T for gram-negative pathogens: meropenem (n = 16), imipenem/cilastatin (n = 5), ertapenem (n = 2), cefepime (n = 13), ceftazidime (n = 2), cefotaxime (n = 1), piperacillin/tazobactam (n = 14).
Utilization Characteristics
Utilization characteristics of C/T by infection type are presented in Table 2. The overall median length of OPAT (IQR) was 21 (14–38) days. By infection type, the median durations of C/T for bacteremia and BJI (IQR) were 41 (28–44) days and 39 (26–42) days, respectively, compared with a median range from 14 days to 26 days for other indications. The median C/T dosage per day (range) was 4.5 (1.125–9.0) g with renally adjusted doses in 23 patients (18%) and >4.5 g C/T daily in 7 patients (3 BJI, 3 RTI, 1 bacteremia). Of the study cohort, 112 patients (89%) received C/T before US approval for the treatment of HABP/VABP at a dose of 3 g every 8 hours. In total, 123 patients (98%) received C/T intermittently, with the remaining 3 (2%) via continuous infusions. C/T was dispensed in elastomeric pumps in 83 patients (66%) for self-administration at home using intermittent dosing over 60 minutes. In addition, ambulatory pumps were used for administration of the total dose of C/T in PVC bags in 42 patients (33%) requiring daily pump bag changes in the OIC. Of these, 39 were programmed for intermittent dosing and 3 for continuous infusions (bacteremia, BJI, cSSTI). Another patient (1%) received a renally adjusted dosage of C/T once daily in the OIC using stationary pumps. Concomitant intravenous antibiotics were provided to 16 patients (13%) including 9 BJI, 5 cSSTI, 1 RTI, 1 UTI. Note, concomitant oral antibiotic use was reported in 22 patients (17%).
| Characteristic . | Overall . | BJI . | cUTI . | RTI . | IAI . | cSSTI . | Bacteremia . |
|---|---|---|---|---|---|---|---|
| (n = 126), No. (%) . | (n = 34), No. (%) . | (n = 29), No. (%) . | (n = 23), No. (%) . | (n = 20), No. (%) . | (n = 17), No. (%) . | (n = 3), No. (%) . | |
| OPAT duration, median (IQR), d | 21 (14–38) | 39 (26–42) | 14 (8–17) | 21 (14–29) | 21 (14–22) | 26 (19–54) | 41 (28–44) |
| C/T dosage per day, median (range) | 4.5 (1.125–9.0) | 4.5 (2.25–9.0) | 4.5 (2.25–4.5) | 4.5 (2.25–9.0) | 4.5 (1.125–4.5) | 4.5 (2.25–4.5) | 4.5 (1.125–9.0) |
| <4.5 g | 23 (18) | 5 (15) | 10 (34) | 2 (9) | 2 (10) | 3 (18) | 1 (33) |
| >4.5 g | 7 (6) | 3 (9) | … | 3 (13) | … | … | 1 (33) |
| Frequency of infusion | … | ||||||
| Intermittent | 123 (98) | 33 (97) | 29 (100) | 23 (100) | 20 (100) | 16 (94) | 2 (67) |
| Continuous | 3 (2) | 1 (3) | … | … | … | 1 (6) | 1 (33) |
| Method of administration | … | ||||||
| Elastomeric pump, self-administration at home | 83 (66) | 28 (82) | 14 (48) | 10 (43) | 16 (80) | 13 (76) | 2 (67) |
| Ambulatory pump, daily pump bag changes in OIC | 42 (33) | 6 (18) | 14 (48) | 13 (57) | 4 (20) | 4 (24) | 1 (33) |
| Stationary pump, daily administration in OIC | 1 (1) | … | … | … | … | … | … |
| Concomitant intravenous antibiotic therapy | 16 (13) | 9 (26) | 1 (3) | 1 (4) | … | 5 (29) | … |
| Characteristic . | Overall . | BJI . | cUTI . | RTI . | IAI . | cSSTI . | Bacteremia . |
|---|---|---|---|---|---|---|---|
| (n = 126), No. (%) . | (n = 34), No. (%) . | (n = 29), No. (%) . | (n = 23), No. (%) . | (n = 20), No. (%) . | (n = 17), No. (%) . | (n = 3), No. (%) . | |
| OPAT duration, median (IQR), d | 21 (14–38) | 39 (26–42) | 14 (8–17) | 21 (14–29) | 21 (14–22) | 26 (19–54) | 41 (28–44) |
| C/T dosage per day, median (range) | 4.5 (1.125–9.0) | 4.5 (2.25–9.0) | 4.5 (2.25–4.5) | 4.5 (2.25–9.0) | 4.5 (1.125–4.5) | 4.5 (2.25–4.5) | 4.5 (1.125–9.0) |
| <4.5 g | 23 (18) | 5 (15) | 10 (34) | 2 (9) | 2 (10) | 3 (18) | 1 (33) |
| >4.5 g | 7 (6) | 3 (9) | … | 3 (13) | … | … | 1 (33) |
| Frequency of infusion | … | ||||||
| Intermittent | 123 (98) | 33 (97) | 29 (100) | 23 (100) | 20 (100) | 16 (94) | 2 (67) |
| Continuous | 3 (2) | 1 (3) | … | … | … | 1 (6) | 1 (33) |
| Method of administration | … | ||||||
| Elastomeric pump, self-administration at home | 83 (66) | 28 (82) | 14 (48) | 10 (43) | 16 (80) | 13 (76) | 2 (67) |
| Ambulatory pump, daily pump bag changes in OIC | 42 (33) | 6 (18) | 14 (48) | 13 (57) | 4 (20) | 4 (24) | 1 (33) |
| Stationary pump, daily administration in OIC | 1 (1) | … | … | … | … | … | … |
| Concomitant intravenous antibiotic therapy | 16 (13) | 9 (26) | 1 (3) | 1 (4) | … | 5 (29) | … |
Abbreviations: C/T, ceftolozane/tazobactam; IQR, interquartile range; OIC, physician office infusion center; OPAT, outpatient antimicrobial therapy.
Values are expressed as number of patients (%), unless otherwise indicated.
Daptomycin (n = 10), vancomycin (n = 5), televancin (n = 1).
| Characteristic . | Overall . | BJI . | cUTI . | RTI . | IAI . | cSSTI . | Bacteremia . |
|---|---|---|---|---|---|---|---|
| (n = 126), No. (%) . | (n = 34), No. (%) . | (n = 29), No. (%) . | (n = 23), No. (%) . | (n = 20), No. (%) . | (n = 17), No. (%) . | (n = 3), No. (%) . | |
| OPAT duration, median (IQR), d | 21 (14–38) | 39 (26–42) | 14 (8–17) | 21 (14–29) | 21 (14–22) | 26 (19–54) | 41 (28–44) |
| C/T dosage per day, median (range) | 4.5 (1.125–9.0) | 4.5 (2.25–9.0) | 4.5 (2.25–4.5) | 4.5 (2.25–9.0) | 4.5 (1.125–4.5) | 4.5 (2.25–4.5) | 4.5 (1.125–9.0) |
| <4.5 g | 23 (18) | 5 (15) | 10 (34) | 2 (9) | 2 (10) | 3 (18) | 1 (33) |
| >4.5 g | 7 (6) | 3 (9) | … | 3 (13) | … | … | 1 (33) |
| Frequency of infusion | … | ||||||
| Intermittent | 123 (98) | 33 (97) | 29 (100) | 23 (100) | 20 (100) | 16 (94) | 2 (67) |
| Continuous | 3 (2) | 1 (3) | … | … | … | 1 (6) | 1 (33) |
| Method of administration | … | ||||||
| Elastomeric pump, self-administration at home | 83 (66) | 28 (82) | 14 (48) | 10 (43) | 16 (80) | 13 (76) | 2 (67) |
| Ambulatory pump, daily pump bag changes in OIC | 42 (33) | 6 (18) | 14 (48) | 13 (57) | 4 (20) | 4 (24) | 1 (33) |
| Stationary pump, daily administration in OIC | 1 (1) | … | … | … | … | … | … |
| Concomitant intravenous antibiotic therapy | 16 (13) | 9 (26) | 1 (3) | 1 (4) | … | 5 (29) | … |
| Characteristic . | Overall . | BJI . | cUTI . | RTI . | IAI . | cSSTI . | Bacteremia . |
|---|---|---|---|---|---|---|---|
| (n = 126), No. (%) . | (n = 34), No. (%) . | (n = 29), No. (%) . | (n = 23), No. (%) . | (n = 20), No. (%) . | (n = 17), No. (%) . | (n = 3), No. (%) . | |
| OPAT duration, median (IQR), d | 21 (14–38) | 39 (26–42) | 14 (8–17) | 21 (14–29) | 21 (14–22) | 26 (19–54) | 41 (28–44) |
| C/T dosage per day, median (range) | 4.5 (1.125–9.0) | 4.5 (2.25–9.0) | 4.5 (2.25–4.5) | 4.5 (2.25–9.0) | 4.5 (1.125–4.5) | 4.5 (2.25–4.5) | 4.5 (1.125–9.0) |
| <4.5 g | 23 (18) | 5 (15) | 10 (34) | 2 (9) | 2 (10) | 3 (18) | 1 (33) |
| >4.5 g | 7 (6) | 3 (9) | … | 3 (13) | … | … | 1 (33) |
| Frequency of infusion | … | ||||||
| Intermittent | 123 (98) | 33 (97) | 29 (100) | 23 (100) | 20 (100) | 16 (94) | 2 (67) |
| Continuous | 3 (2) | 1 (3) | … | … | … | 1 (6) | 1 (33) |
| Method of administration | … | ||||||
| Elastomeric pump, self-administration at home | 83 (66) | 28 (82) | 14 (48) | 10 (43) | 16 (80) | 13 (76) | 2 (67) |
| Ambulatory pump, daily pump bag changes in OIC | 42 (33) | 6 (18) | 14 (48) | 13 (57) | 4 (20) | 4 (24) | 1 (33) |
| Stationary pump, daily administration in OIC | 1 (1) | … | … | … | … | … | … |
| Concomitant intravenous antibiotic therapy | 16 (13) | 9 (26) | 1 (3) | 1 (4) | … | 5 (29) | … |
Abbreviations: C/T, ceftolozane/tazobactam; IQR, interquartile range; OIC, physician office infusion center; OPAT, outpatient antimicrobial therapy.
Values are expressed as number of patients (%), unless otherwise indicated.
Daptomycin (n = 10), vancomycin (n = 5), televancin (n = 1).
Microbiology
A total of 136 aerobic and anerobic gram-negative pathogens were identified in 115 patients. Nineteen patients (16%) had ≥2 gram-negative isolates, 37 patients (32%) had both gram-negative and gram-positive pathogens (15 BJI, 10 cSSTI, 6 IAI, 3 UTI, 2 RTI, 1 bacteremia). There were 4 anerobic pathogens identified, all in patients with additional gram-negative aerobic pathogens. There were no culture data in 11 patients (9%). Table 3 shows the distribution of gram-negative pathogens by infection type and resistance pattern. Overall, P. aeruginosa was most frequently isolated (n = 86, 63%), followed by E. coli (n = 18, 13%) and Klebsiella spp. (n = 9, 7%). P. aeruginosa was also the most prominent pathogen across all infection types, with the exception of IAI, where Enterobacterales species were more common. Enterobacterales included E. coli (n = 18), Klebsiella spp. (n = 9), Enterobacter cloacae (n = 4), Proteus mirabilis (n = 3), Citrobacter koseri (n = 1), and Providencia stuartii (n = 1). Resistant pathogens were identified in 60% (82/136) of all gram-negative pathogens including 18% MDR (n = 24), 12% ESBL (n = 17), 3.7% CR (n = 5), 0.7% MDR/ESBL (n = 1), and 26% MDR/CR (n = 35). Among P. aeruginosa, 57/86 isolates (66%) were MDR and 39/86 isolates (45%) were CR. ESBL producers were detected in 44% of all Enterobacterales (16/36) including 56% E. coli (10/18), 44% Klebsiella spp. (4/9), and 50% Enterobacter cloacae (2/4). Prevotella spp. had ESBL producers isolated in 67% (2/3).
| Characteristic . | No. of Gram-Negative Pathogensa . | P. aeruginosa . | E. coli . | Klebsiella spp. . | Enterobacter cloacae . | Prevotella spp. . | Proteus mirabilis . | Otherb . |
|---|---|---|---|---|---|---|---|---|
| Total No. of pathogens | 136 | 86 | 18 | 9 | 4 | 3 | 3 | 13 |
| Infection type | ||||||||
| Bone and joint | 40 (29) | 25 (29) | 5 (28) | 2 (22) | 1 (25) | 1 (33.3) | 1 (33.3) | 5 (39)b,c,d |
| Urinary tract | 32 (24) | 22 (26) | 4 (22) | 3 (33) | 1 (25) | … | … | 2 (15)e,f |
| Respiratory tract | 24 (18) | 20 (23) | … | 2 (22) | … | 1 (33.3) | … | 1 (8)g |
| Intra-abdominal | 21 (15) | 7 (8) | 7 (39) | 1 (11.5) | 1 (25) | 1 (33.3) | 1 (33.3) | 3 (23)d |
| Skin and soft tissue | 16 (12) | 10 (12) | 2 (11) | 1 (11.5) | … | … | 1 (33.3) | 2 (15)d |
| Bacteremia | 3 (2) | 2 (2) | … | … | 1 (25) | … | … | … |
| No. of resistant pathogens | 82 (60) | 61 (71) | 10 (56) | 5 (55) | 2 (50) | 2 (67) | 2 (15) | |
| MDR | 24 (18) | 22 (26) | … | 1 (11) | … | … | … | 1 (7.5)f |
| ESBL | 17 (12) | … | 9 (50) | 4 (44) | 2 (50) | 2 (67) | … | … |
| CR | 5 (3.7) | 4 (5) | … | … | … | … | … | 1 (7.5)e |
| MDR/ESBL | 1 (0.7) | … | 1 (6) | … | … | … | … | … |
| MDR/CR | 35 (26) | 35 (40) | … | … | … | … | … | … |
| Characteristic . | No. of Gram-Negative Pathogensa . | P. aeruginosa . | E. coli . | Klebsiella spp. . | Enterobacter cloacae . | Prevotella spp. . | Proteus mirabilis . | Otherb . |
|---|---|---|---|---|---|---|---|---|
| Total No. of pathogens | 136 | 86 | 18 | 9 | 4 | 3 | 3 | 13 |
| Infection type | ||||||||
| Bone and joint | 40 (29) | 25 (29) | 5 (28) | 2 (22) | 1 (25) | 1 (33.3) | 1 (33.3) | 5 (39)b,c,d |
| Urinary tract | 32 (24) | 22 (26) | 4 (22) | 3 (33) | 1 (25) | … | … | 2 (15)e,f |
| Respiratory tract | 24 (18) | 20 (23) | … | 2 (22) | … | 1 (33.3) | … | 1 (8)g |
| Intra-abdominal | 21 (15) | 7 (8) | 7 (39) | 1 (11.5) | 1 (25) | 1 (33.3) | 1 (33.3) | 3 (23)d |
| Skin and soft tissue | 16 (12) | 10 (12) | 2 (11) | 1 (11.5) | … | … | 1 (33.3) | 2 (15)d |
| Bacteremia | 3 (2) | 2 (2) | … | … | 1 (25) | … | … | … |
| No. of resistant pathogens | 82 (60) | 61 (71) | 10 (56) | 5 (55) | 2 (50) | 2 (67) | 2 (15) | |
| MDR | 24 (18) | 22 (26) | … | 1 (11) | … | … | … | 1 (7.5)f |
| ESBL | 17 (12) | … | 9 (50) | 4 (44) | 2 (50) | 2 (67) | … | … |
| CR | 5 (3.7) | 4 (5) | … | … | … | … | … | 1 (7.5)e |
| MDR/ESBL | 1 (0.7) | … | 1 (6) | … | … | … | … | … |
| MDR/CR | 35 (26) | 35 (40) | … | … | … | … | … | … |
Abbreviations: CLABSI, central line bloodstream infection; CR, carbapenem-resistant; ESBL, extended-spectrum beta-lactamase; MDR, multidrug-resistant; No., number.
Values represent number (%) of gram-negative isolates based on total no. of pathogens.
Bacteroides spp. (n = 2).
Providencia stuartii (n = 1).
Unspecified gram-negative rods.
Achromobacter nitrificans (n = 1).
Citrobacter koseri (n = 1).
Stenotrophomonas maltophilia (n = 1).
| Characteristic . | No. of Gram-Negative Pathogensa . | P. aeruginosa . | E. coli . | Klebsiella spp. . | Enterobacter cloacae . | Prevotella spp. . | Proteus mirabilis . | Otherb . |
|---|---|---|---|---|---|---|---|---|
| Total No. of pathogens | 136 | 86 | 18 | 9 | 4 | 3 | 3 | 13 |
| Infection type | ||||||||
| Bone and joint | 40 (29) | 25 (29) | 5 (28) | 2 (22) | 1 (25) | 1 (33.3) | 1 (33.3) | 5 (39)b,c,d |
| Urinary tract | 32 (24) | 22 (26) | 4 (22) | 3 (33) | 1 (25) | … | … | 2 (15)e,f |
| Respiratory tract | 24 (18) | 20 (23) | … | 2 (22) | … | 1 (33.3) | … | 1 (8)g |
| Intra-abdominal | 21 (15) | 7 (8) | 7 (39) | 1 (11.5) | 1 (25) | 1 (33.3) | 1 (33.3) | 3 (23)d |
| Skin and soft tissue | 16 (12) | 10 (12) | 2 (11) | 1 (11.5) | … | … | 1 (33.3) | 2 (15)d |
| Bacteremia | 3 (2) | 2 (2) | … | … | 1 (25) | … | … | … |
| No. of resistant pathogens | 82 (60) | 61 (71) | 10 (56) | 5 (55) | 2 (50) | 2 (67) | 2 (15) | |
| MDR | 24 (18) | 22 (26) | … | 1 (11) | … | … | … | 1 (7.5)f |
| ESBL | 17 (12) | … | 9 (50) | 4 (44) | 2 (50) | 2 (67) | … | … |
| CR | 5 (3.7) | 4 (5) | … | … | … | … | … | 1 (7.5)e |
| MDR/ESBL | 1 (0.7) | … | 1 (6) | … | … | … | … | … |
| MDR/CR | 35 (26) | 35 (40) | … | … | … | … | … | … |
| Characteristic . | No. of Gram-Negative Pathogensa . | P. aeruginosa . | E. coli . | Klebsiella spp. . | Enterobacter cloacae . | Prevotella spp. . | Proteus mirabilis . | Otherb . |
|---|---|---|---|---|---|---|---|---|
| Total No. of pathogens | 136 | 86 | 18 | 9 | 4 | 3 | 3 | 13 |
| Infection type | ||||||||
| Bone and joint | 40 (29) | 25 (29) | 5 (28) | 2 (22) | 1 (25) | 1 (33.3) | 1 (33.3) | 5 (39)b,c,d |
| Urinary tract | 32 (24) | 22 (26) | 4 (22) | 3 (33) | 1 (25) | … | … | 2 (15)e,f |
| Respiratory tract | 24 (18) | 20 (23) | … | 2 (22) | … | 1 (33.3) | … | 1 (8)g |
| Intra-abdominal | 21 (15) | 7 (8) | 7 (39) | 1 (11.5) | 1 (25) | 1 (33.3) | 1 (33.3) | 3 (23)d |
| Skin and soft tissue | 16 (12) | 10 (12) | 2 (11) | 1 (11.5) | … | … | 1 (33.3) | 2 (15)d |
| Bacteremia | 3 (2) | 2 (2) | … | … | 1 (25) | … | … | … |
| No. of resistant pathogens | 82 (60) | 61 (71) | 10 (56) | 5 (55) | 2 (50) | 2 (67) | 2 (15) | |
| MDR | 24 (18) | 22 (26) | … | 1 (11) | … | … | … | 1 (7.5)f |
| ESBL | 17 (12) | … | 9 (50) | 4 (44) | 2 (50) | 2 (67) | … | … |
| CR | 5 (3.7) | 4 (5) | … | … | … | … | … | 1 (7.5)e |
| MDR/ESBL | 1 (0.7) | … | 1 (6) | … | … | … | … | … |
| MDR/CR | 35 (26) | 35 (40) | … | … | … | … | … | … |
Abbreviations: CLABSI, central line bloodstream infection; CR, carbapenem-resistant; ESBL, extended-spectrum beta-lactamase; MDR, multidrug-resistant; No., number.
Values represent number (%) of gram-negative isolates based on total no. of pathogens.
Bacteroides spp. (n = 2).
Providencia stuartii (n = 1).
Unspecified gram-negative rods.
Achromobacter nitrificans (n = 1).
Citrobacter koseri (n = 1).
Stenotrophomonas maltophilia (n = 1).
Clinical Outcome
The clinical outcomes of C/T therapy by infection type are shown in Figure 1. Overall clinical success was achieved in 84.7% of patients (105/124). Twenty-nine patients (23%) transitioned to oral antibiotics after completion of C/T (7 BJI, 5 UTI, 5 RTI, 5 IAI, 5 cSSTI, 2 bacteremia). Those requiring transition to oral antibiotics had complicated cases with chronic indwelling prosthetic material or were at a high risk for reinfection. By infection type, successful outcomes were observed in 72.7% of patients with BJI (24/33), 96.6% with UTI (28/29), 95.4% with RTI (21/22), 80.0% with IAI (16/20), 82.4% with cSSTI (14/17), and 66.7% with bacteremia (2/3). Nonsuccessful outcomes were reported in 12 patients (15.3%) due to persistent or recurrent infections (6 BJI, 3 cSSTI, 2 IAI, 1 RTI). Another 7 patients discontinued C/T due to adverse events including skin rash (n = 3), Clostridioides difficile–associated diarrhea (n = 1), dyspnea (n = 1), and nephrotoxicity/hepatotoxicity (n = 1) in a bacteremia patient with advanced heart failure. In addition, 1 patient discontinued due to catheter-related complication. Two patients were nonevaluable for outcome assessment due to transfer of care. Health care resource utilization during C/T therapy occurred in 14 patients (11.1%). Two patients visited the emergency department for OPAT-unrelated reasons, and 12 patients were admitted to the hospital due to worsening infections (n = 8), an adverse event (n = 1), and reasons unrelated to OPAT (n = 3).
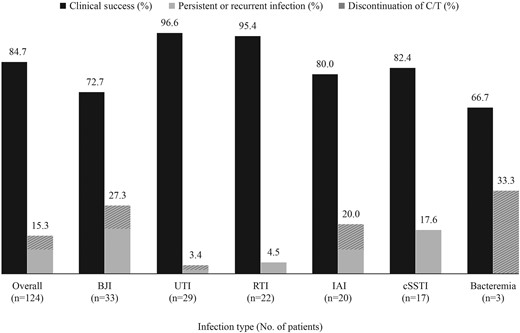
Clinical outcome of C/T by infection type. Abbreviations: BJI, bone and joint infection; cSSTI, complicated skin and skin structure infection; C/T, ceftolozane/tazobactam; IAI, intra-abdominal infection; RTI, respiratory tract infection; UTI, complicated urinary tract infection.
Summary statistics of baseline variables between patients with success (n = 105) and nonsuccess (n = 19) following C/T therapy are displayed in Table 4. Bivariate analysis indicated that patients treated with C/T for UTI were more likely to experience clinical success compared with those with other infections (96.6% vs 81.0%; P = .042). Conversely, BJI patients were less likely to experience clinical success than those treated with C/T for other infection types (72.7% vs 89.0%; P = .026). Five of 9 BJI cases with nonsuccessful outcomes had osteomyelitis secondary to diabetic foot infection. Although the proportion of successful outcomes was low for patients with liver disease (62.5%) and bacteremia (66.7%), the sample size was rather small, including 8 and 3 patients, respectively. Successful outcomes with C/T were observed in 85.9% and 81.8% of patients with P. aeruginosa and Enterobacterales infections, respectively. C/T success rates for resistant infections were 86.0% and 79.4% for MDR and CR P. aeruginosa, respectively, and 90.9% for ESBL-producing Enterobacterales.
| Variable . | Success . | Nonsuccess . | Proportion With Success, . | P Value . |
|---|---|---|---|---|
| (n = 105) . | (n = 19) . | n/N (%) . | ||
| Age ≥65 y | 42 (40.0) | 6 (31.6) | 42/48 (87.5) | .595 |
| Gender | ||||
| Male | 58 (55.2) | 15 (78.9) | 58/73 (79.4) | .053 |
| Female | 47 (44.8) | 4 (21.0) | 47/51 (92.2) | .053 |
| Location before C/T | ||||
| Community | 61 (58.1) | 8 (42.1) | 61/69 (88.4) | .197 |
| Hospital | 44 (41.9) | 11 (57.9) | 44/55 (80.0) | .197 |
| Charlson comorbidity index, median (range) | 4.0 (0–8.0) | 5.0 (3.5–6.5) | … | .922 |
| BMI ≥30 kg/m2 | 43 (40.9) | 11 (57.9) | 43/54 (79.6) | .171 |
| Renal function (CrCl) | ||||
| Normal (>90 mL/min) | 55 (52.4) | 13 (68.4) | 55/68 (80.9) | .196 |
| Mild (51–90 mL/min) | 33 (31.4) | 6 (31.6) | 33/39 (84.6) | .989 |
| Moderate (30–50 mL/min) | 12 (11.4) | 0 | 12/12 (100) | .121 |
| Severe (<30 mL/min) | 3 (2.9) | 0 | 3/3 (100) | .456 |
| Comorbidities | ||||
| Cardiovascular disease | 72 (68.6) | 11 (57.9) | 72/83 (86.7) | .363 |
| Diabetes mellitus | 37 (35.2) | 11 (57.9) | 37/48 (77.1) | .062 |
| Immunocompromised | 41 (39.0) | 7 (36.8) | 41/48 (85.4) | .856 |
| Malignancy (current) | 24 (22.9) | 4 (21.0) | 24/28 (85.7) | .862 |
| Chronic kidney disease | 14 (13.3) | 1 (5.3) | 14/15 (93.3) | .321 |
| Liver disease | 5 (4.8) | 3 (15.8) | 5/8 (62.5) | .071 |
| Infection type | ||||
| Bone and joint | 24 (22.9) | 9 (47.4) | 24/33 (72.7) | .026 |
| Urinary tract | 28 (26.7) | 1 (5.3) | 28/29 (96.6) | .042 |
| Respiratory tract | 21 (20.0) | 1 (5.3) | 21/22 (95.4) | .121 |
| Intra-abdominal | 16 (15.2) | 4 (21.0) | 16/20 (80.0) | .526 |
| Skin and soft tissue | 14 (13.3) | 3 (15.8) | 14/17 (82.4) | .774 |
| Bacteremia | 2 (1.9) | 1 (5.3) | 2/3 (66.7) | .380 |
| Concurrent bacteremia | 9 (7.6) | 3 (15.8) | 9/12 (75.0) | .249 |
| Causative pathogen | ||||
| P. aeruginosa infection | 67 (63.8) | 11 (57.9) | 67/78 (85.9) | .696 |
| MDR | 43 (40.9) | 7 (36.8) | 43/50 (86.0) | 1.000 |
| CR | 27 (25.7) | 7 (36.8) | 27/34 (79.4) | .195 |
| Enterobacterales infection | 18 (17.1) | 4 (21.0) | 18/22 (81.8) | .683 |
| ESBL | 10 (9.5) | 1 (5.3) | 10/11 (90.9) | .586 |
| Variable . | Success . | Nonsuccess . | Proportion With Success, . | P Value . |
|---|---|---|---|---|
| (n = 105) . | (n = 19) . | n/N (%) . | ||
| Age ≥65 y | 42 (40.0) | 6 (31.6) | 42/48 (87.5) | .595 |
| Gender | ||||
| Male | 58 (55.2) | 15 (78.9) | 58/73 (79.4) | .053 |
| Female | 47 (44.8) | 4 (21.0) | 47/51 (92.2) | .053 |
| Location before C/T | ||||
| Community | 61 (58.1) | 8 (42.1) | 61/69 (88.4) | .197 |
| Hospital | 44 (41.9) | 11 (57.9) | 44/55 (80.0) | .197 |
| Charlson comorbidity index, median (range) | 4.0 (0–8.0) | 5.0 (3.5–6.5) | … | .922 |
| BMI ≥30 kg/m2 | 43 (40.9) | 11 (57.9) | 43/54 (79.6) | .171 |
| Renal function (CrCl) | ||||
| Normal (>90 mL/min) | 55 (52.4) | 13 (68.4) | 55/68 (80.9) | .196 |
| Mild (51–90 mL/min) | 33 (31.4) | 6 (31.6) | 33/39 (84.6) | .989 |
| Moderate (30–50 mL/min) | 12 (11.4) | 0 | 12/12 (100) | .121 |
| Severe (<30 mL/min) | 3 (2.9) | 0 | 3/3 (100) | .456 |
| Comorbidities | ||||
| Cardiovascular disease | 72 (68.6) | 11 (57.9) | 72/83 (86.7) | .363 |
| Diabetes mellitus | 37 (35.2) | 11 (57.9) | 37/48 (77.1) | .062 |
| Immunocompromised | 41 (39.0) | 7 (36.8) | 41/48 (85.4) | .856 |
| Malignancy (current) | 24 (22.9) | 4 (21.0) | 24/28 (85.7) | .862 |
| Chronic kidney disease | 14 (13.3) | 1 (5.3) | 14/15 (93.3) | .321 |
| Liver disease | 5 (4.8) | 3 (15.8) | 5/8 (62.5) | .071 |
| Infection type | ||||
| Bone and joint | 24 (22.9) | 9 (47.4) | 24/33 (72.7) | .026 |
| Urinary tract | 28 (26.7) | 1 (5.3) | 28/29 (96.6) | .042 |
| Respiratory tract | 21 (20.0) | 1 (5.3) | 21/22 (95.4) | .121 |
| Intra-abdominal | 16 (15.2) | 4 (21.0) | 16/20 (80.0) | .526 |
| Skin and soft tissue | 14 (13.3) | 3 (15.8) | 14/17 (82.4) | .774 |
| Bacteremia | 2 (1.9) | 1 (5.3) | 2/3 (66.7) | .380 |
| Concurrent bacteremia | 9 (7.6) | 3 (15.8) | 9/12 (75.0) | .249 |
| Causative pathogen | ||||
| P. aeruginosa infection | 67 (63.8) | 11 (57.9) | 67/78 (85.9) | .696 |
| MDR | 43 (40.9) | 7 (36.8) | 43/50 (86.0) | 1.000 |
| CR | 27 (25.7) | 7 (36.8) | 27/34 (79.4) | .195 |
| Enterobacterales infection | 18 (17.1) | 4 (21.0) | 18/22 (81.8) | .683 |
| ESBL | 10 (9.5) | 1 (5.3) | 10/11 (90.9) | .586 |
Abbreviations: BMI, body mass index; CR, carbapenem-resistant; C/T, ceftolozane/tazobactam; ESBL, extended-spectrum beta-lactamase; MDR, multidrug-resistant; OIC, office infusion center.
All values represent number of patients (%) unless otherwise indicated.
Chi-square test or Fisher exact test was used for categorical variables, and Wilcoxon rank test for continues variables.
Due to immunosuppressive medication (chemotherapy, steroids, biologics) or underlying immune deficiency (cancer, AIDS, genetic disorder, autoimmune disease, organ transplant, CKD).
Including E. coli (n = 14), Klebsiella spp. (n = 4), Enterobacter cloacae (n = 3), Citrobacter koseri (n = 1).
Bivariate analysis indicated that patients with bone and joint infections were less likely to experience success compared with patients treated with ceftolozane/tazobactam for other infection types (72.7% vs 89.0%; P = .026).
Bivariate analysis indicated that patients with urinary tract infection were more likely to experience success compared with patients treated with ceftolozane/tazobactam for other infection types (96.6% vs 81.0%; P = .042).
| Variable . | Success . | Nonsuccess . | Proportion With Success, . | P Value . |
|---|---|---|---|---|
| (n = 105) . | (n = 19) . | n/N (%) . | ||
| Age ≥65 y | 42 (40.0) | 6 (31.6) | 42/48 (87.5) | .595 |
| Gender | ||||
| Male | 58 (55.2) | 15 (78.9) | 58/73 (79.4) | .053 |
| Female | 47 (44.8) | 4 (21.0) | 47/51 (92.2) | .053 |
| Location before C/T | ||||
| Community | 61 (58.1) | 8 (42.1) | 61/69 (88.4) | .197 |
| Hospital | 44 (41.9) | 11 (57.9) | 44/55 (80.0) | .197 |
| Charlson comorbidity index, median (range) | 4.0 (0–8.0) | 5.0 (3.5–6.5) | … | .922 |
| BMI ≥30 kg/m2 | 43 (40.9) | 11 (57.9) | 43/54 (79.6) | .171 |
| Renal function (CrCl) | ||||
| Normal (>90 mL/min) | 55 (52.4) | 13 (68.4) | 55/68 (80.9) | .196 |
| Mild (51–90 mL/min) | 33 (31.4) | 6 (31.6) | 33/39 (84.6) | .989 |
| Moderate (30–50 mL/min) | 12 (11.4) | 0 | 12/12 (100) | .121 |
| Severe (<30 mL/min) | 3 (2.9) | 0 | 3/3 (100) | .456 |
| Comorbidities | ||||
| Cardiovascular disease | 72 (68.6) | 11 (57.9) | 72/83 (86.7) | .363 |
| Diabetes mellitus | 37 (35.2) | 11 (57.9) | 37/48 (77.1) | .062 |
| Immunocompromised | 41 (39.0) | 7 (36.8) | 41/48 (85.4) | .856 |
| Malignancy (current) | 24 (22.9) | 4 (21.0) | 24/28 (85.7) | .862 |
| Chronic kidney disease | 14 (13.3) | 1 (5.3) | 14/15 (93.3) | .321 |
| Liver disease | 5 (4.8) | 3 (15.8) | 5/8 (62.5) | .071 |
| Infection type | ||||
| Bone and joint | 24 (22.9) | 9 (47.4) | 24/33 (72.7) | .026 |
| Urinary tract | 28 (26.7) | 1 (5.3) | 28/29 (96.6) | .042 |
| Respiratory tract | 21 (20.0) | 1 (5.3) | 21/22 (95.4) | .121 |
| Intra-abdominal | 16 (15.2) | 4 (21.0) | 16/20 (80.0) | .526 |
| Skin and soft tissue | 14 (13.3) | 3 (15.8) | 14/17 (82.4) | .774 |
| Bacteremia | 2 (1.9) | 1 (5.3) | 2/3 (66.7) | .380 |
| Concurrent bacteremia | 9 (7.6) | 3 (15.8) | 9/12 (75.0) | .249 |
| Causative pathogen | ||||
| P. aeruginosa infection | 67 (63.8) | 11 (57.9) | 67/78 (85.9) | .696 |
| MDR | 43 (40.9) | 7 (36.8) | 43/50 (86.0) | 1.000 |
| CR | 27 (25.7) | 7 (36.8) | 27/34 (79.4) | .195 |
| Enterobacterales infection | 18 (17.1) | 4 (21.0) | 18/22 (81.8) | .683 |
| ESBL | 10 (9.5) | 1 (5.3) | 10/11 (90.9) | .586 |
| Variable . | Success . | Nonsuccess . | Proportion With Success, . | P Value . |
|---|---|---|---|---|
| (n = 105) . | (n = 19) . | n/N (%) . | ||
| Age ≥65 y | 42 (40.0) | 6 (31.6) | 42/48 (87.5) | .595 |
| Gender | ||||
| Male | 58 (55.2) | 15 (78.9) | 58/73 (79.4) | .053 |
| Female | 47 (44.8) | 4 (21.0) | 47/51 (92.2) | .053 |
| Location before C/T | ||||
| Community | 61 (58.1) | 8 (42.1) | 61/69 (88.4) | .197 |
| Hospital | 44 (41.9) | 11 (57.9) | 44/55 (80.0) | .197 |
| Charlson comorbidity index, median (range) | 4.0 (0–8.0) | 5.0 (3.5–6.5) | … | .922 |
| BMI ≥30 kg/m2 | 43 (40.9) | 11 (57.9) | 43/54 (79.6) | .171 |
| Renal function (CrCl) | ||||
| Normal (>90 mL/min) | 55 (52.4) | 13 (68.4) | 55/68 (80.9) | .196 |
| Mild (51–90 mL/min) | 33 (31.4) | 6 (31.6) | 33/39 (84.6) | .989 |
| Moderate (30–50 mL/min) | 12 (11.4) | 0 | 12/12 (100) | .121 |
| Severe (<30 mL/min) | 3 (2.9) | 0 | 3/3 (100) | .456 |
| Comorbidities | ||||
| Cardiovascular disease | 72 (68.6) | 11 (57.9) | 72/83 (86.7) | .363 |
| Diabetes mellitus | 37 (35.2) | 11 (57.9) | 37/48 (77.1) | .062 |
| Immunocompromised | 41 (39.0) | 7 (36.8) | 41/48 (85.4) | .856 |
| Malignancy (current) | 24 (22.9) | 4 (21.0) | 24/28 (85.7) | .862 |
| Chronic kidney disease | 14 (13.3) | 1 (5.3) | 14/15 (93.3) | .321 |
| Liver disease | 5 (4.8) | 3 (15.8) | 5/8 (62.5) | .071 |
| Infection type | ||||
| Bone and joint | 24 (22.9) | 9 (47.4) | 24/33 (72.7) | .026 |
| Urinary tract | 28 (26.7) | 1 (5.3) | 28/29 (96.6) | .042 |
| Respiratory tract | 21 (20.0) | 1 (5.3) | 21/22 (95.4) | .121 |
| Intra-abdominal | 16 (15.2) | 4 (21.0) | 16/20 (80.0) | .526 |
| Skin and soft tissue | 14 (13.3) | 3 (15.8) | 14/17 (82.4) | .774 |
| Bacteremia | 2 (1.9) | 1 (5.3) | 2/3 (66.7) | .380 |
| Concurrent bacteremia | 9 (7.6) | 3 (15.8) | 9/12 (75.0) | .249 |
| Causative pathogen | ||||
| P. aeruginosa infection | 67 (63.8) | 11 (57.9) | 67/78 (85.9) | .696 |
| MDR | 43 (40.9) | 7 (36.8) | 43/50 (86.0) | 1.000 |
| CR | 27 (25.7) | 7 (36.8) | 27/34 (79.4) | .195 |
| Enterobacterales infection | 18 (17.1) | 4 (21.0) | 18/22 (81.8) | .683 |
| ESBL | 10 (9.5) | 1 (5.3) | 10/11 (90.9) | .586 |
Abbreviations: BMI, body mass index; CR, carbapenem-resistant; C/T, ceftolozane/tazobactam; ESBL, extended-spectrum beta-lactamase; MDR, multidrug-resistant; OIC, office infusion center.
All values represent number of patients (%) unless otherwise indicated.
Chi-square test or Fisher exact test was used for categorical variables, and Wilcoxon rank test for continues variables.
Due to immunosuppressive medication (chemotherapy, steroids, biologics) or underlying immune deficiency (cancer, AIDS, genetic disorder, autoimmune disease, organ transplant, CKD).
Including E. coli (n = 14), Klebsiella spp. (n = 4), Enterobacter cloacae (n = 3), Citrobacter koseri (n = 1).
Bivariate analysis indicated that patients with bone and joint infections were less likely to experience success compared with patients treated with ceftolozane/tazobactam for other infection types (72.7% vs 89.0%; P = .026).
Bivariate analysis indicated that patients with urinary tract infection were more likely to experience success compared with patients treated with ceftolozane/tazobactam for other infection types (96.6% vs 81.0%; P = .042).
DISCUSSION
To date, we present the largest real-world multicenter outpatient experience study of C/T for the treatment of complicated infections in US outpatient infusion centers. This study cohort was highly comorbid; 39% were immunocompromised, and half had been previously treated with intravenous antibiotics for the same infection. The overall clinical success rate for all infections at the end of OPAT was 84.7%. The results are comparable to cure rates reported in clinical trials and other observational studies [1–4]. In this study, C/T used in UTI patients showed the highest clinical success, which was above that reported in clinical trials [2]. High rates of success were also observed for patients with RTI, of whom the majority received C/T for respiratory infections other than pneumonia. The clinical success rate for IAI patients was comparable to those reported in clinical trials [1, 3] and other observational studies [8, 10].
We observed varying rates of clinical success for infections, for which there is little historical data. C/T was successfully used in 82% of patients with cSSTI, which was similar to success rates reported in patients with multidrug-resistant organisms [8–10]. Our experience of the use of C/T in the treatment of BJI infections is the largest to date. This cohort consisted primarily of osteomyelitis patients, with 26% receiving concomitant intravenous antibiotics for mixed infections. The overall success rate for the use of C/T in BJI was 72.7%. The majority of patients received standard dosing, with 9% receiving high-dose therapy of C/T, and all completed OPAT successfully. Based on case reports, the cure rate of C/T for osteomyelitis was between 40% and 100%, assessed 3 to 6 months following completion of therapy [15–18]. Future studies are necessary to determine the efficacy of C/T in patients with BJI. Our study cohort receiving C/T for bacteremia was small, with 2 of 3 successfully completing OPAT. The majority of patients in this study were treated before the expanded indication, resulting in a low utilization of the higher C/T dosage.
C/T offers a favorable treatment option for infections due to MDR P. aeruginosa and other resistant pathogens, avoiding agents with higher toxicity [22–24]. This is particularly useful in the nonacute health care setting, but to date, limited data exist on the utilization and outcome of C/T in this setting. The case reports described in the literature have shown successful outcomes with C/T in the outpatient setting primarily against MDR P. aeruginosa infections administered as continuous infusions [6, 11, 14, 16, 18, 25]. Jones and colleagues reported symptomatic and microbiologic resolution in 6/7 and 3/3 patients, respectively, following continuous infusion of C/T for P. aeruginosa infections in the outpatient setting [11]. In addition, clinical resolution was reported in 7 patients receiving continuous infusion of C/T for MDR P. aeruginosa deep-seated infections, of whom 4 of 7 were treated in the outpatient setting [14]. Our study provides additional data on the real-world performance of C/T in the outpatient setting for a variety of infection types using different dosage regimens and administration methods. Utilization of C/T in our cohort was largely related to resistant pathogens. Two-thirds of patients with positive cultures reported at least 1 resistant gram-negative pathogen. P. aeruginosa was the most predominant pathogen, with 71% resistant strains. Of these, 26% were MDR, 5% CR, and 40% were MDR/CR isolates. Other studies have reported treatment of MDR P. aeruginosa infections with C/T in comparable populations, with similar outcomes achieved as in our population [9]. Furthermore, clinical outcomes of C/T against CR P. aeruginosa have been studied [7, 25, 34]. An in vitro study by Teo et al. indicated a low susceptibility rate of 37.9% for C/T against CR P. aeruginosa clinical isolates with geographical variations [34]. Another surveillance study showed that C/T remained 83.1% and 88.9% active against meropenem-nonsusceptible P. aeruginosa collected from patients with lower RTI in intensive care units (ICU) and non-ICU wards, respectively, at 26 US hospitals [35]. Stewart et al. reported clinical success in 1 patient with CR P. aeruginosa [25]. In our setting, successful outcomes with C/T were observed in 79.4% of patients with CR P. aeruginosa infections, comparable to a success rate of 74% reported for hospitalized patients [7]. The rise in gram-negative antimicrobial resistance has led to clinical practice guidelines issued by the Infectious Diseases Society of America for the management P. aeruginosa infections with difficult-to-treat resistance identifying C/T as a preferred agent for UTI and infections outside the urinary tract [20]. Infections caused by Enterobacterales represented the second largest group in our population, with approximately half of the strains being ESBL producers. We observed a high success rate of 90.9% for the treatment of ESBL Enterobacterales infections, which included ESBL E. coli, ESBL Klebsiella spp., and ESBL Enterobacter cloacae. To our knowledge, only 1 other study reported C/T use in different types of ESBL Enterobacterales infections, with a favorable clinical outcome in 83.7% of hospitalized patients [10].
The primary method of administration of C/T was in elastomeric infusion pumps using intermittent dosing. This method allows for self-administration of C/T in the comfort of a patient's home with weekly visits to the OIC. With favorable drug stability over 24 hours at room temperature [29–31], the remainder of patients were able to receive C/T in the OIC, primarily through use of an ambulatory infusion pump with either intermittent or continuous dosing. Most of these patients were federally funded, requiring daily visits to the infusion center [28, 31].
This study has several limitations. We used a retrospective study design to obtain clinical outcomes of C/T in a heterogenous patient cohort, thereby removing control over measured variables. C/T susceptibilies and minimum inhibitory concentrations (MICs) were largely unavailable during the study period due to lack of commercially available testing methods. It should be noted, we assessed clinical outcomes for all infection types at the end of OPAT due to limited clinical and microbiologic follow-up data available in the outpatient setting. This could have resulted in higher reported success rates and under-reported emerging resistance. In addition, recurrence rates for BJIs were not available. Nonetheless, this study provides the largest outpatient experience of C/T to date, including data from 33 geographically diverse OICs, reflecting on practice and utilization of C/T in the real world.
In summary, our data demonstrate that OPAT use of C/T was successful to treat approved indications of IAI and UTI and other complicated infections including BJI, RTI, cSSTI, and bacteremia. C/T was primarily utilized for difficult-to-treat gram-negative infections with a high prevalence of resistant pathogens in a highly comorbid population. We believe these real-world findings support the use of C/T in the outpatient setting for serious gram-negative infections.
Acknowledgments
Financial support. This study was supported by a grant from the Merck Investigator Studies Progam (MISP).
Patient consent. Approval was obtained by an independent institutional review board (Advarra/IntegReview IRB, Austin, TX, USA).
References
Author notes
Potential conflicts of interest. L.J.V. received grant funding from Merck & Co, Inc., Kenilworth, NJ, USA. All other authors report no potential conflicts.





Comments