-
PDF
- Split View
-
Views
-
Cite
Cite
Bliss Green, Jacqueline Meredith, Renee Ackley, Maggie S McCarter, Christopher Polk, 1621. Novel Beta-lactam Beta-lactamase Inhibitors Against Alternative Antibiotics for the Treatment of Complicated Urinary Tract Infections and Pyelonephritis Caused by Carbapenem-resistant Enterobacterales, Open Forum Infectious Diseases, Volume 7, Issue Supplement_1, October 2020, Page S803, https://doi.org/10.1093/ofid/ofaa439.1801
Close - Share Icon Share
Abstract
There is little data on the comparative efficacy or safety of carbapenem-resistant Enterobacterales (CRE)-targeted beta-lactam beta-lactamase inhibitors (BL-BLI), including ceftazidime/avibactam (CZA) and meropenem/vaborbactam (MVB), versus alternative antibiotics for the treatment of CRE complicated urinary tract infections/acute pyelonephritis (cUTI/AP). The objective of this study was to evaluate rates of clinical failure in patients with CRE cUTI/AP treated with CRE-targeted BL-BLI vs. alternative regimens.
This was a multicenter, retrospective cohort study of adults admitted with a CRE cUTI/AP treated with CRE-active antibiotic(s), including combination therapy, for at least 48 hours between January 2012 and June 2019. Exclusion criteria included CRE colonization, non-urinary source co-infection, non-Enterobacterales cUTI/AP, or mortality within 48 hours of index culture. The primary outcome was clinical failure, defined as continued symptoms or recurrence at 30 days from index culture. Secondary outcomes included 90-day recurrence and 30-day readmission. Safety outcomes included treatment-limiting adverse effects, non-treatment limiting nephrotoxicity, and C. difficile infection.
A total of 47 patients were included (BL-BLI, n=16; alternative, n=31). Alternative regimens contained aminoglycosides, carbapenems, polymyxins, and tigecycline and utilized combination therapy more often (32.3% vs. 6.3%, p=0.046). Clinical failure occurred in 12.5% of patients in the BL-BLI group vs. 38.7% in the alternative group (p=0.063). Higher rates of 90-day recurrence (25.8% vs. 18.8%) and 30-day readmissions (51.6% vs. 31.3%) occurred in the alternative group vs. the BL-BLI group but were not statistically significant (Table 2). There were clinically significant rates of nephrotoxicity in the alternative group (45.2%) compared to the BL-BLI group (18.8%), contributing largely to the difference in treatment-limiting adverse effects (29% vs. 0%, p=0.017).
Table 1: Antibiotic Data
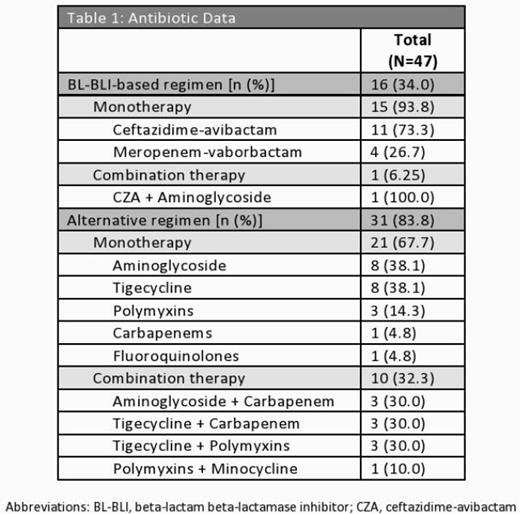
Table 2: Efficacy Outcomes
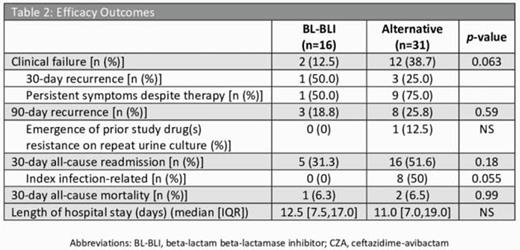
Table 3: Safety Outcomes
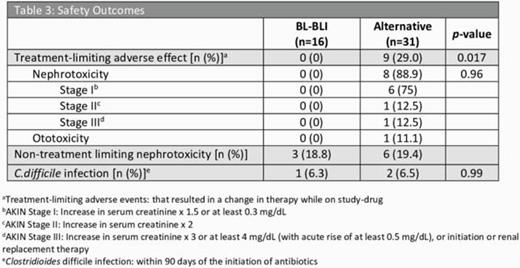
In this retrospective study, no difference in clinical failure resulted among groups; however, there was significantly more treatment-limiting adverse effects in the alternative group compared to the BL-BLI-based regimens, driven by nephrotoxicity.
All Authors: No reported disclosures
- antibiotics
- beta-lactam antibiotics
- carbapenem
- urinary tract infections
- adult
- ceftazidime
- combined modality therapy
- disclosure
- patient readmission
- polymyxins
- pyelonephritis
- safety
- urinary tract
- infections
- mortality
- meropenem
- aminoglycosides
- pyelonephritis, acute
- coinfection
- beta-lactamase inhibitors
- nephrotoxicity
- tigecycline
- microbial colonization
- avibactam
- carbapenem resistance
- vaborbactam
- primary outcome measure





Comments