-
PDF
- Split View
-
Views
-
Cite
Cite
Shweta Anjan, Dimitra Skiada, Miriam Andrea Duque Cuartas, Douglas Salguero, David P Serota, Jose Gonzales-Zamora, Folusakin Ayoade, Laura Beauchamps, Tanya R Quiroz, Jovanna Bertran-Lopez, Patricia Raccamarich, Emily K Montgomerie, Irma Barreto, Lilian M Abbo, Lilian M Abbo, Susanne Doblecki-Lewis, Yanyun Wu, Maria L Alcaide, 549. Clinical Characteristics and Outcomes of Patients with COVID-19 treated with Convalescent Plasma in Miami, Florida, Open Forum Infectious Diseases, Volume 7, Issue Supplement_1, October 2020, Pages S340–S341, https://doi.org/10.1093/ofid/ofaa439.743
Close - Share Icon Share
Abstract
The Coronavirus disease of 2019 (COVID-19) global health crisis caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in unprecedented mortality, impacted society, and strained healthcare systems, yet sufficient data regarding treatment options are lacking. Convalescent plasma, used since 1895 for infectious disease outbreaks, offers promise as a treatment option for COVID-19.
This is a retrospective study of patients diagnosed by a nasopharyngeal swab SARS-CoV-2 reverse transcriptase–polymerase chain reaction (RT-PCR), who received convalescent plasma between April to June 2020 at two large hospitals in Miami, Florida, as part of the US FDA Expanded Access Program for COVID-19 convalescent plasma (CCP).
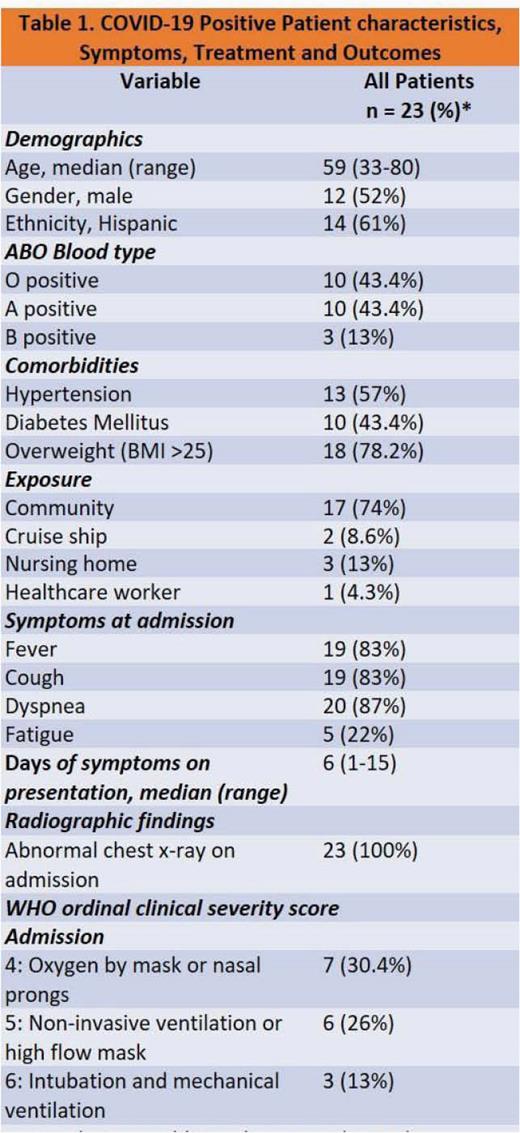
A total of 23 patients received CCP, 13 (57%) had severe COVID-19 disease, while 8 (35%) had critical or critical with multiorgan dysfunction. Median time of follow up was 26 (range, 7–79) days. Overall, 11 (48%) survived to discharge, 6 (26%) died, while 6 (26%) are currently hospitalized. All deaths reported were due to septic shock from secondary infections. 15 (65%) showed improvement in oxygen requirements 7 days post CCP transfusion. Measured inflammatory markers, c-reactive protein, lactate dehydrogenase, ferritin and d-dimer improved 7 days post transfusion in 13 (57%) patients. No adverse events due to the transfusion were reported. 10 (43.4%) patients had a negative SARS-CoV-2 RT-PCR at a median of 14.5 (range, 4–31) days after receiving convalescent plasma.
Administration of convalescent plasma was found to be safe, with favorable outcomes in this small cohort of relatively high acuity patients. Larger studies including control arms are needed to establish the efficacy of convalescent plasma on clinical and virologic outcomes for patients with COVID-19.
Table
All Authors: No reported disclosures
- oxygen
- inflammatory markers
- septic shock
- coronavirus
- communicable diseases
- disclosure
- disease outbreaks
- florida
- follow-up
- plasma
- reverse transcriptase polymerase chain reaction
- united states food and drug administration
- world health
- c-reactive protein
- ferritin
- lactate dehydrogenase
- mortality
- nasopharynx
- multiple organ dysfunction syndrome
- transfusion
- fibrin fragment d substance
- health care systems
- adverse event
- secondary infection
- expanded access
- clear cell papulosis
- sars-cov-2
- covid-19





Comments