-
PDF
- Split View
-
Views
-
Cite
Cite
Truong-Thanh Pham, Romain Garreau, Fabien Craighero, Vincent Cottin, Benoît Ben Said, Sylvain Goutelle, Tristan Ferry, on behalf of the Lyon Bone and Joint Infection Study Group, Seventeen Cases of Daptomycin-Induced Eosinophilic Pneumonia in a Cohort of Patients Treated for Bone and Joint Infections: Proposal for a New Algorithm, Open Forum Infectious Diseases, Volume 9, Issue 11, November 2022, ofac577, https://doi.org/10.1093/ofid/ofac577
Close - Share Icon Share
Abstract
Daptomycin is increasingly used in the treatment of bone and joint infections (BJIs) and may be responsible for daptomycin-induced eosinophilic pneumonia (DIEP), a potentially severe adverse drug reaction. The aim of this study was to describe DIEP in patients treated at a referral center for the management of BJI, and to revisit current definitions of this disease.
Patients treated from 1 January 2012 to 31 March 2021 were included in a prospective cohort (NCT02817711), in which all potential serious adverse events are prospectively recorded. Patients diagnosed with DIEP were retrospectively analyzed using different definitions.
In a total of 4664 patients included in the cohort during the study period, 1021 patients (21.9%) received daptomycin, of whom 17 (1.7%) were diagnosed with DIEP. Most patients were male (n = 11 [64.7%]), and periprosthetic joint infection was the commonest BJI (n = 12 [70.6%]). Only 1 patient had bronchoalveolar lavage (BAL) eosinophil count ≥25%, while most patients had peripheral blood eosinophilia (n = 15 [88.2%]). Chest computed tomography (CT) was compatible with eosinophilic pneumonia in 13 of 14 cases (92.9%). All patients recovered upon discontinuation of daptomycin. Using the different definitions available, only a minority of cases fulfilled existing criteria for DIEP. We propose a new algorithm that includes specific CT scan signs, and systemic instead of BAL eosinophilia.
DIEP is a rare event that requires prompt discontinuation of the causative antibiotic. Current criteria to diagnose definite DIEP are too restrictive and not easily applicable in clinical practice. A new algorithm is proposed here (Lyon algorithm) to facilitate the early identification of DIEP.
Bone and joint infections (BJIs) can be difficult to treat, especially if an implant is present, such as in patients with periprosthetic joint infections (PJIs). These infections are increasingly common and are mainly due to gram-positive organisms such as staphylococci, enterococci, and corynebacteria, that could be resistant to β-lactam antibiotics [1, 2].
Daptomycin is a cyclic lipopeptide antibiotic with exclusive anti-gram-positive activity. Its use was approved by the US Food and Drug Administration (FDA) in 2003 for the following indications [3]: (1) complicated skin and skin structure infections caused by gram-positive cocci, (2) Staphylococcus aureus bloodstream infections, and (3) right-sided S aureus infective endocarditis. Even if daptomycin is not approved in patients with PJI, the latest Infectious Diseases Society of America guidelines for the management of PJIs suggest this antibiotic as an alternative treatment for such infections caused by Staphylococcus or Enterococcus spp [4]. This approach is supported by data from cohort studies, and high bactericidal activity of daptomycin in vitro against both planktonic and biofilm-embedded bacteria [4–6]. However, in this off-label indication, higher doses are used compared to that proposed for complicated skin and skin structure infections and bacteremia (4 and 6 mg/kg/day, respectively) because only a small part (about 10%) of the drug penetrates the bone from the blood, and because of the risk of emergence of resistance at standard doses [6–11].
Even if daptomycin is considered as a safe drug, with less occurrence of adverse events (AEs) in comparison with vancomycin, some specific AEs have been described [12]. Creatinine phosphokinase elevation is the most common AE, occurring in 2%–14% of patients receiving daptomycin [13]. It was associated with antibiotic trough concentrations (Cmin) ≥24.3 mg/L in a study of 108 patients [14]. Less frequently, patients may develop daptomycin-induced eosinophilic pneumonia (DIEP), a potentially serious adverse effect (SAE) that requires prompt discontinuation of daptomycin in all patients and corticosteroid therapy in some of them, when causality is confirmed. Because high doses of daptomycin are used in patients with BJI, the risk and clinical presentation of DIEP need to be evaluated in this specific population.
The aims of this study were to describe the frequency and manifestations of DIEP in patients treated at a referral center for the management of complex BJI, to revisit the current definitions of this disease, and to propose a new algorithm for the diagnosis of DIEP.
METHODS
Study Design and Population
Patients treated from 1 January 2012 to 31 March 2021 in a referral center for complex BJI in Lyon, France (Centre de Référence des Infections Ostéo-Articulaires complexes, www.crioac-lyon.fr), were prospectively included in the Lyon BJI cohort study (NCT02817711): This prospective cohort study includes all patients with BJI, with or without implant, treated in this referral center. The only exclusion criterion is refusal to be included in this cohort study. In this ongoing cohort, off-label use of antimicrobial treatments, as well as occurrence of SAEs, are prospectively recorded. All SAEs are reported to the Centre Régional de Pharmacovigilance of Lyon (a regional reference center that aims to analyze the imputability of a particular drug in each SAE) for analysis. All consecutive cases in whom a diagnosis of DIEP was suspected by the treating clinician were included in this study. We retrospectively analyzed all DIEP cases to exclude patients with another diagnosis, and we included highly suggestive cases of DIEP in this study. Then we applied the different current definitions to discuss criteria for the diagnosis. Patients were informed about the objective of the present study (NCT04414137), and those who refused to participate were excluded.
Study Variables and Definitions
The patients’ demographic characteristics (sex, age, body mass index, American Society of Anesthesiology score, comorbidity such as diabetes, hypertension, chronic lung disease, or chronic kidney disease), BJI (location, type [PJI, osteosynthesis-associated infection, septic arthritis, spondylodiscitis, osteomyelitis, diabetic foot infection], presence or absence of an implant), dose of daptomycin, and usual biological parameters that were prescribed at the physician's discretion were collected. Blood eosinophilia was defined as an eosinophil count >0.5 × 109/L, and low oxygen saturation as an oxygen saturation of <94% on room air without chronic lung disease. Clinical resolution of DIEP was defined as the restoration of baseline respiratory status.
Radiological findings (X-rays and computed tomography [CT] scans) that were prescribed by the treating physicians were all prospectively collected in our electronic medical chart (Centricity Universal Viewer, GE Healthcare) and retrospectively reviewed by a radiology specialist: CT scan features (ground glass opacities, consolidation, septal lines) were graded as absent, mild, moderate, or significant, based on criteria by Jeong et al [15]. Cases were classified based on chest CT as follows: no eosinophilic pneumonia, acute eosinophilic pneumonia, chronic eosinophilic pneumonia, or unclassified eosinophilic pneumonia.
Then, we applied diagnostic criteria of DIEP from the FDA, of those published by Solomon and Schwarz in 2006, Kim et al in 2012, and Phillips et al in 2013, listed in Table 1 [16–19]. Thus, each case of DIEP was retrospectively examined using these different diagnostic criteria.
| FDA [16] . | Solomon and Schwarza [17] . | Phillips et al [18] . | Kim et al [19] . | |||
|---|---|---|---|---|---|---|
| Definite . | Probable . | Possible . | Unlikely . | |||
| Concurrent exposure to daptomycin | Presence of a potential candidate drug or toxin in an appropriate time frame | Concurrent exposure to daptomycin | Concurrent exposure to daptomycin | Concurrent exposure to daptomycin | Concurrent exposure to daptomycin | All other cases that did not meet these criteria |
| - Dyspnea with increased oxygen requirement or requiring MV - Fever | … | Hypoxemia with 1 of the following: dyspnea, cough, fever | - Dyspnea with increased oxygen requirement or requiring MV - Fever | Dyspnea with increased oxygen requirement or requiring MV | … | |
| New infiltrates on CXR or CT scan | Presence of simple, acute, or chronic eosinophilic pneumonia by diagnostic criteria which includes excess of eosinophils either on lung biopsy or BAL (usually ≥25%) in the setting of parenchymal infiltrates | Diffuse bilateral pulmonary infiltrates on CXR or CT scan | New infiltrates on CXR or CT scan | New infiltrates on CXR or CT scan | New infiltrates on CXR or CT scan | |
| BAL with >25% eosinophils | Abnormal BAL eosinophil % of any value, or lung biopsy with histopathology consistent with eosinophilic pneumonia | BAL with >25% eosinophils | BAL with ≤25% eosinophils OR peripheral eosinophilia | … | ||
| Clinical improvement following daptomycin withdrawal | Clinical improvement after cessation of the drug or toxin | Clinical improvement following daptomycin withdrawal | Clinical improvement following daptomycin withdrawal | Clinical improvement following daptomycin withdrawal | Clinical improvement following daptomycin withdrawal or the patient died | |
| … | No other cause of eosinophilic pneumonia such as fungal or parasitic infection | Exclusion of other causes of eosinophilic pneumonia | … | … | … | |
| … | Recurrence of eosinophilic pneumonia with rechallenge to the drug or toxin | … | … | … | … | … |
| FDA [16] . | Solomon and Schwarza [17] . | Phillips et al [18] . | Kim et al [19] . | |||
|---|---|---|---|---|---|---|
| Definite . | Probable . | Possible . | Unlikely . | |||
| Concurrent exposure to daptomycin | Presence of a potential candidate drug or toxin in an appropriate time frame | Concurrent exposure to daptomycin | Concurrent exposure to daptomycin | Concurrent exposure to daptomycin | Concurrent exposure to daptomycin | All other cases that did not meet these criteria |
| - Dyspnea with increased oxygen requirement or requiring MV - Fever | … | Hypoxemia with 1 of the following: dyspnea, cough, fever | - Dyspnea with increased oxygen requirement or requiring MV - Fever | Dyspnea with increased oxygen requirement or requiring MV | … | |
| New infiltrates on CXR or CT scan | Presence of simple, acute, or chronic eosinophilic pneumonia by diagnostic criteria which includes excess of eosinophils either on lung biopsy or BAL (usually ≥25%) in the setting of parenchymal infiltrates | Diffuse bilateral pulmonary infiltrates on CXR or CT scan | New infiltrates on CXR or CT scan | New infiltrates on CXR or CT scan | New infiltrates on CXR or CT scan | |
| BAL with >25% eosinophils | Abnormal BAL eosinophil % of any value, or lung biopsy with histopathology consistent with eosinophilic pneumonia | BAL with >25% eosinophils | BAL with ≤25% eosinophils OR peripheral eosinophilia | … | ||
| Clinical improvement following daptomycin withdrawal | Clinical improvement after cessation of the drug or toxin | Clinical improvement following daptomycin withdrawal | Clinical improvement following daptomycin withdrawal | Clinical improvement following daptomycin withdrawal | Clinical improvement following daptomycin withdrawal or the patient died | |
| … | No other cause of eosinophilic pneumonia such as fungal or parasitic infection | Exclusion of other causes of eosinophilic pneumonia | … | … | … | |
| … | Recurrence of eosinophilic pneumonia with rechallenge to the drug or toxin | … | … | … | … | … |
Abbreviations: BAL, bronchoalveolar lavage; CT, computed tomography; CXR, chest radiograph; FDA, United States Food and Drug Administration; MV, mechanical ventilation.
Not specific to daptomycin-induced eosinophilic pneumonia.
| FDA [16] . | Solomon and Schwarza [17] . | Phillips et al [18] . | Kim et al [19] . | |||
|---|---|---|---|---|---|---|
| Definite . | Probable . | Possible . | Unlikely . | |||
| Concurrent exposure to daptomycin | Presence of a potential candidate drug or toxin in an appropriate time frame | Concurrent exposure to daptomycin | Concurrent exposure to daptomycin | Concurrent exposure to daptomycin | Concurrent exposure to daptomycin | All other cases that did not meet these criteria |
| - Dyspnea with increased oxygen requirement or requiring MV - Fever | … | Hypoxemia with 1 of the following: dyspnea, cough, fever | - Dyspnea with increased oxygen requirement or requiring MV - Fever | Dyspnea with increased oxygen requirement or requiring MV | … | |
| New infiltrates on CXR or CT scan | Presence of simple, acute, or chronic eosinophilic pneumonia by diagnostic criteria which includes excess of eosinophils either on lung biopsy or BAL (usually ≥25%) in the setting of parenchymal infiltrates | Diffuse bilateral pulmonary infiltrates on CXR or CT scan | New infiltrates on CXR or CT scan | New infiltrates on CXR or CT scan | New infiltrates on CXR or CT scan | |
| BAL with >25% eosinophils | Abnormal BAL eosinophil % of any value, or lung biopsy with histopathology consistent with eosinophilic pneumonia | BAL with >25% eosinophils | BAL with ≤25% eosinophils OR peripheral eosinophilia | … | ||
| Clinical improvement following daptomycin withdrawal | Clinical improvement after cessation of the drug or toxin | Clinical improvement following daptomycin withdrawal | Clinical improvement following daptomycin withdrawal | Clinical improvement following daptomycin withdrawal | Clinical improvement following daptomycin withdrawal or the patient died | |
| … | No other cause of eosinophilic pneumonia such as fungal or parasitic infection | Exclusion of other causes of eosinophilic pneumonia | … | … | … | |
| … | Recurrence of eosinophilic pneumonia with rechallenge to the drug or toxin | … | … | … | … | … |
| FDA [16] . | Solomon and Schwarza [17] . | Phillips et al [18] . | Kim et al [19] . | |||
|---|---|---|---|---|---|---|
| Definite . | Probable . | Possible . | Unlikely . | |||
| Concurrent exposure to daptomycin | Presence of a potential candidate drug or toxin in an appropriate time frame | Concurrent exposure to daptomycin | Concurrent exposure to daptomycin | Concurrent exposure to daptomycin | Concurrent exposure to daptomycin | All other cases that did not meet these criteria |
| - Dyspnea with increased oxygen requirement or requiring MV - Fever | … | Hypoxemia with 1 of the following: dyspnea, cough, fever | - Dyspnea with increased oxygen requirement or requiring MV - Fever | Dyspnea with increased oxygen requirement or requiring MV | … | |
| New infiltrates on CXR or CT scan | Presence of simple, acute, or chronic eosinophilic pneumonia by diagnostic criteria which includes excess of eosinophils either on lung biopsy or BAL (usually ≥25%) in the setting of parenchymal infiltrates | Diffuse bilateral pulmonary infiltrates on CXR or CT scan | New infiltrates on CXR or CT scan | New infiltrates on CXR or CT scan | New infiltrates on CXR or CT scan | |
| BAL with >25% eosinophils | Abnormal BAL eosinophil % of any value, or lung biopsy with histopathology consistent with eosinophilic pneumonia | BAL with >25% eosinophils | BAL with ≤25% eosinophils OR peripheral eosinophilia | … | ||
| Clinical improvement following daptomycin withdrawal | Clinical improvement after cessation of the drug or toxin | Clinical improvement following daptomycin withdrawal | Clinical improvement following daptomycin withdrawal | Clinical improvement following daptomycin withdrawal | Clinical improvement following daptomycin withdrawal or the patient died | |
| … | No other cause of eosinophilic pneumonia such as fungal or parasitic infection | Exclusion of other causes of eosinophilic pneumonia | … | … | … | |
| … | Recurrence of eosinophilic pneumonia with rechallenge to the drug or toxin | … | … | … | … | … |
Abbreviations: BAL, bronchoalveolar lavage; CT, computed tomography; CXR, chest radiograph; FDA, United States Food and Drug Administration; MV, mechanical ventilation.
Not specific to daptomycin-induced eosinophilic pneumonia.
Last, to ensure imputability of daptomycin to DIEP, we used the Adverse Drug Reaction Probability Scale, developed in 1981 by Naranjo et al, and other possible drug causes of eosinophilic pneumonitis were evaluated using www.pneumotox.com [20, 21].
Statistical Analysis
Descriptive analyses were done with Stata version 16.1 software (StataCorp, College Station, Texas). Continuous variables were described as mean and standard deviation, or median with interquartile range (IQR) and/or range as appropriate, while categorical variables were described as number and percentage.
Patient Consent Statement
This study was part of the Lyon BJI cohort study (NCT02817711), and patient written consent was obtained for each inclusion. For the present study, patients were informed by letter; those who refused to participate were not included. This study was authorized by the local Commission for Data Protection and Liberties under the number 21_397 and was recorded at ClinicalTrials.gov under the number NCT04414137.
RESULTS
Baseline Characteristics
Among the 4664 patients who were included in the Lyon BJI cohort study between 1 January 2012 and 31 March 2021, 1021 (21.9%) of them received daptomycin, and 22 (2.2%) of them had a suspected DIEP. Five cases were retrospectively excluded because they had an alternative diagnosis: cryptogenic organizing pneumonia, pulmonary embolism, acute respiratory distress syndrome (ARDS), aspiration pneumonia, or drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome (not attributed to daptomycin). Highly suggestive diagnosis of DIEP was considered in 17 (1.7%) patients, in whom the Naranjo scale was between 6 and 8; the AEs’ imputability to daptomycin was considered as probable.
The majority of patients were male (n = 11 [64.7%]) with a median age of 76.0 years (IQR, 71.0–79.0) (Table 2). Most patients had hypertension (n = 12 [70.6%]); only a minority had diabetes (n = 5 [29.4%]) and chronic pulmonary disease (n = 3 [17.7%]) (Table 2).
Patient Characteristics at the Time of Daptomycin-Induced Eosinophilic Pneumonia
| Characteristic . | No. (%) (N = 17) . |
|---|---|
| Age, y, median (IQR) | 76.0 (71.0–79.0) |
| Female sex | 6 (35.3) |
| BMI, kg/m2, median (IQR) | 28.4 (24.7–30.1) |
| ASA score, median (IQR) | 2.0 (2.0–3.0) |
| Comorbidities | |
| Hypertension | 12 (70.6) |
| Diabetes | 5 (29.4) |
| Chronic kidney disease | 4 (23.5) |
| Baseline creatinine, µmol/L, median (IQR) | 77.0 (72.0–98.0) |
| Baseline eGFR, mL/min/1.73 m2, median (IQR) | 73.0 (60.2–85.0) |
| Chronic lung disease | 3 (17.7) |
| Smoker | 2 (11.8) |
| Statin treatment, discontinued | 6 (35.3), 5/6 (83.3) |
| Type of infection | |
| Periprosthetic joint infection | 12 (70.6) |
| Osteosynthesis-associated infection | 2 (11.8) |
| Osteomyelitis | 2 (11.8) |
| Chronic limb ulcers | 1 (5.9) |
| Infection localization | |
| Lower limb | 13 (76.5) |
| Upper limb | 2 (11.8) |
| Sternum | 1 (5.9) |
| Mandibula | 1 (5.9) |
| Daptomycin therapy | |
| Initial daily dose, mg, median (IQR) | 700 (500–700) |
| Initial daily dose, mg/kg, median (IQR; range)a | 8.2 (6.8–8.6; 5.6–10) |
| Trough concentration, mg/L, median (IQR)b | 23.5 (22.2–30.1) |
| Length of treatment, d, median (IQR) | 22.0 (18.0–25.0; 13–78) |
| Repeated exposure | 1 (5.9) |
| DIEP | |
| Days of daptomycin to DIEP, median (IQR) | 18.0 (16.0–23.0; 3–78) |
| Clinical findings | |
| Fever | 10 (58.8) |
| Dyspnea | 13 (76.5) |
| Cough | 9 (52.9) |
| Hypoxemia | 13/15 (86.7) |
| Crackles | 17 (100) |
| Biological findings | |
| CRP, mg/L, median (range) | 232.9 (22.9–423.7) |
| Eosinophil count (×109 cells/mL), median (range) | 1.1 (.31–5.0) |
| Eosinophil %, median (IQR) | 13.9 (3.6–31.0) |
| Concomitant CPK elevation | 3 (17.6) |
| Radiological findings on CT scanc | |
| Bilateral involvement | 13 (100) |
| Localization | |
| Central | 0 |
| Peripheral | 5 (38.5) |
| Combined | 8 (61.5) |
| Ground glass | |
| None | 1 (7.7) |
| Mild | 2 (15.4) |
| Moderate | 4 (30.8) |
| Significant | 6 (46.2) |
| Condensation | |
| None | 0 |
| Mild | 6 (46.2) |
| Moderate | 2 (15.4) |
| Significant | 5 (38.5) |
| Septal lines | |
| None | 5 (38.5) |
| Mild | 3 (23.1) |
| Moderate | 2 (15.4) |
| Significant | 3 (23.1) |
| Specific radiological classification | |
| AEP | 8 (61.5) |
| CEP | 4 (30.8) |
| Combined AEP/CEP | 1 (7.7) |
| Bronchoalveolar lavaged | |
| BAL eosinophil, %, median (IQR; range) | 7.5 (1.3–10.0; 0–35) |
| Characteristic . | No. (%) (N = 17) . |
|---|---|
| Age, y, median (IQR) | 76.0 (71.0–79.0) |
| Female sex | 6 (35.3) |
| BMI, kg/m2, median (IQR) | 28.4 (24.7–30.1) |
| ASA score, median (IQR) | 2.0 (2.0–3.0) |
| Comorbidities | |
| Hypertension | 12 (70.6) |
| Diabetes | 5 (29.4) |
| Chronic kidney disease | 4 (23.5) |
| Baseline creatinine, µmol/L, median (IQR) | 77.0 (72.0–98.0) |
| Baseline eGFR, mL/min/1.73 m2, median (IQR) | 73.0 (60.2–85.0) |
| Chronic lung disease | 3 (17.7) |
| Smoker | 2 (11.8) |
| Statin treatment, discontinued | 6 (35.3), 5/6 (83.3) |
| Type of infection | |
| Periprosthetic joint infection | 12 (70.6) |
| Osteosynthesis-associated infection | 2 (11.8) |
| Osteomyelitis | 2 (11.8) |
| Chronic limb ulcers | 1 (5.9) |
| Infection localization | |
| Lower limb | 13 (76.5) |
| Upper limb | 2 (11.8) |
| Sternum | 1 (5.9) |
| Mandibula | 1 (5.9) |
| Daptomycin therapy | |
| Initial daily dose, mg, median (IQR) | 700 (500–700) |
| Initial daily dose, mg/kg, median (IQR; range)a | 8.2 (6.8–8.6; 5.6–10) |
| Trough concentration, mg/L, median (IQR)b | 23.5 (22.2–30.1) |
| Length of treatment, d, median (IQR) | 22.0 (18.0–25.0; 13–78) |
| Repeated exposure | 1 (5.9) |
| DIEP | |
| Days of daptomycin to DIEP, median (IQR) | 18.0 (16.0–23.0; 3–78) |
| Clinical findings | |
| Fever | 10 (58.8) |
| Dyspnea | 13 (76.5) |
| Cough | 9 (52.9) |
| Hypoxemia | 13/15 (86.7) |
| Crackles | 17 (100) |
| Biological findings | |
| CRP, mg/L, median (range) | 232.9 (22.9–423.7) |
| Eosinophil count (×109 cells/mL), median (range) | 1.1 (.31–5.0) |
| Eosinophil %, median (IQR) | 13.9 (3.6–31.0) |
| Concomitant CPK elevation | 3 (17.6) |
| Radiological findings on CT scanc | |
| Bilateral involvement | 13 (100) |
| Localization | |
| Central | 0 |
| Peripheral | 5 (38.5) |
| Combined | 8 (61.5) |
| Ground glass | |
| None | 1 (7.7) |
| Mild | 2 (15.4) |
| Moderate | 4 (30.8) |
| Significant | 6 (46.2) |
| Condensation | |
| None | 0 |
| Mild | 6 (46.2) |
| Moderate | 2 (15.4) |
| Significant | 5 (38.5) |
| Septal lines | |
| None | 5 (38.5) |
| Mild | 3 (23.1) |
| Moderate | 2 (15.4) |
| Significant | 3 (23.1) |
| Specific radiological classification | |
| AEP | 8 (61.5) |
| CEP | 4 (30.8) |
| Combined AEP/CEP | 1 (7.7) |
| Bronchoalveolar lavaged | |
| BAL eosinophil, %, median (IQR; range) | 7.5 (1.3–10.0; 0–35) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ASA, American Society of Anesthesiology; BAL, bronchoalveolar lavage; BMI, body mass index; CPK, creatinine phosphokinase; CRP, C-reactive protein; CT, computed tomography; DIEP, daptomycin-induced eosinophilic pneumonia; eGFR, estimated glomerular filtration rate; IQR, interquartile range.
Based on actual body weight.
Trough concentrations of daptomycin were available for 9 patients.
Chest CT scans were performed in 13 of 17 (72.2%) patients.
Bronchoalveolar lavage was performed in 10 of 17 (58.8%) patients.
Patient Characteristics at the Time of Daptomycin-Induced Eosinophilic Pneumonia
| Characteristic . | No. (%) (N = 17) . |
|---|---|
| Age, y, median (IQR) | 76.0 (71.0–79.0) |
| Female sex | 6 (35.3) |
| BMI, kg/m2, median (IQR) | 28.4 (24.7–30.1) |
| ASA score, median (IQR) | 2.0 (2.0–3.0) |
| Comorbidities | |
| Hypertension | 12 (70.6) |
| Diabetes | 5 (29.4) |
| Chronic kidney disease | 4 (23.5) |
| Baseline creatinine, µmol/L, median (IQR) | 77.0 (72.0–98.0) |
| Baseline eGFR, mL/min/1.73 m2, median (IQR) | 73.0 (60.2–85.0) |
| Chronic lung disease | 3 (17.7) |
| Smoker | 2 (11.8) |
| Statin treatment, discontinued | 6 (35.3), 5/6 (83.3) |
| Type of infection | |
| Periprosthetic joint infection | 12 (70.6) |
| Osteosynthesis-associated infection | 2 (11.8) |
| Osteomyelitis | 2 (11.8) |
| Chronic limb ulcers | 1 (5.9) |
| Infection localization | |
| Lower limb | 13 (76.5) |
| Upper limb | 2 (11.8) |
| Sternum | 1 (5.9) |
| Mandibula | 1 (5.9) |
| Daptomycin therapy | |
| Initial daily dose, mg, median (IQR) | 700 (500–700) |
| Initial daily dose, mg/kg, median (IQR; range)a | 8.2 (6.8–8.6; 5.6–10) |
| Trough concentration, mg/L, median (IQR)b | 23.5 (22.2–30.1) |
| Length of treatment, d, median (IQR) | 22.0 (18.0–25.0; 13–78) |
| Repeated exposure | 1 (5.9) |
| DIEP | |
| Days of daptomycin to DIEP, median (IQR) | 18.0 (16.0–23.0; 3–78) |
| Clinical findings | |
| Fever | 10 (58.8) |
| Dyspnea | 13 (76.5) |
| Cough | 9 (52.9) |
| Hypoxemia | 13/15 (86.7) |
| Crackles | 17 (100) |
| Biological findings | |
| CRP, mg/L, median (range) | 232.9 (22.9–423.7) |
| Eosinophil count (×109 cells/mL), median (range) | 1.1 (.31–5.0) |
| Eosinophil %, median (IQR) | 13.9 (3.6–31.0) |
| Concomitant CPK elevation | 3 (17.6) |
| Radiological findings on CT scanc | |
| Bilateral involvement | 13 (100) |
| Localization | |
| Central | 0 |
| Peripheral | 5 (38.5) |
| Combined | 8 (61.5) |
| Ground glass | |
| None | 1 (7.7) |
| Mild | 2 (15.4) |
| Moderate | 4 (30.8) |
| Significant | 6 (46.2) |
| Condensation | |
| None | 0 |
| Mild | 6 (46.2) |
| Moderate | 2 (15.4) |
| Significant | 5 (38.5) |
| Septal lines | |
| None | 5 (38.5) |
| Mild | 3 (23.1) |
| Moderate | 2 (15.4) |
| Significant | 3 (23.1) |
| Specific radiological classification | |
| AEP | 8 (61.5) |
| CEP | 4 (30.8) |
| Combined AEP/CEP | 1 (7.7) |
| Bronchoalveolar lavaged | |
| BAL eosinophil, %, median (IQR; range) | 7.5 (1.3–10.0; 0–35) |
| Characteristic . | No. (%) (N = 17) . |
|---|---|
| Age, y, median (IQR) | 76.0 (71.0–79.0) |
| Female sex | 6 (35.3) |
| BMI, kg/m2, median (IQR) | 28.4 (24.7–30.1) |
| ASA score, median (IQR) | 2.0 (2.0–3.0) |
| Comorbidities | |
| Hypertension | 12 (70.6) |
| Diabetes | 5 (29.4) |
| Chronic kidney disease | 4 (23.5) |
| Baseline creatinine, µmol/L, median (IQR) | 77.0 (72.0–98.0) |
| Baseline eGFR, mL/min/1.73 m2, median (IQR) | 73.0 (60.2–85.0) |
| Chronic lung disease | 3 (17.7) |
| Smoker | 2 (11.8) |
| Statin treatment, discontinued | 6 (35.3), 5/6 (83.3) |
| Type of infection | |
| Periprosthetic joint infection | 12 (70.6) |
| Osteosynthesis-associated infection | 2 (11.8) |
| Osteomyelitis | 2 (11.8) |
| Chronic limb ulcers | 1 (5.9) |
| Infection localization | |
| Lower limb | 13 (76.5) |
| Upper limb | 2 (11.8) |
| Sternum | 1 (5.9) |
| Mandibula | 1 (5.9) |
| Daptomycin therapy | |
| Initial daily dose, mg, median (IQR) | 700 (500–700) |
| Initial daily dose, mg/kg, median (IQR; range)a | 8.2 (6.8–8.6; 5.6–10) |
| Trough concentration, mg/L, median (IQR)b | 23.5 (22.2–30.1) |
| Length of treatment, d, median (IQR) | 22.0 (18.0–25.0; 13–78) |
| Repeated exposure | 1 (5.9) |
| DIEP | |
| Days of daptomycin to DIEP, median (IQR) | 18.0 (16.0–23.0; 3–78) |
| Clinical findings | |
| Fever | 10 (58.8) |
| Dyspnea | 13 (76.5) |
| Cough | 9 (52.9) |
| Hypoxemia | 13/15 (86.7) |
| Crackles | 17 (100) |
| Biological findings | |
| CRP, mg/L, median (range) | 232.9 (22.9–423.7) |
| Eosinophil count (×109 cells/mL), median (range) | 1.1 (.31–5.0) |
| Eosinophil %, median (IQR) | 13.9 (3.6–31.0) |
| Concomitant CPK elevation | 3 (17.6) |
| Radiological findings on CT scanc | |
| Bilateral involvement | 13 (100) |
| Localization | |
| Central | 0 |
| Peripheral | 5 (38.5) |
| Combined | 8 (61.5) |
| Ground glass | |
| None | 1 (7.7) |
| Mild | 2 (15.4) |
| Moderate | 4 (30.8) |
| Significant | 6 (46.2) |
| Condensation | |
| None | 0 |
| Mild | 6 (46.2) |
| Moderate | 2 (15.4) |
| Significant | 5 (38.5) |
| Septal lines | |
| None | 5 (38.5) |
| Mild | 3 (23.1) |
| Moderate | 2 (15.4) |
| Significant | 3 (23.1) |
| Specific radiological classification | |
| AEP | 8 (61.5) |
| CEP | 4 (30.8) |
| Combined AEP/CEP | 1 (7.7) |
| Bronchoalveolar lavaged | |
| BAL eosinophil, %, median (IQR; range) | 7.5 (1.3–10.0; 0–35) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ASA, American Society of Anesthesiology; BAL, bronchoalveolar lavage; BMI, body mass index; CPK, creatinine phosphokinase; CRP, C-reactive protein; CT, computed tomography; DIEP, daptomycin-induced eosinophilic pneumonia; eGFR, estimated glomerular filtration rate; IQR, interquartile range.
Based on actual body weight.
Trough concentrations of daptomycin were available for 9 patients.
Chest CT scans were performed in 13 of 17 (72.2%) patients.
Bronchoalveolar lavage was performed in 10 of 17 (58.8%) patients.
Most patients had normal renal function (median creatinine clearance, 73.0 mL/minute/1.73 m2 [IQR, 60.2–85.0]): only 4 patients had chronic kidney disease with estimated glomerular filtration rate (eGFR) <60 mL/minute/1.73 m2 (of which 3 patients had eGFR 55 mL/minute/1.73 m2 and only 1 had eGFR 34.5 mL/minute/1.73 m2). Median creatinine was 77.0 μmol/L (IQR, 72.0–98.0).
Periprosthetic joint infections were the majority of BJIs (n = 12 [70.6%]), followed by osteosynthesis-associated infection (n = 2 [11.8%]) and osteomyelitis (n = 8). In most cases, an orthopedic implant was present (n = 14 [82.4%]) and the infection was chronic (n = 12 [70.6%]), monomicrobial (n = 11 [64.7%]), and involved the lower limb (n = 13 [76.5%]). In all cases, surgery had been performed.
Daptomycin was prescribed with a median initial daily dose of 700 mg (IQR, 500–700; range, 350–1000), or 8.2 mg/kg (IQR, 6.8–8.6; range, 5.6–10.0) based on actual body weight, and empirically in 8 (47.1%) patients. The median treatment duration until the onset of symptoms suggestive of DIEP was 18.0 days (IQR, 16.0–23.0), while the total treatment length was 22.0 days (IQR, 18.0–25.0).
Clinical and Biological Presentation
In most cases, patients presented with fever (n = 10 [58.8%]), dyspnea (n = 13 [76.5%]), and hypoxemia (n = 13/15 [86.7%]). Cough was a symptom in half of the cases (n = 9 [52.9%]). In contrast, crackles were present in all cases (n = 17 [100%]). Two patients required noninvasive ventilation, and 1 patient had to be intubated due to poor clinical evolution (Table 2; Supplementary Table 1).
CRP at diagnosis was high, with a median of 232.9 mg/L (IQR, 84.3–272.0), and the vast majority of patients had blood eosinophilia (n = 15 [88.2%]), with a median of 1.1 × 109 cells/mL (IQR, 0.7–2.0), that is, 13.9% (IQR, 6.0%–18.0%).
BAL was performed in 10 patients (58.8%), 3 of whom had 0 or 1% of eosinophils. Despite the presence of peripheral blood eosinophilia in 9 of the patients, 7 patients had a neutrophilic BAL cell count, 1 had an eosinophilic BAL pattern (> 25%), 1 a mixed neutrophilic and eosinophilic pattern, and 1 a mixed neutrophilic and lymphocytic pattern (Supplementary Table 2). Overall, the median eosinophil percentage in BAL was 7.5% (IQR, 1.3%–10.0%; range, 0–35%).
Finally, only 1 case had a subsequent reexposure, at 3 months: this led to an increase in eosinophils without pneumonia, prompting its discontinuation after 12 days. Interestingly, 1 patient had a 14-day exposure to daptomycin 6 months prior to DIEP, without any adverse event reported during that past exposure.
Clinical evolution was rapidly favorable in all patients upon discontinuation of daptomycin. In addition, oral prednisone therapy (1 mg/kg/day) was initiated in 2 patients at the time of the diagnosis of DIEP and continued for 20 and 24 days, respectively. Corticosteroid therapy was introduced due to the severity of clinical manifestations, and not because of a lack of response to daptomycin discontinuation. There were no deaths attributed to DIEP.
Radiological Findings
Of the 17 patients with suspected DIEP, 14 had a chest CT scan available for review, and 13 (92.9%) had radiological findings suggestive of drug-induced iatrogenic pneumonia. One patient without imaging abnormalities had CT scan performed at an early stage of the disease (<24 hours). Bilateral involvement was present in all cases, with mostly peripheral lesions only (n = 8/13 [61.5%]), or combined (central and peripheral) lesions: there was no central involvement alone. Ground glass opacities was found in nearly all patients (n = 12/13 [92.3%]), with moderate (n = 4/13 [30.8%]) to significant (n = 6/13 [46.2%]) lesions. Condensation and septal lines were also found (Table 2; Figure 1). Finally, 8 (61.5%) CT scans showed with a pattern suggestive of acute eosinophilic pneumonia; in 1 case, a combined acute and chronic involvement was found.
![Different computed tomography (CT) patterns of daptomycin-induced eosinophilic pneumonia, with specific signs on CT scan based on criteria by Jeong et al [15]. A, Diffuse ground glass opacities and air-space consolidations compatible with chronic eosinophilic pneumonia. B and C, Same patient with multiple bilateral infiltrates compatible with chronic eosinophilic pneumonia (B) and central ground glass opacities with interlobular septal lines suggestive of acute eosinophilic pneumonia (C).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ofid/9/11/10.1093_ofid_ofac577/1/m_ofac577f1.jpeg?Expires=1747905911&Signature=DO3iyNeIo5QddCZVjOKLUhXpMRpJzOMl4YzvVf0Gp5yzW3y-uTnZV1hwdtfJjsaH6lmmvhUKeOdM5e22UgbO4QTYwvEMiiUpXRCJxzkrhttVb-KfUetdye6Klz6GO2ugjCZWBhi6G7XOcJmjc8FRiydVEC3ESTFSywRRntwCRBMSd1pLzRxkfbdMv3ChEVOfwS4KbjaWE5VHaeuywBelw~zJqCem8eY7DdYcjA7SYEYTMT-2zyX28hoF82RgNbz6TWeHWq1GBCYwoiy2CDCIaZvvYwvena1ZIMMjE56~FdvzvKOqvuqqm6NUGHwI3ukF2jK38XbT7mCtL4iQZPUKCA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Different computed tomography (CT) patterns of daptomycin-induced eosinophilic pneumonia, with specific signs on CT scan based on criteria by Jeong et al [15]. A, Diffuse ground glass opacities and air-space consolidations compatible with chronic eosinophilic pneumonia. B and C, Same patient with multiple bilateral infiltrates compatible with chronic eosinophilic pneumonia (B) and central ground glass opacities with interlobular septal lines suggestive of acute eosinophilic pneumonia (C).
Applicability of Existing Criteria
No cases in our cohort met the FDA or Solomon and Schwarz criteria of eosinophilic pneumonia, which need to perform a BAL and to demonstrate BAL eosinophilia ≥25%. Similarly, no patient in this series met the classification by Kim et al, which requires all FDA criteria to be met. However, 13 (76.5%) patients were classified as probable and 4 (23.5%) as possible when limiting the severe BAL criterion to ≤25% and considering peripheral eosinophilia as an alternative to BAL eosinophilia. Finally, it should be noted that fever was absent in the only case with eosinophil count >25% in BAL, which also failed to meet all diagnostic criteria. In contrast, 8 (47.1%) patients fulfilled the 6 criteria by Phillips et al (Tables 1 and 3).
Number of Positive Criteria by Definitions of Daptomycin-Induced Eosinophilic Pneumonia, Depending on the Previous Published Criteria, and Depending on the French Referral Centre for Complex Bone and Joint Infections (CRIOAc) Lyon Criteria Proposed Here
| Patient ID . | FDA [16] . | Solomon and Schwarz [17] . | Phillips et al [18] . | Kim et al [19] . | Lyon Algorithm . |
|---|---|---|---|---|---|
| No. of Criteria in a Total of 6; Final Diagnosis of DIEP . | No. of Criteria in a Total of 5; Final Diagnosis of DIEP . | No. of Criteria in a Total of 6; Final Diagnosis of DIEP . | Final Diagnosis of DIEPa . | Final Diagnosis of DIEPa . | |
| 1 | 5; No | 4; No | 6; Yes | Probable | Yes, definite |
| 2 | 4; No | 3; No | 6; Yes | Probable | Yes, definite |
| 3 | 4; No | 3; No | 4; No | Probable | Probable |
| 4 | 5; No | 3; No | 6; Yes | Probable | Yes, definite |
| 5 | 3; No | 3; No | 4; No | Possible | Probable |
| 6 | 5; No | 3; No | 5; No | Probable | Yes, definite |
| 7 | 5; No | 3; No | 6; Yes | Probable | Yes, definite |
| 8 | 4; No | 3; No | 6; Yes | Probable | Yes, definite |
| 9 | 3; No | 3; No | 4; No | Possible | Yes, definite |
| 10 | 5; No | 3; No | 5; No | Probable | Yes, definite |
| 11 | 4; No | 3; No | 5; No | Probable | Probable |
| 12 | 5; No | 3; No | 6; Yes | Probable | Yes, definite |
| 13 | 5; No | 3; No | 6; Yes | Probable | Yes, definite |
| 14 | 4; No | 3; No | 4; No | Possible | Yes, definite |
| 15 | 4; No | 3; No | 5; No | Probable | Yes, definite |
| 16 | 5; No | 3; No | 6; Yes | Probable | Yes, definite |
| 17 | 4; No | 3; No | 4; No | Possible | Yes, definite |
| Patient ID . | FDA [16] . | Solomon and Schwarz [17] . | Phillips et al [18] . | Kim et al [19] . | Lyon Algorithm . |
|---|---|---|---|---|---|
| No. of Criteria in a Total of 6; Final Diagnosis of DIEP . | No. of Criteria in a Total of 5; Final Diagnosis of DIEP . | No. of Criteria in a Total of 6; Final Diagnosis of DIEP . | Final Diagnosis of DIEPa . | Final Diagnosis of DIEPa . | |
| 1 | 5; No | 4; No | 6; Yes | Probable | Yes, definite |
| 2 | 4; No | 3; No | 6; Yes | Probable | Yes, definite |
| 3 | 4; No | 3; No | 4; No | Probable | Probable |
| 4 | 5; No | 3; No | 6; Yes | Probable | Yes, definite |
| 5 | 3; No | 3; No | 4; No | Possible | Probable |
| 6 | 5; No | 3; No | 5; No | Probable | Yes, definite |
| 7 | 5; No | 3; No | 6; Yes | Probable | Yes, definite |
| 8 | 4; No | 3; No | 6; Yes | Probable | Yes, definite |
| 9 | 3; No | 3; No | 4; No | Possible | Yes, definite |
| 10 | 5; No | 3; No | 5; No | Probable | Yes, definite |
| 11 | 4; No | 3; No | 5; No | Probable | Probable |
| 12 | 5; No | 3; No | 6; Yes | Probable | Yes, definite |
| 13 | 5; No | 3; No | 6; Yes | Probable | Yes, definite |
| 14 | 4; No | 3; No | 4; No | Possible | Yes, definite |
| 15 | 4; No | 3; No | 5; No | Probable | Yes, definite |
| 16 | 5; No | 3; No | 6; Yes | Probable | Yes, definite |
| 17 | 4; No | 3; No | 4; No | Possible | Yes, definite |
Red color indicates patients without the diagnosis of DIEP; orange color indicates patients with probable or possible diagnosis of DIEP; green indicates patients with a final diagnosis of DIEP.
Abbreviations: DIEP, daptomycin-induced eosinophilic pneumonia; FDA, United States Food and Drug Administration.
Definite, probable, possible, or unlikely.
Number of Positive Criteria by Definitions of Daptomycin-Induced Eosinophilic Pneumonia, Depending on the Previous Published Criteria, and Depending on the French Referral Centre for Complex Bone and Joint Infections (CRIOAc) Lyon Criteria Proposed Here
| Patient ID . | FDA [16] . | Solomon and Schwarz [17] . | Phillips et al [18] . | Kim et al [19] . | Lyon Algorithm . |
|---|---|---|---|---|---|
| No. of Criteria in a Total of 6; Final Diagnosis of DIEP . | No. of Criteria in a Total of 5; Final Diagnosis of DIEP . | No. of Criteria in a Total of 6; Final Diagnosis of DIEP . | Final Diagnosis of DIEPa . | Final Diagnosis of DIEPa . | |
| 1 | 5; No | 4; No | 6; Yes | Probable | Yes, definite |
| 2 | 4; No | 3; No | 6; Yes | Probable | Yes, definite |
| 3 | 4; No | 3; No | 4; No | Probable | Probable |
| 4 | 5; No | 3; No | 6; Yes | Probable | Yes, definite |
| 5 | 3; No | 3; No | 4; No | Possible | Probable |
| 6 | 5; No | 3; No | 5; No | Probable | Yes, definite |
| 7 | 5; No | 3; No | 6; Yes | Probable | Yes, definite |
| 8 | 4; No | 3; No | 6; Yes | Probable | Yes, definite |
| 9 | 3; No | 3; No | 4; No | Possible | Yes, definite |
| 10 | 5; No | 3; No | 5; No | Probable | Yes, definite |
| 11 | 4; No | 3; No | 5; No | Probable | Probable |
| 12 | 5; No | 3; No | 6; Yes | Probable | Yes, definite |
| 13 | 5; No | 3; No | 6; Yes | Probable | Yes, definite |
| 14 | 4; No | 3; No | 4; No | Possible | Yes, definite |
| 15 | 4; No | 3; No | 5; No | Probable | Yes, definite |
| 16 | 5; No | 3; No | 6; Yes | Probable | Yes, definite |
| 17 | 4; No | 3; No | 4; No | Possible | Yes, definite |
| Patient ID . | FDA [16] . | Solomon and Schwarz [17] . | Phillips et al [18] . | Kim et al [19] . | Lyon Algorithm . |
|---|---|---|---|---|---|
| No. of Criteria in a Total of 6; Final Diagnosis of DIEP . | No. of Criteria in a Total of 5; Final Diagnosis of DIEP . | No. of Criteria in a Total of 6; Final Diagnosis of DIEP . | Final Diagnosis of DIEPa . | Final Diagnosis of DIEPa . | |
| 1 | 5; No | 4; No | 6; Yes | Probable | Yes, definite |
| 2 | 4; No | 3; No | 6; Yes | Probable | Yes, definite |
| 3 | 4; No | 3; No | 4; No | Probable | Probable |
| 4 | 5; No | 3; No | 6; Yes | Probable | Yes, definite |
| 5 | 3; No | 3; No | 4; No | Possible | Probable |
| 6 | 5; No | 3; No | 5; No | Probable | Yes, definite |
| 7 | 5; No | 3; No | 6; Yes | Probable | Yes, definite |
| 8 | 4; No | 3; No | 6; Yes | Probable | Yes, definite |
| 9 | 3; No | 3; No | 4; No | Possible | Yes, definite |
| 10 | 5; No | 3; No | 5; No | Probable | Yes, definite |
| 11 | 4; No | 3; No | 5; No | Probable | Probable |
| 12 | 5; No | 3; No | 6; Yes | Probable | Yes, definite |
| 13 | 5; No | 3; No | 6; Yes | Probable | Yes, definite |
| 14 | 4; No | 3; No | 4; No | Possible | Yes, definite |
| 15 | 4; No | 3; No | 5; No | Probable | Yes, definite |
| 16 | 5; No | 3; No | 6; Yes | Probable | Yes, definite |
| 17 | 4; No | 3; No | 4; No | Possible | Yes, definite |
Red color indicates patients without the diagnosis of DIEP; orange color indicates patients with probable or possible diagnosis of DIEP; green indicates patients with a final diagnosis of DIEP.
Abbreviations: DIEP, daptomycin-induced eosinophilic pneumonia; FDA, United States Food and Drug Administration.
Definite, probable, possible, or unlikely.
DISCUSSION
Daptomycin-induced eosinophilic pneumonia is a rare but potentially severe event if not diagnosed in time and if daptomycin is not discontinued. In a review of 196 cases of drug-induced eosinophilic pneumonia, daptomycin accounted for most cases [22].
The pathogenesis of DIEP is not known. However, as daptomycin is inactivated by pulmonary surfactant (resulting in inhibition of its antibacterial activity), one hypothesis is that this interaction may cause pulmonary lesions by sequestration of daptomycin in the alveolar space; it would act as an antigen presented by alveolar macrophages to T-helper 2 cells resulting in the release of interleukin 5 in parallel with the secretion of eotaxin by macrophages, leading to the migration of eosinophils to the lungs [19, 23]. Therefore, accumulation of daptomycin in the lung, and/or repeated daptomycin exposure may promote the occurrence of DIEP.
In the management of BJI, daptomycin is increasingly used at doses >6 mg/kg that is the maximum dose for the approved indications in the drug label [24]. The use of higher dosage may be associated with an increased risk of AE [6]. Among our cohort, only 1.7% of patients who received daptomycin for BJI developed a DIEP whereas they received a median daily dose of 8.2 mg/kg. This is lower than the 4.8% found in a recently published study by Soldevila-Boixader et al [25]. Unfortunately, in that study, dosages were only expressed in milligrams or total cumulative doses, and there is no information of dosages in milligrams per kilogram, which seems more relevant. Indeed, as we know, sex is a factor influencing daptomycin clearance, and a residual concentration >24.3 mg/L has been shown to be associated with an increased risk of myotoxicity [11, 14]. A study considering pharmacokinetic and pharmacodynamic aspects of daptomycin instead of total cumulative doses could help identifying patients at risk of DIEP.
Because all cases in this series resolved upon discontinuation of daptomycin, we consider that the diagnosis of DIEP can be considered definite. However, when diagnostic criteria of the FDA, Solomon and Schwarz, or Kim et al were used for the diagnosis of DIEP in our patients, none of our cases would be classified as definite DIEP. Thus, the applicability of these definitions is limited in clinical practice when a case of DIEP is suspected. The present series suggests that DIEP, as many cases of drug-induced pneumonitis, may be difficult to classify and may often not meet diagnostic criteria of eosinophilic pneumonia. In our opinion, the published criteria, which are based on the diagnosis of eosinophilic pneumonia in general, are too restrictive. Typically, the criterion of >25% eosinophils in BAL seems poorly sensitive and difficult to collect in clinical practice [26]. The combination of peripheral blood eosinophilia and absence (eg, <5%) or only mild (eg, <25%) eosinophilia in BAL is unusual, and if confirmed, could represent a unique feature of DIEP among drug-induced interstitial lung diseases. The approaches of Phillips et al and Kim et al seem to be more sensitive, considering any abnormal eosinophil count in BAL and/or blood eosinophilia. Indeed, nearly 90% of patients had a blood eosinophilia, a criterion not included in the other 2 definitions. Importantly, peripheral blood eosinophilia may be lacking at presentation of idiopathic eosinophilic pneumonia, with the eosinophil count rising within days after presentation, which was not observed in this series [27–29]. Moreover, fever is mandatory for the FDA definition, although only half of the cases had this symptom. A strict implementation of FDA criteria would be likely to result in underdiagnosis of DIEP, with a risk of inadequate management of the condition with potential delay in drug discontinuation, which is the key intervention.
Reexposure to daptomycin as a diagnostic criterion for Solomon and Schwarz is also questionable. Although this is a classical criterion in pharmacovigilance analysis, rechallenge is generally discouraged and should be avoided, considering the potential severity of DIEP [30].
Typically, acute eosinophilic pneumonia is characterized radiologically by bilateral pleural effusion, interlobular septal thickening, and alveolar consolidations, while chronic eosinophilic pneumonia presents with a combination of ground glass opacities and/or consolidations with upper lobe and peripheral predominance [15, 30]. These radiological findings may also be suggestive of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia. The presentation, however, could be markedly different—a peripheral distribution with a posterior predominance especially in inferior lobes in SARS-CoV-2 infections, and involving mainly the upper lobes in DIEP [31]. This may help to differentiate between the 2 diseases, although a bronchoscopy is most often needed to make the final diagnosis [32].
Although the vast majority of patients developed their symptoms within 1 month of starting daptomycin (except for 2 patients, at 39 and 78 days), 5 had radiological patterns suggestive of chronic rather than acute eosinophilic pneumonia. Moreover, 1 patient had a radiological pattern consistent with a combination of both acute and chronic eosinophilic pneumonia features. These findings suggest that the radiologic presentation of DIEP is variable and can borrow features of either acute or eosinophilic pneumonia irrespective of the timeframe relative to treatment initiation and symptom onset.
Interestingly, all patients in this series but 1 had imaging features of eosinophilic pneumonia on CT scan, whereas published definitions of DIEP suggest only “new bilateral infiltrates on X-ray or CT scan,” without any details [15]. In our opinion, chest X-rays are not specific enough for the diagnosis of DIEP. For example, in our cohort, some X-rays suspected acute lung edema or bacterial pneumonia with alveolar condensations, or interstitial infiltrates without further diagnostic input; in contrast, CT scan is the most relevant imaging when eosinophilic pneumonia is suspected. We believe that CT scan contribution could be more significant in the future, to increase or decrease the diagnostic probability of DIEP. Of note, in the case of the patient without suggestive findings on the CT scan, it turns out that CT was performed very soon after the onset of symptoms (<24 hours), whereas BAL was done 1 week later, showing >25% eosinophils. It is therefore possible that the CT scan was done too soon. Thus, we suggest that CT scan should have a more important place in the identification and management of DIEP, as in any drug-induced iatrogenic pneumonia.
Our study has several limitations: first, as it was a monocentric study, DIEP was a rare SAE, accounting for only 17 patients treated with daptomycin. Second, it was a retrospective analysis of prospectively included cases: Clinicians in charge of patients decided or not to include the patients, yet some DIEP may have been missed. Last, BAL was not performed in all patients (10 of 17 cases). In our opinion, this underlies the limit of the previous definitions in which a bronchoscopy is mandatory for the diagnosis of DIEP, whereas it cannot be systematically performed in clinical practice.
From these perspectives, we suggest a new algorithm (called Lyon algorithm) for the approach to suspected DIEP (Figure 2) based on new or refined criteria. We believe that the radiological contribution to the diagnosis, together with any abnormality of peripheric eosinophils or in BAL, are strong indicators of DIEP. Thus, in the presence of suggestive radiological findings on CT scan and any abnormal eosinophil count (peripheric or BAL), and the improvement of the patient’s clinical condition upon daptomycin discontinuation, the diagnosis of DIEP could be considered definite, even if BAL is not performed nor conclusive. All but 3 of the present cases meet the proposed definition of definite DIEP, whereas 3 are classified as probable DIEP in the absence of a CT scan available. Prospective evaluation of the proposed algorithm and criteria in an independent cohort is warranted, especially as this algorithm has only been tested on 17 cases of DIEP in our institution. We believe that it will be more useful than the previous definitions for clinicians in daily practice to diagnose DIEP in patients treated with daptomycin.
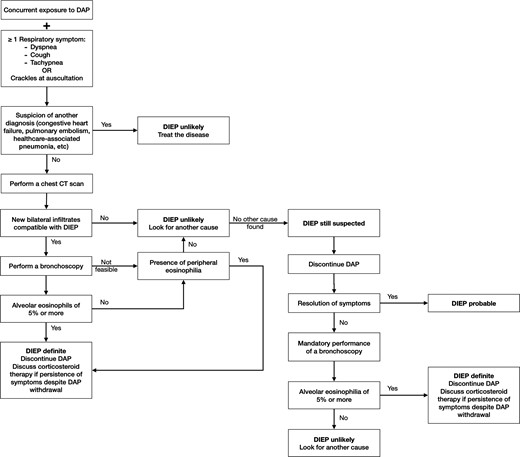
Proposed Lyon algorithm definition for diagnosis and management of suspected daptomycin-induced eosinophilic pneumonia. Abbreviations: CT, computed tomography; DAP, daptomycin; DIEP, daptomycin-induced eosinophilic pneumonia.
CONCLUSIONS
DIEP is a potentially life-threatening adverse event that should be suspected in any patient receiving daptomycin and presenting respiratory symptoms or increased eosinophils. Daptomycin should be promptly discontinued if DIEP is suspected. As available criteria seem not clinically accessible and/or poorly sensitive to diagnose all DIEP, we proposed a new algorithm based on our experience of DIEP in patients with BJI treated by high doses (>6 mg/kg) of daptomycin. Further studies should be conducted to validate and refine this algorithm, and to better understand the pathogenesis and determinants of DIEP.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. Study design: T.-T. P., R. G., S. G., T. F. Data collection: T.-T. P. Data analysis: T.-T. P., V. C., T. F. Statistics: T.-T. P. Drafting article: T-.T. P. Critical revision of the article: All authors.
Lyon Bone and Joint Infection Study Group. Coordinator: Tristan Ferry. Infectious Diseases Specialists: Tristan Ferry, Florent Valour, Thomas Perpoint, Florence Ader, Sandrine Roux, Agathe Becker, Claire Triffault-Fillit, Anne Conrad, Cécile Pouderoux, Pierre Chauvelot, Paul Chabert, Johanna Lippman, Evelyne Braun. Surgeons: Sébastien Lustig, Elvire Servien, Cécile Batailler, Stanislas Gunst, Axel Schmidt, Eliott Sappey-Marinier, Quentin Ode, Michel-Henry Fessy, Anthony Viste, Jean-Luc Besse, Philippe Chaudier, Lucie Louboutin, Adrien Van Haecke, Marcelle Mercier, Vincent Belgaid, Aram Gazarian, Arnaud Walch, Antoine Bertani, Frédéric Rongieras, Sébastien Martres, Franck Trouillet, Cédric Barrey, Ali Mojallal, Sophie Brosset, Camille Hanriat, Hélène Person, Philippe Céruse, Carine Fuchsmann, Arnaud Gleizal. Anesthesiologists: Frédéric Aubrun, Mikhail Dziadzko, Caroline Macabéo, Dana Patrascu. Microbiologists: Frederic Laurent, Laetitia Beraud, Tiphaine Roussel-Gaillard, Céline Dupieux, Camille Kolenda, Jérôme Josse. Imaging: Fabien Craighero, Loic Boussel, Jean-Baptiste Pialat, Isabelle Morelec. Pharmacokinetics/pharmacodynamics specialists: Michel Tod, Marie-Claude Gagnieu, Sylvain Goutelle. Clinical research assistant and database manager: Eugénie Mabrut.
Financial support. This work was funded by Hospices Civils de Lyon and T.-T. Pham received a fellowship grant from Geneva University Hospitals.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Potential conflicts of interest. The authors: No reported conflicts of interest.





Comments