-
PDF
- Split View
-
Views
-
Cite
Cite
Yosuke Ebisu, Yoichiro Natori, Gemma Rosello, Shweta Anjan, Jacques Simkins, Jose F Camargo, Michele I Morris, Octavio V Martinez, Lilian M Abbo, Mycobacterium abscessus Infections in Solid Organ Transplant Recipients: Single-Center Experience in the United States, 2013–2018, Open Forum Infectious Diseases, Volume 9, Issue 7, July 2022, ofac254, https://doi.org/10.1093/ofid/ofac254
Close - Share Icon Share
Abstract
Mycobacterium abscessus is increasingly recognized as a human pathogen causing life-threatening infections in immunocompromised patients. There is a paucity of data around this topic in solid organ transplant (SOT) recipients.
This work was a single-center retrospective cohort study of all SOT recipients with a positive culture for M abscessus between 2013 and 2018.
A total of 20 patients (55% female) met inclusion criteria, including 1 kidney recipient (5.0%), 2 liver recipients (10.0%), 12 lung recipients (60.0%), 1 heart recipient (5.0%), and 4 combined organ recipients (20.0%). The median time from SOT to infection was 100 days (range, 30–431 days). Thirteen (65.0%) patients (1 kidney, 1 heart, 7 lung, 1 liver, 1 intestine, and 2 multivisceral) were treated with a median duration of 185 antibiotic days (range, 20–523 days). Among them, M abscessus was isolated from respiratory samples in 8 and nonrespiratory samples in 5; 4 of 13 (30.8%) patients had treatment failure and 3 of 13 (23.1%) had unrelated deaths within 1 year after diagnosis. Seven patients (5 lung transplant recipients) with the organism isolated from respiratory samples were not treated as their cultures represented airway colonization or contamination; of those, 2 (28.6%) died (unrelated to infection) and 5 (71.4%) were alive without the infection after 1 year of follow-up.
Mycobacterium abscessus infections affect SOT recipients with a high proportion of clinical failures. However, in lung recipients, not all positive cultures correlated with infection, and without treatment some patients had good clinical outcomes. Thus, differentiating colonization from infection is important, and infection prevention measures and novel therapeutic agents are needed for SOT recipients.
Mycobacterium abscessus complex organisms are found ubiquitously in the environment in water, soil and dust, shower heads, and ice machines [1, 2]. It is a species of rapidly growing mycobacteria, first reported by Moore and Frerichs in 1953 [3]. After it was separated from the Mycobacterium chelonae group, the infections caused by M abscessus strains were recognized to have higher fatality rates than any other rapidly growing mycobacteria [3]. Mycobacterium abscessus causes a wide spectrum of infections in both immunocompetent and immunocompromised patients [4, 5] and has been associated with hospital outbreaks [6].
Mycobacterium abscessus infections have been reported among lung transplant recipients as well as other solid organ transplant (SOT) recipients. The estimated reported incidence of nontuberculous mycobacterial (NTM) infection is 0.16%–0.55%, 0.04%, 2.8%, and 0.46%–4.4% in kidney, liver, heart, and lung transplant recipients, respectively [7]. Aitken et al reported the outbreak of M abscessus subspecies massiliense at a cystic fibrosis clinic and the possibility of patient-to-patient infection transmission [8]. Moreover, M abscessus infections are associated with high mortality among SOT recipients. Huang et al and Longworth et al suggested that early NTM infection after transplantation was related to higher mortality [9, 10]. In 2019, the guidelines for the management of NTM infection in SOT recipients were published by the Infectious Diseases Community of Practice of the American Society of Transplantation. The recommendation for M abscessus infection is to treat with at least 3 effective antibiotics in combination therapy including azithromycin, with caution concerning drug-drug interactions and potential drug toxicity [11].
Although clinicians should start at least 3 antibiotics for treating this serious infection, with the risk of drug-drug interaction and potential drug toxicity, we do not have enough data about the clinical course and outcome of M abscessus infection among SOT recipients. Most data about M abscessus infection have arisen mainly from case reports, case series, and observational studies because of the rarity of this infection. For optimizing the management of M abscessus infection and improving the mortality among SOT recipients, we need more data about this infection.
To expand on this topic, the purpose of this study was to describe clinical characteristics and outcomes of SOT patients infected with M abscessus at a large transplant center in Miami, Florida.
METHODS
This is a descriptive retrospective cohort study of SOT recipients who had a positive culture for M abscessus group strains at the Miami Transplant Institute and Jackson Memorial Hospital, which is a 1558-licensed bed tertiary care teaching hospital located in Miami, Florida, between 1 January 2013 and 30 September 2018. The clinical samples were sent to the Mycobacterial Laboratory at National Jewish Hospital in Denver, Colorado, for susceptibility and identification. Not all M abscessus isolates were identified to the subspecies level, as this identification was requested by the individual treating physician. All cultures without subspecies were reported as M abscessus group. A list of all SOT recipients who had a positive M abscessus culture during the study period in our hospital was obtained from the corresponding hospital microbiology records. Data collected included patients’ demographics, comorbidities (eg, cystic fibrosis, chronic bronchitis, interstitial lung disease, diabetes, and liver cirrhosis), date and type of transplantation, immunosuppressive regimen including induction therapy and maintenance therapy, history of acute rejection and treatment, site of infection, antimicrobial susceptibilities, antimicrobial therapy, and clinical outcomes via retrospective review of electronic medical records. For patients having multiple positive cultures corresponding to a single or multiple episodes of infections, only the first isolate of M abscessus was counted. The study was approved by the Institutional Review Board at University of Miami.
Definitions
Currently there is no consensus on the definition of treatment failure for M abscessus complex infections. In this study, we apply the definitions in the study conducted by Sfeir et al to our study [5]. We defined early treatment failure as death and/or refractory infection characterized either by persistent positive culture for M abscessus within 12 weeks of treatment initiation and/or lack of radiographic improvement [5]. Culture results and radiograph findings were evaluated within 12 weeks of treatment. Acute kidney injury was defined based on the RIFLE (Risk, Injury, Failure, Loss, End-Stage Renal Disease) criteria [12]. To define M abscessus infection, we followed the American Thoracic Society/Infectious Diseases Society of America (ATS/IDSA) criteria for NTM disease during the study period for respiratory isolates [13] (Supplementary Table 1). Also, we recognized M abscessus cultured from sterile site with clinical syndrome as true infection. Disseminated infection was defined as the presence of at least 2 sites of M abscessus infection [5]. If patients had positive culture result but did not meet ATS/IDSA criteria for NTM disease, we define the patients as colonization.
Only 1 isolate per patient was included for susceptibility testing, adopting the Clinical and Laboratory Standards Institute (CLSI) guidelines [14]. Identification of M abscessus isolates was performed at the Mycobacteriology Laboratory at National Jewish Health in Denver, Colorado. The broth microdilution method with cation-supplemented Mueller-Hinton broth was used as recommended by the CLSI [14].
Statistical Analysis
For survival analysis, we constructed Kaplan-Meier survival curves to compare the 1-year survival of the lung transplant recipients with M abscessus infection and non–lung transplant recipients with M abscessus infection. We compared 1-year survival of the recipients with M abscessus infection and with M abscessus colonization in all SOT and lung transplantation. Statistical analysis was done with R software [15, 16].
RESULTS
Patient Characteristics in All SOT and Lung Transplant Recipients
The final study cohort included 20 SOT recipients (1 kidney, 2 liver, 12 lung, 1 heart, 2 multivisceral, 1 intestine, 1 kidney-pancreas) with positive cultures for M abscessus. Tables 1–3 show demographic data, medical history, site of infection, time to diagnosis from transplant, time to rejection from transplantation to M abscessus diagnosis, rejection after initiation of treatment, the initial treatment regimen, and clinical outcome. In this cohort, there were 9 male and 11 female patients with a median age of 61 years (range, 11–73 years). Among our 20 patients, 13 (65.0%) were treated with a median treatment duration of 185 days (range, 20–523 days); there were 4 patients who had short duration of antibiotic treatment (20–91 days); 1 patient was subsequently deemed to have colonization rather than an infection and treatment was stopped, and the other 3 patients had unrelated deaths so that they were unable to complete treatment for M abscessus infection. Among the 13 patients treated with antibiotics, a total of 5 (5/13 [38.5%]) patients died. All 7 patients who did not receive treatment were alive and free from M abscessus infection. Four patients (20.0%) had treatment failure, 2 of whom (50.0%) died subsequently. Among our 20 patients, the infectious disease (ID) service was consulted in 17 cases (85.0%); the 3 cases without ID consult were lung transplant recipients, and all were alive without infection.
| Characteristic . | All Patients (n = 20) . | Lung Transplant Recipients (n = 12) . |
|---|---|---|
| Age, y, median (range) | 61 (11–73) | 64 (47–72) |
| Sex | ||
| Male | 9 (45.0) | 5 (41.7) |
| Female | 11 (55.0) | 7 (58.3) |
| Race/ethnicity | ||
| White | 12 (60.0) | 7 (58.3) |
| Hispanic | 3 (15.0) | 2 (16.7) |
| African American | 4 (20.0) | 3 (25.0) |
| Others | 1 (5.0) | 0 (0) |
| Time to diagnosis, d, XXX (range) | 100 (0–3497) | 100 (0–1382) |
| Site of M abscessus infection | ||
| Respiratory | 15 (75.0) | 12 (100) |
| Abdominal fluid | 1 (5.0) | 0 (0) |
| Deep tissue culture | 1 (5.0) | 0 (0) |
| Blood | 1 (5.0) | 0 (0) |
| Disseminated Blood + bone Blood + pleural effusion | 2 (10.0) 1 1 | 0 (0) 0 (0) 0 (0) |
| Organ transplant | ||
| Kidney | 1 (5.0) | 0 (0) |
| Liver | 2 (10.0) | 0 (0) |
| Lung | 12 (60.0) | 12 (100) |
| Heart | 1 (5.0) | 0 (0) |
| Intestine/multivisceral | 3 (15.0) | 0 (0) |
| Kidney and pancreas | 1 (5.0) | 0 (0) |
| Immunosuppression induction | ||
| Methylprednisone | 10 (50.0) | 8 (66.7) |
| ATG + methylprednisone | 2 (10.0) | 0 (0) |
| Methylprednisone + rituximab + tacrolimus | 1 (5.0) | 0 (0) |
| Methylprednisone + basiliximab | 2 (10.0) | 2 (16.7) |
| ATG + daclizumab | 1 (5.0) | 0 (0) |
| Not obtained | 4 (20.0) | 2 (16.7) |
| Immunosuppression maintenance | ||
| MMF/MPA + prednisone + tacrolimus | 11 (55.0) | 9 (75.0) |
| MMF/MPA + methylprednisone + tacrolimus | 1 (5.0) | 0 (0) |
| MMF/MPA + tacrolimus | 1 (10.0) | 0 (0) |
| Tacrolimus + mercaptopurine | 1 (5.0) | 0 (0) |
| Prednisone + tacrolimus | 2 (10.0) | 2 (16.7) |
| Tacrolimus | 2 (10.0) | 0 (0) |
| Prednisone | 1 (5.0) | 1 (8.3) |
| No data | 1 (5.0) | 0 |
| Rejection | ||
| Rejection before the treatmenta | 3 (15.0) | 2 (16.7) |
| Time from transplantation to rejection, d, median (range) | 298 (82–343) | 213 (82–343) |
| Rejection after M abscessus diagnosisa | 7 (35.0) | 3 (25.0) |
| Time from diagnosis to rejection, d, median (range) | 59 (0–476) | 59 (0–131) |
| Outcome | ||
| Patients treated with antibiotics | 13 (65.0) | 7 (58.3) |
| Duration of therapy, d, XXX (range) | 185 (20–523) | 186 (20–296) |
| Treatment failure | 4/13 (30.8) | 3/7 (42.9) |
| Characteristic . | All Patients (n = 20) . | Lung Transplant Recipients (n = 12) . |
|---|---|---|
| Age, y, median (range) | 61 (11–73) | 64 (47–72) |
| Sex | ||
| Male | 9 (45.0) | 5 (41.7) |
| Female | 11 (55.0) | 7 (58.3) |
| Race/ethnicity | ||
| White | 12 (60.0) | 7 (58.3) |
| Hispanic | 3 (15.0) | 2 (16.7) |
| African American | 4 (20.0) | 3 (25.0) |
| Others | 1 (5.0) | 0 (0) |
| Time to diagnosis, d, XXX (range) | 100 (0–3497) | 100 (0–1382) |
| Site of M abscessus infection | ||
| Respiratory | 15 (75.0) | 12 (100) |
| Abdominal fluid | 1 (5.0) | 0 (0) |
| Deep tissue culture | 1 (5.0) | 0 (0) |
| Blood | 1 (5.0) | 0 (0) |
| Disseminated Blood + bone Blood + pleural effusion | 2 (10.0) 1 1 | 0 (0) 0 (0) 0 (0) |
| Organ transplant | ||
| Kidney | 1 (5.0) | 0 (0) |
| Liver | 2 (10.0) | 0 (0) |
| Lung | 12 (60.0) | 12 (100) |
| Heart | 1 (5.0) | 0 (0) |
| Intestine/multivisceral | 3 (15.0) | 0 (0) |
| Kidney and pancreas | 1 (5.0) | 0 (0) |
| Immunosuppression induction | ||
| Methylprednisone | 10 (50.0) | 8 (66.7) |
| ATG + methylprednisone | 2 (10.0) | 0 (0) |
| Methylprednisone + rituximab + tacrolimus | 1 (5.0) | 0 (0) |
| Methylprednisone + basiliximab | 2 (10.0) | 2 (16.7) |
| ATG + daclizumab | 1 (5.0) | 0 (0) |
| Not obtained | 4 (20.0) | 2 (16.7) |
| Immunosuppression maintenance | ||
| MMF/MPA + prednisone + tacrolimus | 11 (55.0) | 9 (75.0) |
| MMF/MPA + methylprednisone + tacrolimus | 1 (5.0) | 0 (0) |
| MMF/MPA + tacrolimus | 1 (10.0) | 0 (0) |
| Tacrolimus + mercaptopurine | 1 (5.0) | 0 (0) |
| Prednisone + tacrolimus | 2 (10.0) | 2 (16.7) |
| Tacrolimus | 2 (10.0) | 0 (0) |
| Prednisone | 1 (5.0) | 1 (8.3) |
| No data | 1 (5.0) | 0 |
| Rejection | ||
| Rejection before the treatmenta | 3 (15.0) | 2 (16.7) |
| Time from transplantation to rejection, d, median (range) | 298 (82–343) | 213 (82–343) |
| Rejection after M abscessus diagnosisa | 7 (35.0) | 3 (25.0) |
| Time from diagnosis to rejection, d, median (range) | 59 (0–476) | 59 (0–131) |
| Outcome | ||
| Patients treated with antibiotics | 13 (65.0) | 7 (58.3) |
| Duration of therapy, d, XXX (range) | 185 (20–523) | 186 (20–296) |
| Treatment failure | 4/13 (30.8) | 3/7 (42.9) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ATG, antithymocyte globulin; M abscessus, Mycobacterium abscessus; MMF, mycophenolic mofetil; MPA, mycophenolic acid.
Patients who had rejection on the day when Mycobacterium abscessus infection was diagnosed were categorized in “rejection during M abscessus treatment.”
| Characteristic . | All Patients (n = 20) . | Lung Transplant Recipients (n = 12) . |
|---|---|---|
| Age, y, median (range) | 61 (11–73) | 64 (47–72) |
| Sex | ||
| Male | 9 (45.0) | 5 (41.7) |
| Female | 11 (55.0) | 7 (58.3) |
| Race/ethnicity | ||
| White | 12 (60.0) | 7 (58.3) |
| Hispanic | 3 (15.0) | 2 (16.7) |
| African American | 4 (20.0) | 3 (25.0) |
| Others | 1 (5.0) | 0 (0) |
| Time to diagnosis, d, XXX (range) | 100 (0–3497) | 100 (0–1382) |
| Site of M abscessus infection | ||
| Respiratory | 15 (75.0) | 12 (100) |
| Abdominal fluid | 1 (5.0) | 0 (0) |
| Deep tissue culture | 1 (5.0) | 0 (0) |
| Blood | 1 (5.0) | 0 (0) |
| Disseminated Blood + bone Blood + pleural effusion | 2 (10.0) 1 1 | 0 (0) 0 (0) 0 (0) |
| Organ transplant | ||
| Kidney | 1 (5.0) | 0 (0) |
| Liver | 2 (10.0) | 0 (0) |
| Lung | 12 (60.0) | 12 (100) |
| Heart | 1 (5.0) | 0 (0) |
| Intestine/multivisceral | 3 (15.0) | 0 (0) |
| Kidney and pancreas | 1 (5.0) | 0 (0) |
| Immunosuppression induction | ||
| Methylprednisone | 10 (50.0) | 8 (66.7) |
| ATG + methylprednisone | 2 (10.0) | 0 (0) |
| Methylprednisone + rituximab + tacrolimus | 1 (5.0) | 0 (0) |
| Methylprednisone + basiliximab | 2 (10.0) | 2 (16.7) |
| ATG + daclizumab | 1 (5.0) | 0 (0) |
| Not obtained | 4 (20.0) | 2 (16.7) |
| Immunosuppression maintenance | ||
| MMF/MPA + prednisone + tacrolimus | 11 (55.0) | 9 (75.0) |
| MMF/MPA + methylprednisone + tacrolimus | 1 (5.0) | 0 (0) |
| MMF/MPA + tacrolimus | 1 (10.0) | 0 (0) |
| Tacrolimus + mercaptopurine | 1 (5.0) | 0 (0) |
| Prednisone + tacrolimus | 2 (10.0) | 2 (16.7) |
| Tacrolimus | 2 (10.0) | 0 (0) |
| Prednisone | 1 (5.0) | 1 (8.3) |
| No data | 1 (5.0) | 0 |
| Rejection | ||
| Rejection before the treatmenta | 3 (15.0) | 2 (16.7) |
| Time from transplantation to rejection, d, median (range) | 298 (82–343) | 213 (82–343) |
| Rejection after M abscessus diagnosisa | 7 (35.0) | 3 (25.0) |
| Time from diagnosis to rejection, d, median (range) | 59 (0–476) | 59 (0–131) |
| Outcome | ||
| Patients treated with antibiotics | 13 (65.0) | 7 (58.3) |
| Duration of therapy, d, XXX (range) | 185 (20–523) | 186 (20–296) |
| Treatment failure | 4/13 (30.8) | 3/7 (42.9) |
| Characteristic . | All Patients (n = 20) . | Lung Transplant Recipients (n = 12) . |
|---|---|---|
| Age, y, median (range) | 61 (11–73) | 64 (47–72) |
| Sex | ||
| Male | 9 (45.0) | 5 (41.7) |
| Female | 11 (55.0) | 7 (58.3) |
| Race/ethnicity | ||
| White | 12 (60.0) | 7 (58.3) |
| Hispanic | 3 (15.0) | 2 (16.7) |
| African American | 4 (20.0) | 3 (25.0) |
| Others | 1 (5.0) | 0 (0) |
| Time to diagnosis, d, XXX (range) | 100 (0–3497) | 100 (0–1382) |
| Site of M abscessus infection | ||
| Respiratory | 15 (75.0) | 12 (100) |
| Abdominal fluid | 1 (5.0) | 0 (0) |
| Deep tissue culture | 1 (5.0) | 0 (0) |
| Blood | 1 (5.0) | 0 (0) |
| Disseminated Blood + bone Blood + pleural effusion | 2 (10.0) 1 1 | 0 (0) 0 (0) 0 (0) |
| Organ transplant | ||
| Kidney | 1 (5.0) | 0 (0) |
| Liver | 2 (10.0) | 0 (0) |
| Lung | 12 (60.0) | 12 (100) |
| Heart | 1 (5.0) | 0 (0) |
| Intestine/multivisceral | 3 (15.0) | 0 (0) |
| Kidney and pancreas | 1 (5.0) | 0 (0) |
| Immunosuppression induction | ||
| Methylprednisone | 10 (50.0) | 8 (66.7) |
| ATG + methylprednisone | 2 (10.0) | 0 (0) |
| Methylprednisone + rituximab + tacrolimus | 1 (5.0) | 0 (0) |
| Methylprednisone + basiliximab | 2 (10.0) | 2 (16.7) |
| ATG + daclizumab | 1 (5.0) | 0 (0) |
| Not obtained | 4 (20.0) | 2 (16.7) |
| Immunosuppression maintenance | ||
| MMF/MPA + prednisone + tacrolimus | 11 (55.0) | 9 (75.0) |
| MMF/MPA + methylprednisone + tacrolimus | 1 (5.0) | 0 (0) |
| MMF/MPA + tacrolimus | 1 (10.0) | 0 (0) |
| Tacrolimus + mercaptopurine | 1 (5.0) | 0 (0) |
| Prednisone + tacrolimus | 2 (10.0) | 2 (16.7) |
| Tacrolimus | 2 (10.0) | 0 (0) |
| Prednisone | 1 (5.0) | 1 (8.3) |
| No data | 1 (5.0) | 0 |
| Rejection | ||
| Rejection before the treatmenta | 3 (15.0) | 2 (16.7) |
| Time from transplantation to rejection, d, median (range) | 298 (82–343) | 213 (82–343) |
| Rejection after M abscessus diagnosisa | 7 (35.0) | 3 (25.0) |
| Time from diagnosis to rejection, d, median (range) | 59 (0–476) | 59 (0–131) |
| Outcome | ||
| Patients treated with antibiotics | 13 (65.0) | 7 (58.3) |
| Duration of therapy, d, XXX (range) | 185 (20–523) | 186 (20–296) |
| Treatment failure | 4/13 (30.8) | 3/7 (42.9) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ATG, antithymocyte globulin; M abscessus, Mycobacterium abscessus; MMF, mycophenolic mofetil; MPA, mycophenolic acid.
Patients who had rejection on the day when Mycobacterium abscessus infection was diagnosed were categorized in “rejection during M abscessus treatment.”
| No. . | Age/Sex . | Diseases . | Transplant . | Site of Infection (Culture) . | No. of Culture Positivity . | Finding in Chest Imaging . | ATS/IDSA Diagnostic Criteria . | Time to Diagnosis, d . | Rejection Before Treatment . | ID Consult . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 48/M | End-stage intestinal failure, liver failure, chronic pancreatitis, enterocutaneous fistula | Multivisceral | Bacteremia (blood culture) | 1 | NA | NA | 17 | No | Yes |
| 2 | 63/F | Bronchiectasis, GERD | Lung (double) | BAL culture | 1 | Abnormal findings in CXR | No | 470 | Yes | No |
| 3 | 63/F | Cirrhosis due to chronic hepatitis C, HCC, DM, and GERD | Liver | Wound infection (deep wound culture) | 1 | NA | NA | 31 | No | Yes |
| 4 | 73/M | ESRD due to hypertension | Kidney | Pneumonia (sputum culture) | 1 | Multiple nodular lesions in chest CT | No | 2158 | No | Yes |
| 5 | 48/M | Stage 4 sarcoidosis, bronchiectasis | Lung (double) | NA (BAL culture) | 5 | Abnormal findings in CXR | Yes | 24 | No | Yes |
| 6 | 11/F | Cystic fibrosis | Liver | NA (sputum culture) | 2 | Pulmonary nodule in chest CT | Yes | 3497 | No | Yes |
| 7 | 69/M | Pulmonary fibrosis, GERD | Lung (double) | Pneumonia (BAL culture) | 3 | Worsening opacifications in CXR | Yes | 0 | No | Yes |
| 8 | 53/F | Short bowel syndrome, DM | Intestine | Intra-abdominal infection (abdominal fluid culture) | 1 | NA | NA | 118 | No | Yes |
| 9 | 70/M | Pulmonary fibrosis, DM | Lung (double) | NA (BAL culture) | 1 | No acute cardiopulmonary issues in CXR | No | 281 | No | No |
| 10 | 60/F | COPD | Lung (double) | NA (BAL culture) | 1 | Worsening consolidation in right upper lung | Yes | 44 | No | Yes |
| 11 | 68/F | COPD, DM | Lung (double) | Pneumonia (BAL culture) | 3 | No acute cardiopulmonary issues in CXR | No | 242 | No | Yes |
| 12 | 65/F | Primary sarcoidosis and COPD, GERD | Lung (single) | Disseminated infection (BAL culture/pleural effusion culture) | 1 | Consolidation, bronchopleural fistula, and hemopneumothorax, anastomosis breakdown in chest CT | Yes | 11 | No | Yes |
| 13 | 34/M | Dilated cardiomyopathy, GERD | Heart | Bacteremia (blood culture) | 12 | NA | NA | 314 | Yes | Yes |
| 14 | 60/M | Pulmonary fibrosis | Lung (single) | Pneumonia (BAL culture) | 2 | Postsurgical change and pulmonary nodules in chest CT | Yes | 82 | Yes | Yes |
| 15 | 61/M | Pulmonary fibrosis, GERD | Lung (double) | Pneumonia (BAL culture) | 8 | Mild perihilar density in CXR | Yes | 66 | No | Yes |
| 16 | 47/F | Lymphangioleiomyomatosis | Lung (double) | Pneumonia (BAL culture) | 1 | Tree-in-bud and ground glass opacity in chest CT | Yes | 195 | No | Yes |
| 17 | 65/F | COPD | Lung (double) | Pneumonia (BAL culture) | 9 | Consolidations in CXR | Yes | 56 | No | Yes |
| 18 | 55/F | Short bowel syndrome, pseudo-obstruction and DM | Multivisceral | Disseminated infection (blood culture/bone culture) | 1 | NA | NA | 56 | No | Yes |
| 19 | 72/F | Pulmonary fibrosis, bronchiectasis, DM, and GERD | Lung (single) | NA (BAL culture) | 1 | NA | NA | 1382 | No | No |
| 20 | 56/M | DM (type 1), ESRD | Kidney and pancreas | NA (throat swab culture) | 1 | NA | NA | 1690 | No | Yes |
| No. . | Age/Sex . | Diseases . | Transplant . | Site of Infection (Culture) . | No. of Culture Positivity . | Finding in Chest Imaging . | ATS/IDSA Diagnostic Criteria . | Time to Diagnosis, d . | Rejection Before Treatment . | ID Consult . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 48/M | End-stage intestinal failure, liver failure, chronic pancreatitis, enterocutaneous fistula | Multivisceral | Bacteremia (blood culture) | 1 | NA | NA | 17 | No | Yes |
| 2 | 63/F | Bronchiectasis, GERD | Lung (double) | BAL culture | 1 | Abnormal findings in CXR | No | 470 | Yes | No |
| 3 | 63/F | Cirrhosis due to chronic hepatitis C, HCC, DM, and GERD | Liver | Wound infection (deep wound culture) | 1 | NA | NA | 31 | No | Yes |
| 4 | 73/M | ESRD due to hypertension | Kidney | Pneumonia (sputum culture) | 1 | Multiple nodular lesions in chest CT | No | 2158 | No | Yes |
| 5 | 48/M | Stage 4 sarcoidosis, bronchiectasis | Lung (double) | NA (BAL culture) | 5 | Abnormal findings in CXR | Yes | 24 | No | Yes |
| 6 | 11/F | Cystic fibrosis | Liver | NA (sputum culture) | 2 | Pulmonary nodule in chest CT | Yes | 3497 | No | Yes |
| 7 | 69/M | Pulmonary fibrosis, GERD | Lung (double) | Pneumonia (BAL culture) | 3 | Worsening opacifications in CXR | Yes | 0 | No | Yes |
| 8 | 53/F | Short bowel syndrome, DM | Intestine | Intra-abdominal infection (abdominal fluid culture) | 1 | NA | NA | 118 | No | Yes |
| 9 | 70/M | Pulmonary fibrosis, DM | Lung (double) | NA (BAL culture) | 1 | No acute cardiopulmonary issues in CXR | No | 281 | No | No |
| 10 | 60/F | COPD | Lung (double) | NA (BAL culture) | 1 | Worsening consolidation in right upper lung | Yes | 44 | No | Yes |
| 11 | 68/F | COPD, DM | Lung (double) | Pneumonia (BAL culture) | 3 | No acute cardiopulmonary issues in CXR | No | 242 | No | Yes |
| 12 | 65/F | Primary sarcoidosis and COPD, GERD | Lung (single) | Disseminated infection (BAL culture/pleural effusion culture) | 1 | Consolidation, bronchopleural fistula, and hemopneumothorax, anastomosis breakdown in chest CT | Yes | 11 | No | Yes |
| 13 | 34/M | Dilated cardiomyopathy, GERD | Heart | Bacteremia (blood culture) | 12 | NA | NA | 314 | Yes | Yes |
| 14 | 60/M | Pulmonary fibrosis | Lung (single) | Pneumonia (BAL culture) | 2 | Postsurgical change and pulmonary nodules in chest CT | Yes | 82 | Yes | Yes |
| 15 | 61/M | Pulmonary fibrosis, GERD | Lung (double) | Pneumonia (BAL culture) | 8 | Mild perihilar density in CXR | Yes | 66 | No | Yes |
| 16 | 47/F | Lymphangioleiomyomatosis | Lung (double) | Pneumonia (BAL culture) | 1 | Tree-in-bud and ground glass opacity in chest CT | Yes | 195 | No | Yes |
| 17 | 65/F | COPD | Lung (double) | Pneumonia (BAL culture) | 9 | Consolidations in CXR | Yes | 56 | No | Yes |
| 18 | 55/F | Short bowel syndrome, pseudo-obstruction and DM | Multivisceral | Disseminated infection (blood culture/bone culture) | 1 | NA | NA | 56 | No | Yes |
| 19 | 72/F | Pulmonary fibrosis, bronchiectasis, DM, and GERD | Lung (single) | NA (BAL culture) | 1 | NA | NA | 1382 | No | No |
| 20 | 56/M | DM (type 1), ESRD | Kidney and pancreas | NA (throat swab culture) | 1 | NA | NA | 1690 | No | Yes |
Abbreviations: ATS/IDSA, American Thoracic Society/Infectious Diseases Society of America; BAL, bronchoalveolar lavage; COPD, chronic obstructive pulmonary disease; CT, Computerized Tomography; CXR, chest radiograph; DM, diabetes mellitus; ESRD, end-stage renal disease; GERD, gastroesophageal reflex disease; HCC, hepatocellular carcinoma; ID, infectious diseases; NA, not applicable.
| No. . | Age/Sex . | Diseases . | Transplant . | Site of Infection (Culture) . | No. of Culture Positivity . | Finding in Chest Imaging . | ATS/IDSA Diagnostic Criteria . | Time to Diagnosis, d . | Rejection Before Treatment . | ID Consult . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 48/M | End-stage intestinal failure, liver failure, chronic pancreatitis, enterocutaneous fistula | Multivisceral | Bacteremia (blood culture) | 1 | NA | NA | 17 | No | Yes |
| 2 | 63/F | Bronchiectasis, GERD | Lung (double) | BAL culture | 1 | Abnormal findings in CXR | No | 470 | Yes | No |
| 3 | 63/F | Cirrhosis due to chronic hepatitis C, HCC, DM, and GERD | Liver | Wound infection (deep wound culture) | 1 | NA | NA | 31 | No | Yes |
| 4 | 73/M | ESRD due to hypertension | Kidney | Pneumonia (sputum culture) | 1 | Multiple nodular lesions in chest CT | No | 2158 | No | Yes |
| 5 | 48/M | Stage 4 sarcoidosis, bronchiectasis | Lung (double) | NA (BAL culture) | 5 | Abnormal findings in CXR | Yes | 24 | No | Yes |
| 6 | 11/F | Cystic fibrosis | Liver | NA (sputum culture) | 2 | Pulmonary nodule in chest CT | Yes | 3497 | No | Yes |
| 7 | 69/M | Pulmonary fibrosis, GERD | Lung (double) | Pneumonia (BAL culture) | 3 | Worsening opacifications in CXR | Yes | 0 | No | Yes |
| 8 | 53/F | Short bowel syndrome, DM | Intestine | Intra-abdominal infection (abdominal fluid culture) | 1 | NA | NA | 118 | No | Yes |
| 9 | 70/M | Pulmonary fibrosis, DM | Lung (double) | NA (BAL culture) | 1 | No acute cardiopulmonary issues in CXR | No | 281 | No | No |
| 10 | 60/F | COPD | Lung (double) | NA (BAL culture) | 1 | Worsening consolidation in right upper lung | Yes | 44 | No | Yes |
| 11 | 68/F | COPD, DM | Lung (double) | Pneumonia (BAL culture) | 3 | No acute cardiopulmonary issues in CXR | No | 242 | No | Yes |
| 12 | 65/F | Primary sarcoidosis and COPD, GERD | Lung (single) | Disseminated infection (BAL culture/pleural effusion culture) | 1 | Consolidation, bronchopleural fistula, and hemopneumothorax, anastomosis breakdown in chest CT | Yes | 11 | No | Yes |
| 13 | 34/M | Dilated cardiomyopathy, GERD | Heart | Bacteremia (blood culture) | 12 | NA | NA | 314 | Yes | Yes |
| 14 | 60/M | Pulmonary fibrosis | Lung (single) | Pneumonia (BAL culture) | 2 | Postsurgical change and pulmonary nodules in chest CT | Yes | 82 | Yes | Yes |
| 15 | 61/M | Pulmonary fibrosis, GERD | Lung (double) | Pneumonia (BAL culture) | 8 | Mild perihilar density in CXR | Yes | 66 | No | Yes |
| 16 | 47/F | Lymphangioleiomyomatosis | Lung (double) | Pneumonia (BAL culture) | 1 | Tree-in-bud and ground glass opacity in chest CT | Yes | 195 | No | Yes |
| 17 | 65/F | COPD | Lung (double) | Pneumonia (BAL culture) | 9 | Consolidations in CXR | Yes | 56 | No | Yes |
| 18 | 55/F | Short bowel syndrome, pseudo-obstruction and DM | Multivisceral | Disseminated infection (blood culture/bone culture) | 1 | NA | NA | 56 | No | Yes |
| 19 | 72/F | Pulmonary fibrosis, bronchiectasis, DM, and GERD | Lung (single) | NA (BAL culture) | 1 | NA | NA | 1382 | No | No |
| 20 | 56/M | DM (type 1), ESRD | Kidney and pancreas | NA (throat swab culture) | 1 | NA | NA | 1690 | No | Yes |
| No. . | Age/Sex . | Diseases . | Transplant . | Site of Infection (Culture) . | No. of Culture Positivity . | Finding in Chest Imaging . | ATS/IDSA Diagnostic Criteria . | Time to Diagnosis, d . | Rejection Before Treatment . | ID Consult . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 48/M | End-stage intestinal failure, liver failure, chronic pancreatitis, enterocutaneous fistula | Multivisceral | Bacteremia (blood culture) | 1 | NA | NA | 17 | No | Yes |
| 2 | 63/F | Bronchiectasis, GERD | Lung (double) | BAL culture | 1 | Abnormal findings in CXR | No | 470 | Yes | No |
| 3 | 63/F | Cirrhosis due to chronic hepatitis C, HCC, DM, and GERD | Liver | Wound infection (deep wound culture) | 1 | NA | NA | 31 | No | Yes |
| 4 | 73/M | ESRD due to hypertension | Kidney | Pneumonia (sputum culture) | 1 | Multiple nodular lesions in chest CT | No | 2158 | No | Yes |
| 5 | 48/M | Stage 4 sarcoidosis, bronchiectasis | Lung (double) | NA (BAL culture) | 5 | Abnormal findings in CXR | Yes | 24 | No | Yes |
| 6 | 11/F | Cystic fibrosis | Liver | NA (sputum culture) | 2 | Pulmonary nodule in chest CT | Yes | 3497 | No | Yes |
| 7 | 69/M | Pulmonary fibrosis, GERD | Lung (double) | Pneumonia (BAL culture) | 3 | Worsening opacifications in CXR | Yes | 0 | No | Yes |
| 8 | 53/F | Short bowel syndrome, DM | Intestine | Intra-abdominal infection (abdominal fluid culture) | 1 | NA | NA | 118 | No | Yes |
| 9 | 70/M | Pulmonary fibrosis, DM | Lung (double) | NA (BAL culture) | 1 | No acute cardiopulmonary issues in CXR | No | 281 | No | No |
| 10 | 60/F | COPD | Lung (double) | NA (BAL culture) | 1 | Worsening consolidation in right upper lung | Yes | 44 | No | Yes |
| 11 | 68/F | COPD, DM | Lung (double) | Pneumonia (BAL culture) | 3 | No acute cardiopulmonary issues in CXR | No | 242 | No | Yes |
| 12 | 65/F | Primary sarcoidosis and COPD, GERD | Lung (single) | Disseminated infection (BAL culture/pleural effusion culture) | 1 | Consolidation, bronchopleural fistula, and hemopneumothorax, anastomosis breakdown in chest CT | Yes | 11 | No | Yes |
| 13 | 34/M | Dilated cardiomyopathy, GERD | Heart | Bacteremia (blood culture) | 12 | NA | NA | 314 | Yes | Yes |
| 14 | 60/M | Pulmonary fibrosis | Lung (single) | Pneumonia (BAL culture) | 2 | Postsurgical change and pulmonary nodules in chest CT | Yes | 82 | Yes | Yes |
| 15 | 61/M | Pulmonary fibrosis, GERD | Lung (double) | Pneumonia (BAL culture) | 8 | Mild perihilar density in CXR | Yes | 66 | No | Yes |
| 16 | 47/F | Lymphangioleiomyomatosis | Lung (double) | Pneumonia (BAL culture) | 1 | Tree-in-bud and ground glass opacity in chest CT | Yes | 195 | No | Yes |
| 17 | 65/F | COPD | Lung (double) | Pneumonia (BAL culture) | 9 | Consolidations in CXR | Yes | 56 | No | Yes |
| 18 | 55/F | Short bowel syndrome, pseudo-obstruction and DM | Multivisceral | Disseminated infection (blood culture/bone culture) | 1 | NA | NA | 56 | No | Yes |
| 19 | 72/F | Pulmonary fibrosis, bronchiectasis, DM, and GERD | Lung (single) | NA (BAL culture) | 1 | NA | NA | 1382 | No | No |
| 20 | 56/M | DM (type 1), ESRD | Kidney and pancreas | NA (throat swab culture) | 1 | NA | NA | 1690 | No | Yes |
Abbreviations: ATS/IDSA, American Thoracic Society/Infectious Diseases Society of America; BAL, bronchoalveolar lavage; COPD, chronic obstructive pulmonary disease; CT, Computerized Tomography; CXR, chest radiograph; DM, diabetes mellitus; ESRD, end-stage renal disease; GERD, gastroesophageal reflex disease; HCC, hepatocellular carcinoma; ID, infectious diseases; NA, not applicable.
| No. . | Initial Regimen . | Comment . | Duration of Therapy, d . | Treatment Failure . | Rejection After Treatment . | Outcome . |
|---|---|---|---|---|---|---|
| 1 | Clarithromycin, levofloxacin, and tigecycline | After initial treatment, tigecycline caused transaminitis and levofloxacin was stopped based on susceptibility result. But treatment was terminated in about 25 d because it was thought that M abscessus was contaminated. | 25 | No | Yes | Alive |
| 2 | Not treated | Not treated | Not treated | No | Yes | Alive |
| 3 | Azithromycin, cefoxitin, and tigecycline | Initial regimen was continued for 3 mo, and then it was stepped down to azithromycin and tigecycline. | 297 | No | Yes | Dead |
| 4 | Azithromycin, imipenem-cilastatin, and tigecycline | After the initial treatment was started, imipenem-cilastatin was switched to cefoxitin 2 d later. Then the patient was discharged to other facility and there was no further follow-up in our system. | NA | NA | NA | NA |
| 5 | Not treated | Not treated | Not treated | No | No | Alive |
| 6 | Not treated | Not treated | Not treated | No | No | Alive |
| 7 | Azithromycin, cefoxitin, and tigecycline | Initial treatment was continued without any significant side effect. | 20 | No | No | Dead |
| 8 | Azithromycin, cefoxitin, and tigecycline | Initial regimen was switched to azithromycin, imipenem-cilastatin, and cefoxitin because of transaminitis. The patient had been on the 3 medications for 8 mo and then azithromycin and imipenem-cilastatin were continued additional 10 mo for relapse. | 523 | No | Yes | Alive |
| 9 | Not treated | The patient was not treated as infection. Azithromycin was given as prophylaxis. | 202 | No | Yes | Alive |
| 10 | Not treated | Not treated | Not treated | No | Yes | Alive |
| 11 | Azithromycin, cefoxitin, and tigecycline | The initial regimen was switched to azithromycin, imipenem-cilastatin, and linezolid in 4 d. But the further information was missing. | NA | No | No | Alive |
| 12 | Azithromycin, imipenem-cilastatin, and cefoxitin | The initial regimen was switched to azithromycin, tigecycline, and amikacin. The patient died of the event not related to M abscessus infection. | 87 | Yes | No | Dead |
| 13 | Azithromycin, cefoxitin, and amikacin | After the initial treatment, amikacin was stopped in 1 mo, then the regimen was switched to azithromycin, cefoxitin, and tigecycline. The treatment was stopped in 4 mo. After the treatment, the infection relapsed. | 136 | No | Yes | Dead |
| 14 | Azithromycin, cefoxitin, and tigecycline | The initial treatment was switched to azithromycin, imipenem-cilastatin, and inhaled amikacin because tigecycline caused transaminitis and imipenem-cilastatin was started for treating Achromobacter infection. | 184 | Yes | No | Alive |
| 15 | Azithromycin, cefoxitin, and amikacin | The initial treatment was switched to azithromycin, cefoxitin, and tigecycline because amikacin caused hearing loss. After that, tigecycline caused transaminitis and it was switched to inhale amikacin. Inhale amikacin was complicated with bronchospasm. | 186 | No | No | Alive |
| 16 | Azithromycin, cefoxitin, and tigecycline | The initial regimen was continued, but tigecycline caused nausea and vomiting. Then the dose of tigecycline was decreased, but it caused transaminitis. The regimen was switched to azithromycin, cefoxitin, and inhaled amikacin. | 220 | No | Yes | Alive |
| 17 | Azithromycin, imipenem-cilastatin, and tigecycline | The initial regimen was complicated with transaminitis likely due to azithromycin and/or tigecycline, and it was switched to inhaled amikacin, imipenem-cilastatin and linezolid. After that, inhaled amikacin was stopped because of worsening respiratory status, and linezolid caused thrombocytopenia. Imipenem-cilastatin and azithromycin were continued. | 296 | Yes | No | Alive |
| 18 | Clarithromycin, tigecycline, and amikacin | After the initial treatment, clarithromycin was switched to azithromycin and tigecycline was switched to cefoxitin due to pancreatitis. And amikacin was stopped because of acute kidney injury. Meantime cefoxitin was initiated but finally clofazimine and imipenem-cilastatin were continued. | 91 | Yes | No | Dead |
| 19 | Not treated | Not treated | Not treated | NA | No | Alive |
| 20 | Not treated | Not treated | Not treated | NA | No | Alive |
| No. . | Initial Regimen . | Comment . | Duration of Therapy, d . | Treatment Failure . | Rejection After Treatment . | Outcome . |
|---|---|---|---|---|---|---|
| 1 | Clarithromycin, levofloxacin, and tigecycline | After initial treatment, tigecycline caused transaminitis and levofloxacin was stopped based on susceptibility result. But treatment was terminated in about 25 d because it was thought that M abscessus was contaminated. | 25 | No | Yes | Alive |
| 2 | Not treated | Not treated | Not treated | No | Yes | Alive |
| 3 | Azithromycin, cefoxitin, and tigecycline | Initial regimen was continued for 3 mo, and then it was stepped down to azithromycin and tigecycline. | 297 | No | Yes | Dead |
| 4 | Azithromycin, imipenem-cilastatin, and tigecycline | After the initial treatment was started, imipenem-cilastatin was switched to cefoxitin 2 d later. Then the patient was discharged to other facility and there was no further follow-up in our system. | NA | NA | NA | NA |
| 5 | Not treated | Not treated | Not treated | No | No | Alive |
| 6 | Not treated | Not treated | Not treated | No | No | Alive |
| 7 | Azithromycin, cefoxitin, and tigecycline | Initial treatment was continued without any significant side effect. | 20 | No | No | Dead |
| 8 | Azithromycin, cefoxitin, and tigecycline | Initial regimen was switched to azithromycin, imipenem-cilastatin, and cefoxitin because of transaminitis. The patient had been on the 3 medications for 8 mo and then azithromycin and imipenem-cilastatin were continued additional 10 mo for relapse. | 523 | No | Yes | Alive |
| 9 | Not treated | The patient was not treated as infection. Azithromycin was given as prophylaxis. | 202 | No | Yes | Alive |
| 10 | Not treated | Not treated | Not treated | No | Yes | Alive |
| 11 | Azithromycin, cefoxitin, and tigecycline | The initial regimen was switched to azithromycin, imipenem-cilastatin, and linezolid in 4 d. But the further information was missing. | NA | No | No | Alive |
| 12 | Azithromycin, imipenem-cilastatin, and cefoxitin | The initial regimen was switched to azithromycin, tigecycline, and amikacin. The patient died of the event not related to M abscessus infection. | 87 | Yes | No | Dead |
| 13 | Azithromycin, cefoxitin, and amikacin | After the initial treatment, amikacin was stopped in 1 mo, then the regimen was switched to azithromycin, cefoxitin, and tigecycline. The treatment was stopped in 4 mo. After the treatment, the infection relapsed. | 136 | No | Yes | Dead |
| 14 | Azithromycin, cefoxitin, and tigecycline | The initial treatment was switched to azithromycin, imipenem-cilastatin, and inhaled amikacin because tigecycline caused transaminitis and imipenem-cilastatin was started for treating Achromobacter infection. | 184 | Yes | No | Alive |
| 15 | Azithromycin, cefoxitin, and amikacin | The initial treatment was switched to azithromycin, cefoxitin, and tigecycline because amikacin caused hearing loss. After that, tigecycline caused transaminitis and it was switched to inhale amikacin. Inhale amikacin was complicated with bronchospasm. | 186 | No | No | Alive |
| 16 | Azithromycin, cefoxitin, and tigecycline | The initial regimen was continued, but tigecycline caused nausea and vomiting. Then the dose of tigecycline was decreased, but it caused transaminitis. The regimen was switched to azithromycin, cefoxitin, and inhaled amikacin. | 220 | No | Yes | Alive |
| 17 | Azithromycin, imipenem-cilastatin, and tigecycline | The initial regimen was complicated with transaminitis likely due to azithromycin and/or tigecycline, and it was switched to inhaled amikacin, imipenem-cilastatin and linezolid. After that, inhaled amikacin was stopped because of worsening respiratory status, and linezolid caused thrombocytopenia. Imipenem-cilastatin and azithromycin were continued. | 296 | Yes | No | Alive |
| 18 | Clarithromycin, tigecycline, and amikacin | After the initial treatment, clarithromycin was switched to azithromycin and tigecycline was switched to cefoxitin due to pancreatitis. And amikacin was stopped because of acute kidney injury. Meantime cefoxitin was initiated but finally clofazimine and imipenem-cilastatin were continued. | 91 | Yes | No | Dead |
| 19 | Not treated | Not treated | Not treated | NA | No | Alive |
| 20 | Not treated | Not treated | Not treated | NA | No | Alive |
Abbreviations: M abscessus, Mycobacterium abscessus; NA, not applicable.
| No. . | Initial Regimen . | Comment . | Duration of Therapy, d . | Treatment Failure . | Rejection After Treatment . | Outcome . |
|---|---|---|---|---|---|---|
| 1 | Clarithromycin, levofloxacin, and tigecycline | After initial treatment, tigecycline caused transaminitis and levofloxacin was stopped based on susceptibility result. But treatment was terminated in about 25 d because it was thought that M abscessus was contaminated. | 25 | No | Yes | Alive |
| 2 | Not treated | Not treated | Not treated | No | Yes | Alive |
| 3 | Azithromycin, cefoxitin, and tigecycline | Initial regimen was continued for 3 mo, and then it was stepped down to azithromycin and tigecycline. | 297 | No | Yes | Dead |
| 4 | Azithromycin, imipenem-cilastatin, and tigecycline | After the initial treatment was started, imipenem-cilastatin was switched to cefoxitin 2 d later. Then the patient was discharged to other facility and there was no further follow-up in our system. | NA | NA | NA | NA |
| 5 | Not treated | Not treated | Not treated | No | No | Alive |
| 6 | Not treated | Not treated | Not treated | No | No | Alive |
| 7 | Azithromycin, cefoxitin, and tigecycline | Initial treatment was continued without any significant side effect. | 20 | No | No | Dead |
| 8 | Azithromycin, cefoxitin, and tigecycline | Initial regimen was switched to azithromycin, imipenem-cilastatin, and cefoxitin because of transaminitis. The patient had been on the 3 medications for 8 mo and then azithromycin and imipenem-cilastatin were continued additional 10 mo for relapse. | 523 | No | Yes | Alive |
| 9 | Not treated | The patient was not treated as infection. Azithromycin was given as prophylaxis. | 202 | No | Yes | Alive |
| 10 | Not treated | Not treated | Not treated | No | Yes | Alive |
| 11 | Azithromycin, cefoxitin, and tigecycline | The initial regimen was switched to azithromycin, imipenem-cilastatin, and linezolid in 4 d. But the further information was missing. | NA | No | No | Alive |
| 12 | Azithromycin, imipenem-cilastatin, and cefoxitin | The initial regimen was switched to azithromycin, tigecycline, and amikacin. The patient died of the event not related to M abscessus infection. | 87 | Yes | No | Dead |
| 13 | Azithromycin, cefoxitin, and amikacin | After the initial treatment, amikacin was stopped in 1 mo, then the regimen was switched to azithromycin, cefoxitin, and tigecycline. The treatment was stopped in 4 mo. After the treatment, the infection relapsed. | 136 | No | Yes | Dead |
| 14 | Azithromycin, cefoxitin, and tigecycline | The initial treatment was switched to azithromycin, imipenem-cilastatin, and inhaled amikacin because tigecycline caused transaminitis and imipenem-cilastatin was started for treating Achromobacter infection. | 184 | Yes | No | Alive |
| 15 | Azithromycin, cefoxitin, and amikacin | The initial treatment was switched to azithromycin, cefoxitin, and tigecycline because amikacin caused hearing loss. After that, tigecycline caused transaminitis and it was switched to inhale amikacin. Inhale amikacin was complicated with bronchospasm. | 186 | No | No | Alive |
| 16 | Azithromycin, cefoxitin, and tigecycline | The initial regimen was continued, but tigecycline caused nausea and vomiting. Then the dose of tigecycline was decreased, but it caused transaminitis. The regimen was switched to azithromycin, cefoxitin, and inhaled amikacin. | 220 | No | Yes | Alive |
| 17 | Azithromycin, imipenem-cilastatin, and tigecycline | The initial regimen was complicated with transaminitis likely due to azithromycin and/or tigecycline, and it was switched to inhaled amikacin, imipenem-cilastatin and linezolid. After that, inhaled amikacin was stopped because of worsening respiratory status, and linezolid caused thrombocytopenia. Imipenem-cilastatin and azithromycin were continued. | 296 | Yes | No | Alive |
| 18 | Clarithromycin, tigecycline, and amikacin | After the initial treatment, clarithromycin was switched to azithromycin and tigecycline was switched to cefoxitin due to pancreatitis. And amikacin was stopped because of acute kidney injury. Meantime cefoxitin was initiated but finally clofazimine and imipenem-cilastatin were continued. | 91 | Yes | No | Dead |
| 19 | Not treated | Not treated | Not treated | NA | No | Alive |
| 20 | Not treated | Not treated | Not treated | NA | No | Alive |
| No. . | Initial Regimen . | Comment . | Duration of Therapy, d . | Treatment Failure . | Rejection After Treatment . | Outcome . |
|---|---|---|---|---|---|---|
| 1 | Clarithromycin, levofloxacin, and tigecycline | After initial treatment, tigecycline caused transaminitis and levofloxacin was stopped based on susceptibility result. But treatment was terminated in about 25 d because it was thought that M abscessus was contaminated. | 25 | No | Yes | Alive |
| 2 | Not treated | Not treated | Not treated | No | Yes | Alive |
| 3 | Azithromycin, cefoxitin, and tigecycline | Initial regimen was continued for 3 mo, and then it was stepped down to azithromycin and tigecycline. | 297 | No | Yes | Dead |
| 4 | Azithromycin, imipenem-cilastatin, and tigecycline | After the initial treatment was started, imipenem-cilastatin was switched to cefoxitin 2 d later. Then the patient was discharged to other facility and there was no further follow-up in our system. | NA | NA | NA | NA |
| 5 | Not treated | Not treated | Not treated | No | No | Alive |
| 6 | Not treated | Not treated | Not treated | No | No | Alive |
| 7 | Azithromycin, cefoxitin, and tigecycline | Initial treatment was continued without any significant side effect. | 20 | No | No | Dead |
| 8 | Azithromycin, cefoxitin, and tigecycline | Initial regimen was switched to azithromycin, imipenem-cilastatin, and cefoxitin because of transaminitis. The patient had been on the 3 medications for 8 mo and then azithromycin and imipenem-cilastatin were continued additional 10 mo for relapse. | 523 | No | Yes | Alive |
| 9 | Not treated | The patient was not treated as infection. Azithromycin was given as prophylaxis. | 202 | No | Yes | Alive |
| 10 | Not treated | Not treated | Not treated | No | Yes | Alive |
| 11 | Azithromycin, cefoxitin, and tigecycline | The initial regimen was switched to azithromycin, imipenem-cilastatin, and linezolid in 4 d. But the further information was missing. | NA | No | No | Alive |
| 12 | Azithromycin, imipenem-cilastatin, and cefoxitin | The initial regimen was switched to azithromycin, tigecycline, and amikacin. The patient died of the event not related to M abscessus infection. | 87 | Yes | No | Dead |
| 13 | Azithromycin, cefoxitin, and amikacin | After the initial treatment, amikacin was stopped in 1 mo, then the regimen was switched to azithromycin, cefoxitin, and tigecycline. The treatment was stopped in 4 mo. After the treatment, the infection relapsed. | 136 | No | Yes | Dead |
| 14 | Azithromycin, cefoxitin, and tigecycline | The initial treatment was switched to azithromycin, imipenem-cilastatin, and inhaled amikacin because tigecycline caused transaminitis and imipenem-cilastatin was started for treating Achromobacter infection. | 184 | Yes | No | Alive |
| 15 | Azithromycin, cefoxitin, and amikacin | The initial treatment was switched to azithromycin, cefoxitin, and tigecycline because amikacin caused hearing loss. After that, tigecycline caused transaminitis and it was switched to inhale amikacin. Inhale amikacin was complicated with bronchospasm. | 186 | No | No | Alive |
| 16 | Azithromycin, cefoxitin, and tigecycline | The initial regimen was continued, but tigecycline caused nausea and vomiting. Then the dose of tigecycline was decreased, but it caused transaminitis. The regimen was switched to azithromycin, cefoxitin, and inhaled amikacin. | 220 | No | Yes | Alive |
| 17 | Azithromycin, imipenem-cilastatin, and tigecycline | The initial regimen was complicated with transaminitis likely due to azithromycin and/or tigecycline, and it was switched to inhaled amikacin, imipenem-cilastatin and linezolid. After that, inhaled amikacin was stopped because of worsening respiratory status, and linezolid caused thrombocytopenia. Imipenem-cilastatin and azithromycin were continued. | 296 | Yes | No | Alive |
| 18 | Clarithromycin, tigecycline, and amikacin | After the initial treatment, clarithromycin was switched to azithromycin and tigecycline was switched to cefoxitin due to pancreatitis. And amikacin was stopped because of acute kidney injury. Meantime cefoxitin was initiated but finally clofazimine and imipenem-cilastatin were continued. | 91 | Yes | No | Dead |
| 19 | Not treated | Not treated | Not treated | NA | No | Alive |
| 20 | Not treated | Not treated | Not treated | NA | No | Alive |
Abbreviations: M abscessus, Mycobacterium abscessus; NA, not applicable.
Lung transplant recipients included 5 males and 7 females with a median age of 60 years (range, 47–70 years). Seven patients (58.3%) were treated with antibiotics with a median treatment duration of 186 days (range, 20–296 days); 2 (16.7%) had unrelated deaths. Three (25.0%) patients had treatment failure, 1 of whom died. Five patients (41.7%) were not treated and were alive without evidence of M abscessus infections within 1 year of follow-up. In lung transplant recipients, 8 of 12 patients (66.7%) met the ATS/IDSA criteria for diagnosis of NTM infection and only 6 of 8 patients (75.0%) who met the criteria were treated with antibiotics. The 2 patients who met ATS/IDSA criteria were not treated by their transplant pulmonary team and were never referred to ID.
Antibiotic Treatment in All SOT Recipients
Initial antibiotic management of all our patients is summarized in Table 3. The combination of azithromycin, cefoxitin and tigecycline was prescribed in 6 patients (46.2%). All patients tolerated azithromycin, clarithromycin, cefoxitin, and imipenem-cilastatin. Tigecycline use was frequently associated with adverse effects such as gastrointestinal upset (2/6 [33.3%]), transaminitis (4/6 [66.7%]), and pancreatitis (2/6 [33.3%]), and amikacin caused acute kidney injury (1/3 [33.3%]) and hearing loss (1/3 [33.3%]). The 1 patient who had a linezolid-based regimen developed thrombocytopenia.
Rejection in All SOT and Lung Transplant Recipients
In our cohort, a total 8 of 20 (40.0%) patients had rejection with a median time from transplantation to rejection of 192 days (range, 63–594 days); 3 (15.0%) patients had rejection prior to M abscessus diagnosis, and 7 (35.0%) patients had it after M abscessus diagnosis (2 patients had rejection twice, before and after the diagnosis). The median time from transplantation to rejection in the 3 patients was 298 days (range, 82–343 days) and the median time from diagnosis to rejection in the 7 patients was 59 days (range, 0–476 days). In lung transplant recipients, a total 4 of 12 (33.3%) patients had rejection after transplantation with a median time from transplantation to rejection of 192 days (range, 82–343 days); 2 (16.7%) patients had rejection before the M abscessus diagnosis in a median of 213 days (range, 82–343 days) from the transplantation, and 3 (25.0%) patients had rejection after the diagnosis at a median of 59 days (range, 0–131 days) from the diagnosis (1 patient had rejection before and after the diagnosis).
Survival in All SOT and Lung Transplant Recipients
There was no significant difference in survival during the first year after the diagnosis among lung transplant recipients and non–lung transplant recipients (0.833 and 0.875, respectively; Figure 1B). The patients with M abscessus colonization had better survival than those with infection in all SOT recipients (1.0 and 0.786, respectively) and lung transplant recipients (1.0 and 0.786, respectively), but it was not statistically significant (Figure 1C and 1D).
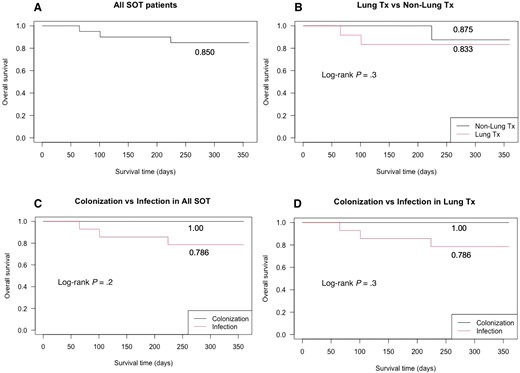
One-year survival after transplantation. A, One-year survival in all solid organ transplant (SOT) recipients with Mycobacterium abscessus infection. B, One-year survival in M abscessus infection, lung transplant (Tx) recipients vs non–lung Tx recipients. C, One-year survival in M abscessus infection, colonization vs infection in all SOT recipients, We defined colonization as the patients who had positive M abscessus culture but did not meet American Thoracic Society/Infectious Diseases Society of America criteria. D, One-year survival in M abscessus infection, colonization vs infection in lung Tx recipients.
Microbiology
In our facility, not all of the isolates were sent for subspecies identification or susceptibilities identification as the laboratory protocol depends upon each provider’s request and clinical decision. Among our 20 cases, 12 had the subspecies identification (Supplementary Table 2) of M abscessus massiliense (n = 7), M abscessus bolletii (n = 4), and M abscessus abscessus (n = 1). Susceptibilities were available for 14 cases (Supplementary Table 3). Based on these results, we calculated the proportion of susceptible isolates as (number of cases with M abscessus susceptible to the specific antibiotic) / (number of cases that have the antibiotic susceptibility test result for the specific antibiotic). Amikacin, azithromycin, clarithromycin, kanamycin, and tigecycline were the most active antibiotics against M abscessus infection in our facility (100% for each agent). Other drugs such as clofazimine, linezolid, cefoxitin, imipenem-cilastatin, ciprofloxacin, and moxifloxacin were more likely to be resistant in vitro (percentage susceptible: 92.0%, 36.3%, 9.1%, 7.1%, 0%, and 0%, respectively).
DISCUSSION
The incidence, risk factors, and outcome of NTM infection in SOT recipients are not fully defined, and the incidence is still uncertain. The purpose of the present study was to reveal the clinical course and outcomes of M abscessus infection. We demonstrated that both treatment failure rate and mortality rate were high in M abscessus infection; treatment failure rate was 4 of 13 (30.8%) and 3 of 7 (42.9%) in all SOT recipients and lung transplant recipients, respectively, and 1-year survival was 85.0% and 83.3% in in all SOT recipients and lung transplant recipients, respectively. Five of 12 lung transplant recipients with positive respiratory culture with M abscessus were not treated. Further review revealed that all of them were alive and free from M abscessus infection at a median of 818 days after positive culture (range, 228–1910 days).
There was a high treatment failure rate in our cohort, which was related to the concomitant infections and immunosuppression as the previous studies addressed [9, 10, 17]. We also found that more patients had adverse events from the initial antibiotic regimens (Supplementary Table 4). Regarding mortality, Longworth et al reported that NTM infection after SOT transplantation decreased 3-year survival after transplantation and there was no difference in mortality between lung transplant recipients and non–lung transplant recipients with NTM infection [10]. In our study, we calculated 1-year survival and there was no significant difference between lung transplant recipients and non–lung transplant recipients (Figure 1B). It is possible that some of lung transplant included in our cohort had colonization, not infection, overestimating better survival outcomes because our data support that patients with M abscessus colonization had better survival than those with the infection and we cannot perfectly differentiate M abscessus colonization from the infection at the first evaluation (Figure 1C and 1D). And lung transplant recipients are clinically diagnosed earlier as there is a low threshold to perform diagnostic bronchoscopies in these patients. In this regard, this practice can cause lead time bias and we need to interpret the survival outcome carefully.
It must be emphasized that in lung transplant recipients whose respiratory culture showed M abscessus, 41.7% of patients were free from M abscessus infection without treatment. As we mentioned above, our study and the previous study by Osmani et al applied ATS/IDSA criteria of NTM infection for diagnosis [13, 22]. In our study, 5 lung transplant recipients with positive M abscessus respiratory culture did not receive treatment, but all of them were alive. And 2 of them did meet the ATS/IDSA criteria but did not receive the treatment because the patients were clinically stable when the ID physicians evaluated them several days after the organism grew from the culture. These 2 patients were alive and free from M abscessus infection. The fact may suggest that the current ATS/IDSA criteria of NTM infection were not applicable in lung transplant recipients and that lung transplant recipients who have positive respiratory cultures with M abscessus do not always need treatment because M abscessus growing in respiratory samples just means its colonization, not infection. In lung transplant recipients, it was difficult to differentiate true infection from colonization, especially early after lung transplantation. Considering the high treatment failure rate and mortality rate, aggressive treatment is recommended, but the combination therapy against M abscessus can cause drug-drug interaction and adverse events simultaneously. Therefore, clinical decision making in some of these cases is not always straightforward, and it should be done on a case-by-case basis weighing risks and benefits as well as evidence of infection vs colonization. Better diagnostic criteria are required to optimize the chance of cure and lower the adverse events. In our cohort, the lung transplant recipients with M abscessus colonization tended to have a lower number of positive culture and shorter time from transplantation and the first positive culture result (Figure 2), although the data were preliminary and need to be confirmed by larger studies.
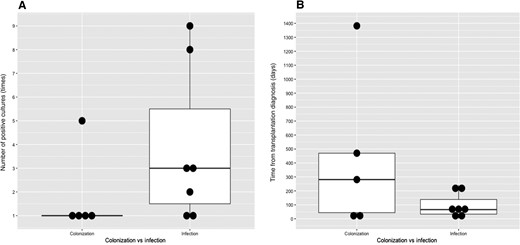
Colonization vs infection in lung transplant recipients. A, Number of positive cultures. B, Time from transplantation to diagnosis.
It is also important to highlight that 8 of 20 (40.0%) patients had rejection after the diagnosis of M abscessus infection. We speculate that the infection itself and/or lowering the level of immunosuppression—due to dose reduction for managing the infection or drug-drug interaction with antibiotics—could have triggered rejection. Further investigations are needed to evaluate the relationship between M abscessus infections and rejection.
Our study has limitations. First, our cohort had more patients with M abscessus infection compared with other studies, but the number of patients was still small. And the clinical course, treatment failure rate, and mortality rate varied widely between these studies (Table 4), so our data including treatment failure rate and mortality rate can be under- or overestimated. Second, there is no validated diagnostic criteria in SOT recipients, especially in lung transplant recipients, for M abscessus infection and we may treat the patients with M abscessus colonization, not M abscessus infection, which might result in a lower mortality rate. Although our data had these limitations, we identified important implications for the diagnosis of M abscessus infections in transplant recipients. Taken all together, culture positivity in respiratory samples does not always suggest infection. In this regard, we recommend caution and thoughtful criteria to confirm the diagnosis of the lung infection and not based on a single respiratory culture balancing the risks and benefits in each individual patient.
| Study . | Study Design . | No. of Patients . | Age/Sex and Type of Transplantation (Indication for Transplantation) . | Site of Infection . | Time from Transplantation to Infection . | Clinical Course/Treatment Failure . | Mortality . |
|---|---|---|---|---|---|---|---|
| Cherneko et al [17] | Survey | 17 | 17 Lung 57/F (bronchiectasis) 29/M (CF) 33/M (PAH) 49/M (COPD) 65/M (IPM) 38/M (CF) 37/M (CF) 40/F (Ei) 48/M (COPD) 66/M (IPF) 53/M (COPD) 60/M (A) 25/F (IPF) 39/M (CF) 54/F (Sclero) 42/F (benign metastasizing leiomyoma) 66/M (IPF) | Lung Cut lesion Lung Lung Lung Lung Lung Cut lesion Lung Lung Lung Lung Lung Lung Lung Lung Lung | 18.5 mo (range, 1–111 mo) | One patient was not treated. Among the treated 16 patients, 1 patient did not respond to the treatment. | 58.8% (10/17) were alive and cured, and 41.2% (7/17) have died. Median follow-up after identification of an organism was 835 d. Mean follow-up was 1207.1 d. |
| Garrison et al [18] | Case series | 3 | 73/F kidney 35/M kidney 28/F multivisceral | SSTI PD catheter-related infection SSTI, and septic arthritis at sternoclavicular joint | 12 wk after Tx 2 mo after Tx 9 mo after Tx | Recurrence of infection in 20 mo after completion of therapy No recurrence, but the patient was back to hemodialysis 11 mo after M abscessus infection. Clinical course was complicated with varicella zoster infection, intestinal graft rejection, and multiorgan failure, and finally died 14 mo after transplantation. | Alive Alive, but graft loss Dead |
| Morales et al [19] | Case series | 7 | 4 Lung 15/F (CF) 19/M (CF) 57/M (COPD) 54/M (pulmonary fibrosis) 2 Kidney 61/F (polycystosis) 58/M (renal failure) 1 Heart 69/M (DCM) | 4 Lung Nodules in both knees SSTI and disseminated infection Stenosis in LMB and pneumonia Pneumonia 2 Kidney Panniculitis SSTI 1 Heart Pneumonia | 24 mo (range, 7 d–276 mo) | Not reported | Not reported |
| Huang et al [20] | Retrospective cohort | 4 | 49/F lung 56/F lung 33/M lung 57/M lung | Lung infection | 614 d 487 d 583 d 83 d | NTM clearance by 3 mo, progressive graft dysfunction NTM clearance by 6 mo, complete resolution Unknown NTM clearance, progressive graft dysfunction NTM clearance by 3 mo, progressive graft dysfunction | Dead/postoperative day 1161 Alive/postoperative day 2109 Dead/postoperative day 612 Dead/postoperative day 436 |
| Richey et al [21] | Case report | 1 | 1 Heart 49/M (nonischemic cardiomyopathy) | Lead infection | <3 mo | No treatment failure | Alive at 18 mo after diagnosis |
| Osamani et al [22] | Case series | 9 | 9 Lung 76/M (IPF) 69/M (IPF) 40/F (CF) 53/M (IPF) 43/M (IPF) 68/M (COPD) 70/M (IPF) 72/F (IPF) 65/F (COPD) | Pneumonia (M abscessus was isolated from respiratory samples) | 7.5 mo (range, 3 d–13 mo) | 4/9 (44.4%) | Median survival 39 mo (range, 11–96 mo) |
| Ose et al [23] | Case series | 2 | 2 Lung 38/F (bronchial ectasia) 39/F (lymphangioleiomyomatosis) | 2 Lung Pneumonia Pneumonia | 82 mo after transplantation 58 mo after transplantation | No treatment failure | Alive at 22 mo after treatment Alive at 12 mo after treatment |
| Study . | Study Design . | No. of Patients . | Age/Sex and Type of Transplantation (Indication for Transplantation) . | Site of Infection . | Time from Transplantation to Infection . | Clinical Course/Treatment Failure . | Mortality . |
|---|---|---|---|---|---|---|---|
| Cherneko et al [17] | Survey | 17 | 17 Lung 57/F (bronchiectasis) 29/M (CF) 33/M (PAH) 49/M (COPD) 65/M (IPM) 38/M (CF) 37/M (CF) 40/F (Ei) 48/M (COPD) 66/M (IPF) 53/M (COPD) 60/M (A) 25/F (IPF) 39/M (CF) 54/F (Sclero) 42/F (benign metastasizing leiomyoma) 66/M (IPF) | Lung Cut lesion Lung Lung Lung Lung Lung Cut lesion Lung Lung Lung Lung Lung Lung Lung Lung Lung | 18.5 mo (range, 1–111 mo) | One patient was not treated. Among the treated 16 patients, 1 patient did not respond to the treatment. | 58.8% (10/17) were alive and cured, and 41.2% (7/17) have died. Median follow-up after identification of an organism was 835 d. Mean follow-up was 1207.1 d. |
| Garrison et al [18] | Case series | 3 | 73/F kidney 35/M kidney 28/F multivisceral | SSTI PD catheter-related infection SSTI, and septic arthritis at sternoclavicular joint | 12 wk after Tx 2 mo after Tx 9 mo after Tx | Recurrence of infection in 20 mo after completion of therapy No recurrence, but the patient was back to hemodialysis 11 mo after M abscessus infection. Clinical course was complicated with varicella zoster infection, intestinal graft rejection, and multiorgan failure, and finally died 14 mo after transplantation. | Alive Alive, but graft loss Dead |
| Morales et al [19] | Case series | 7 | 4 Lung 15/F (CF) 19/M (CF) 57/M (COPD) 54/M (pulmonary fibrosis) 2 Kidney 61/F (polycystosis) 58/M (renal failure) 1 Heart 69/M (DCM) | 4 Lung Nodules in both knees SSTI and disseminated infection Stenosis in LMB and pneumonia Pneumonia 2 Kidney Panniculitis SSTI 1 Heart Pneumonia | 24 mo (range, 7 d–276 mo) | Not reported | Not reported |
| Huang et al [20] | Retrospective cohort | 4 | 49/F lung 56/F lung 33/M lung 57/M lung | Lung infection | 614 d 487 d 583 d 83 d | NTM clearance by 3 mo, progressive graft dysfunction NTM clearance by 6 mo, complete resolution Unknown NTM clearance, progressive graft dysfunction NTM clearance by 3 mo, progressive graft dysfunction | Dead/postoperative day 1161 Alive/postoperative day 2109 Dead/postoperative day 612 Dead/postoperative day 436 |
| Richey et al [21] | Case report | 1 | 1 Heart 49/M (nonischemic cardiomyopathy) | Lead infection | <3 mo | No treatment failure | Alive at 18 mo after diagnosis |
| Osamani et al [22] | Case series | 9 | 9 Lung 76/M (IPF) 69/M (IPF) 40/F (CF) 53/M (IPF) 43/M (IPF) 68/M (COPD) 70/M (IPF) 72/F (IPF) 65/F (COPD) | Pneumonia (M abscessus was isolated from respiratory samples) | 7.5 mo (range, 3 d–13 mo) | 4/9 (44.4%) | Median survival 39 mo (range, 11–96 mo) |
| Ose et al [23] | Case series | 2 | 2 Lung 38/F (bronchial ectasia) 39/F (lymphangioleiomyomatosis) | 2 Lung Pneumonia Pneumonia | 82 mo after transplantation 58 mo after transplantation | No treatment failure | Alive at 22 mo after treatment Alive at 12 mo after treatment |
We performed a literature review of Mycobacterium abscessus infection and summarized the findings: age, site of infection, time from transplantation to infection, outcome, and mortality.
Abbreviations: A, α1-antitrypsin deficiency; CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; DCM, dilated cardiomyopathy; Ei, xxx; IPF, idiopathic pulmonary fibrosis; IPAH, idiopathic pulmonary arterial hypertension; IPM, xxx; LMB, xxx; NTM, nontuberculous mycobacteria, PD, peritoneal dialysis, Sclero, scleroderma; SSTI, skin and soft tissue infection; Tx, transplantation.
| Study . | Study Design . | No. of Patients . | Age/Sex and Type of Transplantation (Indication for Transplantation) . | Site of Infection . | Time from Transplantation to Infection . | Clinical Course/Treatment Failure . | Mortality . |
|---|---|---|---|---|---|---|---|
| Cherneko et al [17] | Survey | 17 | 17 Lung 57/F (bronchiectasis) 29/M (CF) 33/M (PAH) 49/M (COPD) 65/M (IPM) 38/M (CF) 37/M (CF) 40/F (Ei) 48/M (COPD) 66/M (IPF) 53/M (COPD) 60/M (A) 25/F (IPF) 39/M (CF) 54/F (Sclero) 42/F (benign metastasizing leiomyoma) 66/M (IPF) | Lung Cut lesion Lung Lung Lung Lung Lung Cut lesion Lung Lung Lung Lung Lung Lung Lung Lung Lung | 18.5 mo (range, 1–111 mo) | One patient was not treated. Among the treated 16 patients, 1 patient did not respond to the treatment. | 58.8% (10/17) were alive and cured, and 41.2% (7/17) have died. Median follow-up after identification of an organism was 835 d. Mean follow-up was 1207.1 d. |
| Garrison et al [18] | Case series | 3 | 73/F kidney 35/M kidney 28/F multivisceral | SSTI PD catheter-related infection SSTI, and septic arthritis at sternoclavicular joint | 12 wk after Tx 2 mo after Tx 9 mo after Tx | Recurrence of infection in 20 mo after completion of therapy No recurrence, but the patient was back to hemodialysis 11 mo after M abscessus infection. Clinical course was complicated with varicella zoster infection, intestinal graft rejection, and multiorgan failure, and finally died 14 mo after transplantation. | Alive Alive, but graft loss Dead |
| Morales et al [19] | Case series | 7 | 4 Lung 15/F (CF) 19/M (CF) 57/M (COPD) 54/M (pulmonary fibrosis) 2 Kidney 61/F (polycystosis) 58/M (renal failure) 1 Heart 69/M (DCM) | 4 Lung Nodules in both knees SSTI and disseminated infection Stenosis in LMB and pneumonia Pneumonia 2 Kidney Panniculitis SSTI 1 Heart Pneumonia | 24 mo (range, 7 d–276 mo) | Not reported | Not reported |
| Huang et al [20] | Retrospective cohort | 4 | 49/F lung 56/F lung 33/M lung 57/M lung | Lung infection | 614 d 487 d 583 d 83 d | NTM clearance by 3 mo, progressive graft dysfunction NTM clearance by 6 mo, complete resolution Unknown NTM clearance, progressive graft dysfunction NTM clearance by 3 mo, progressive graft dysfunction | Dead/postoperative day 1161 Alive/postoperative day 2109 Dead/postoperative day 612 Dead/postoperative day 436 |
| Richey et al [21] | Case report | 1 | 1 Heart 49/M (nonischemic cardiomyopathy) | Lead infection | <3 mo | No treatment failure | Alive at 18 mo after diagnosis |
| Osamani et al [22] | Case series | 9 | 9 Lung 76/M (IPF) 69/M (IPF) 40/F (CF) 53/M (IPF) 43/M (IPF) 68/M (COPD) 70/M (IPF) 72/F (IPF) 65/F (COPD) | Pneumonia (M abscessus was isolated from respiratory samples) | 7.5 mo (range, 3 d–13 mo) | 4/9 (44.4%) | Median survival 39 mo (range, 11–96 mo) |
| Ose et al [23] | Case series | 2 | 2 Lung 38/F (bronchial ectasia) 39/F (lymphangioleiomyomatosis) | 2 Lung Pneumonia Pneumonia | 82 mo after transplantation 58 mo after transplantation | No treatment failure | Alive at 22 mo after treatment Alive at 12 mo after treatment |
| Study . | Study Design . | No. of Patients . | Age/Sex and Type of Transplantation (Indication for Transplantation) . | Site of Infection . | Time from Transplantation to Infection . | Clinical Course/Treatment Failure . | Mortality . |
|---|---|---|---|---|---|---|---|
| Cherneko et al [17] | Survey | 17 | 17 Lung 57/F (bronchiectasis) 29/M (CF) 33/M (PAH) 49/M (COPD) 65/M (IPM) 38/M (CF) 37/M (CF) 40/F (Ei) 48/M (COPD) 66/M (IPF) 53/M (COPD) 60/M (A) 25/F (IPF) 39/M (CF) 54/F (Sclero) 42/F (benign metastasizing leiomyoma) 66/M (IPF) | Lung Cut lesion Lung Lung Lung Lung Lung Cut lesion Lung Lung Lung Lung Lung Lung Lung Lung Lung | 18.5 mo (range, 1–111 mo) | One patient was not treated. Among the treated 16 patients, 1 patient did not respond to the treatment. | 58.8% (10/17) were alive and cured, and 41.2% (7/17) have died. Median follow-up after identification of an organism was 835 d. Mean follow-up was 1207.1 d. |
| Garrison et al [18] | Case series | 3 | 73/F kidney 35/M kidney 28/F multivisceral | SSTI PD catheter-related infection SSTI, and septic arthritis at sternoclavicular joint | 12 wk after Tx 2 mo after Tx 9 mo after Tx | Recurrence of infection in 20 mo after completion of therapy No recurrence, but the patient was back to hemodialysis 11 mo after M abscessus infection. Clinical course was complicated with varicella zoster infection, intestinal graft rejection, and multiorgan failure, and finally died 14 mo after transplantation. | Alive Alive, but graft loss Dead |
| Morales et al [19] | Case series | 7 | 4 Lung 15/F (CF) 19/M (CF) 57/M (COPD) 54/M (pulmonary fibrosis) 2 Kidney 61/F (polycystosis) 58/M (renal failure) 1 Heart 69/M (DCM) | 4 Lung Nodules in both knees SSTI and disseminated infection Stenosis in LMB and pneumonia Pneumonia 2 Kidney Panniculitis SSTI 1 Heart Pneumonia | 24 mo (range, 7 d–276 mo) | Not reported | Not reported |
| Huang et al [20] | Retrospective cohort | 4 | 49/F lung 56/F lung 33/M lung 57/M lung | Lung infection | 614 d 487 d 583 d 83 d | NTM clearance by 3 mo, progressive graft dysfunction NTM clearance by 6 mo, complete resolution Unknown NTM clearance, progressive graft dysfunction NTM clearance by 3 mo, progressive graft dysfunction | Dead/postoperative day 1161 Alive/postoperative day 2109 Dead/postoperative day 612 Dead/postoperative day 436 |
| Richey et al [21] | Case report | 1 | 1 Heart 49/M (nonischemic cardiomyopathy) | Lead infection | <3 mo | No treatment failure | Alive at 18 mo after diagnosis |
| Osamani et al [22] | Case series | 9 | 9 Lung 76/M (IPF) 69/M (IPF) 40/F (CF) 53/M (IPF) 43/M (IPF) 68/M (COPD) 70/M (IPF) 72/F (IPF) 65/F (COPD) | Pneumonia (M abscessus was isolated from respiratory samples) | 7.5 mo (range, 3 d–13 mo) | 4/9 (44.4%) | Median survival 39 mo (range, 11–96 mo) |
| Ose et al [23] | Case series | 2 | 2 Lung 38/F (bronchial ectasia) 39/F (lymphangioleiomyomatosis) | 2 Lung Pneumonia Pneumonia | 82 mo after transplantation 58 mo after transplantation | No treatment failure | Alive at 22 mo after treatment Alive at 12 mo after treatment |
We performed a literature review of Mycobacterium abscessus infection and summarized the findings: age, site of infection, time from transplantation to infection, outcome, and mortality.
Abbreviations: A, α1-antitrypsin deficiency; CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; DCM, dilated cardiomyopathy; Ei, xxx; IPF, idiopathic pulmonary fibrosis; IPAH, idiopathic pulmonary arterial hypertension; IPM, xxx; LMB, xxx; NTM, nontuberculous mycobacteria, PD, peritoneal dialysis, Sclero, scleroderma; SSTI, skin and soft tissue infection; Tx, transplantation.
In conclusion, our data showed that M abscessus infections are associated with a high number of treatment failures and potentially increased mortality in SOT recipients. Further studies are needed to improve infection prevention strategies, develop better diagnostic criteria to distinguish colonization vs infection in lung transplant recipients, design novel agents to improve clinical outcomes, and minimize adverse events in this challenging patient population.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Patient consent. This research project was approved and conducted under the local institutional review board approval; written consent was waived due to practical issues.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Y. E. and Y. N. contributed equally to this work.





Comments