-
PDF
- Split View
-
Views
-
Cite
Cite
Despoina M Galetaki, Molly Hayes, Alexander Newman, Craig L K Boge, Caitlin W Elgarten, Jason L Freedman, Timothy S Olson, Brian T Fisher, 1745. Retrospective Cohort Analysis to Determine the Incidence of CMV Infection and Disease in Allogeneic Hematopoietic Cell Transplant Recipients at an Academic Children’s Hospital, Open Forum Infectious Diseases, Volume 6, Issue Supplement_2, October 2019, Pages S639–S640, https://doi.org/10.1093/ofid/ofz360.1608
Close - Share Icon Share
Abstract
Data on cytomegalovirus (CMV) infection and disease by donor (D)/recipient (R) status or prophylaxis regimen in pediatric hematopoietic cell transplant (HCT) recipients are limited. There is an absence of data on adverse events (AE) attributable to prophylaxis.
A single-center cohort (N = 352) of allogeneic HCT episodes between January 2004 and June 2017 was assembled. Exclusion criteria were CMV PCR positivity 30 days before HCT, lack of CMV surveillance (<2 blood PCRs in the 30 days post HCT), or unknown D/R CMV status. CMV prophylaxis was recommended for CMV D+ or R+ patients with ≥1 of the following factors: T-cell depletion, cord blood product, or exposure to distal alemtuzumab. The CMV prophylaxis regimen was standard-dose acyclovir from day −7 to +7, then foscarnet to engraftment, and then valganciclovir to day +100 (acyc → fos → valgan). If a patient did not meet criteria for CMV prophylaxis but was HSV IgG positive then standard-dose acyclovir was given from day −7 to the end of study follow-up (SD-acyc). All remaining patients did not receive antiviral prophylaxis. Outcomes of CMV infection and CMV disease by day +180 were captured. AEs attributable to antiviral prophylaxis were also identified. An AE was attributed to an antiviral prophylaxis medication if the dose was reduced or stopped. AEs were only reported in HCT episodes with complete medical records (n = 221).
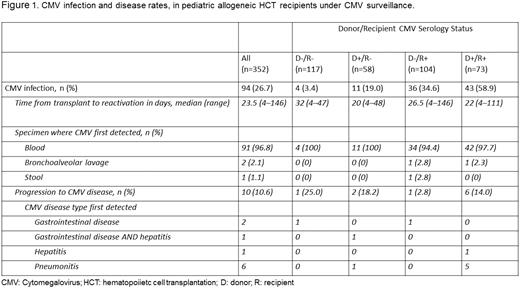
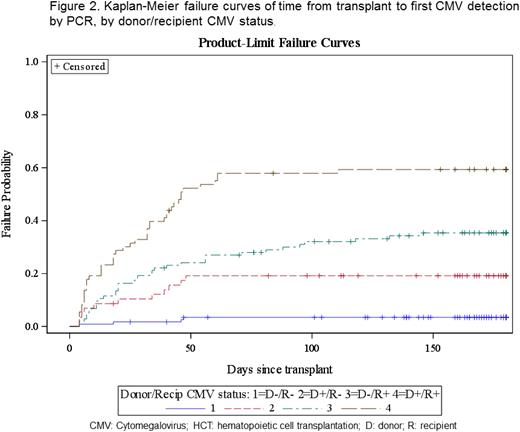
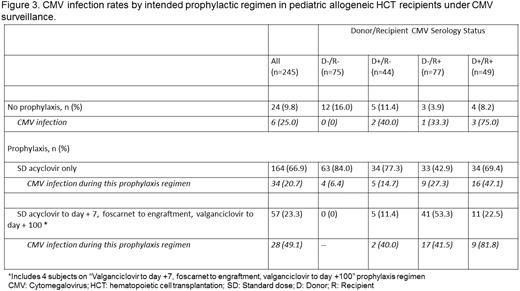
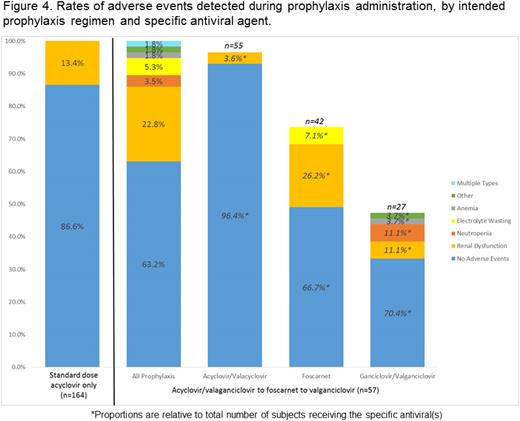
The CMV infection rate was 26.7%, with a median time to detection of 23.5 days (range: 4–146). CMV infection was common in D+/R+ (58.9%) and D−/R+ (34.6%) patients. Just under 11% of CMV infections progressed to disease (Figures 1 and 2). Breakthrough CMV infection occurred in 49.1% of patients despite acyc → fos → valgan (Figure 3) at a median of 11 days from HCT (range: 4–132). The attributable AE rate was 13.4% and 36.8% for SD-acyc and acyc → fos → valgan, respectively (Figure 4).
CMV infection was common in D+/R+ and D−/R+ patients, and a substantial proportion progressed to disease. Breakthrough infection persisted despite acyc → fos → valgan prophylaxis and AEs attributable to this regimen were common. CMV infection in R+ patients was frequent even in the absence of additional risk factors. Studies of novel prophylaxis approaches are needed and should include R+ patients regardless of other factors.
All authors: No reported disclosures.
Session: 167. Transplant ID: CMV
Friday, October 4, 2019: 12:15 PM
- polymerase chain reaction
- medical records
- acyclovir
- hematopoietic stem cell transplantation
- disclosure
- follow-up
- foscarnet
- hospitals, pediatric
- pediatrics
- t-lymphocytes
- immunoglobulin g
- infections
- cytomegalovirus infections
- cytomegalovirus
- valganciclovir
- alemtuzumab
- engraftment
- surveillance, medical
- umbilical cord blood
- allogeneic hematopoietic stem cell transplant
- antiviral prophylaxis
- adverse event
- prevention
- donors





Comments