-
PDF
- Split View
-
Views
-
Cite
Cite
Mark H Wilcox, Galia Rahav, Erik R Dubberke, Lori Gabryelski, Kerrie Davies, Claire Berry, Karen Eves, Misoo C Ellison, Dalya Guris, Mary Beth Dorr, Influence of Diagnostic Method on Outcomes in Phase 3 Clinical Trials of Bezlotoxumab for the Prevention of Recurrent Clostridioides difficile Infection: A Post Hoc Analysis of MODIFY I/II, Open Forum Infectious Diseases, Volume 6, Issue 8, August 2019, ofz293, https://doi.org/10.1093/ofid/ofz293
Close - Share Icon Share
Abstract
The optimum diagnostic test method for Clostridioides difficile infection (CDI) remains controversial due to variation in accuracy in identifying true CDI. This post hoc analysis examined the impact of CDI diagnostic testing methodology on efficacy outcomes in phase 3 MODIFY I/II trials.
In MODIFY I/II (NCT01241552/NCT01513239), participants received bezlotoxumab (10 mg/kg) or placebo during anti-CDI treatment for primary/recurrent CDI (rCDI). Using MODIFY I/II pooled data, initial clinical cure (ICC) and rCDI were assessed in participants diagnosed at baseline using direct detection methods (enzyme immunoassay [EIA]/cell cytotoxicity assay [CCA]) or indirect methods to determine toxin-producing ability (toxin gene polymerase chain reaction [tgPCR]/toxigenic culture).
Of 1554 participants who received bezlotoxumab or placebo in MODIFY I/II, 781 (50.3%) and 773 (49.7%) were diagnosed by tgPCR/toxigenic culture and toxin EIA/CCA, respectively. Participants diagnosed by toxin EIA/CCA were more likely to be inpatients, older, and have severe CDI. In bezlotoxumab recipients, ICC rates were slightly higher in the toxin EIA/CCA subgroup (81.7%) vs tgPCR/toxigenic culture (78.4%). Bezlotoxumab significantly reduced the rCDI rate vs placebo in both subgroups; however, the magnitude of reduction was substantially larger in participants diagnosed by toxin EIA/CCA (relative difference, –46.6%) vs tgPCR/toxigenic culture (–29.1%). In bezlotoxumab recipients, the rCDI rate was lower in the toxin EIA/CCA subgroup (17.6%) vs tgPCR/toxigenic culture (23.6%; absolute difference, –6.0%; 95% confidence interval, –12.4 to 0.3; relative difference, –25.4%).
Diagnostic tests that detect fecal C. difficile toxins are of fundamental importance to accurately diagnosing CDI, including in clinical trial design, ensuring that therapeutic efficacy is not underestimated.
Clostridioides difficile is a Gram-positive, spore-forming anaerobe that resides naturally in the large intestine of up to 15% of the adult population [1]. Disruption of the normal intestinal microbiota, most commonly due to treatment with broad-spectrum antibiotics, can lead to the overgrowth of C. difficile [2, 3]. The subsequent production of C. difficile toxin A and B causes colonic inflammation, resulting in mild to severe diarrhea and abdominal pain and more serious manifestations, including fulminant colitis, severe leukocytosis, and death [3, 4].
There has been a marked increase in the incidence and severity of C. difficile infection (CDI) in recent years [5–7]. Although antibiotic treatment for primary CDI is often successful, it has been reported that ~25% of individuals experience recurrent CDI after completing initial antibiotic therapy [8, 9]. After the first recurrent episode, individuals have a 38%–45% probability of a second recurrence, with increasing risk with further recurrences [10, 11].
Bezlotoxumab (MK-6072), a fully human monoclonal antibody that binds to C. difficile toxin B, is indicated to prevent recurrence of CDI (rCDI) in at-risk adults receiving antibacterial drug treatment for primary or recurrent CDI [12]. In the MODIFY I/II phase 3 trials, a single infusion of bezlotoxumab administered with or without actoxumab (a monoclonal antibody against C. difficile toxin A) resulted in a significantly lower rCDI rate over 12 weeks compared with placebo [13].
The study design of the MODIFY trials, including the definition of CDI and diagnostic methods used, was based on the 2010 Infectious Diseases Society of America/Society for Healthcare Epidemiology of America (IDSA/SHEA) CDI guidelines [14]. Due to the absence of an optimal CDI diagnostic test at the time, these guidelines included several test methods in their recommendations. Stool culture followed by the identification of a toxigenic isolate (known as toxigenic culture) and cell cytotoxicity assay (CCA) testing were considered the gold standard; however, these methods were deemed impractical for routine diagnosis due to a long turnaround time [14]. Enzyme immunoassay (EIA) was considered to be most important clinically, but was limited by a lack of sensitivity [14]. Polymerase chain reaction (PCR) testing was described as a rapid, sensitive, and specific method, but more evidence was required to evaluate its utilization in routine diagnostic testing [14]. Consequently, a range of test methods was permitted for use by the local laboratories to diagnose CDI in participants enrolled in MODIFY I/II.
Since the 2010 IDSA/SHEA CDI guidelines were published, further research has clearly shown that the presence of toxigenic C. difficile alone (ie, toxin-negative) is not always indicative of clinical disease. Indeed, 4%–29% of hospitalized individuals are asymptomatic carriers of toxigenic C. difficile [1]. Furthermore, multiple studies have correlated the presence of C. difficile toxins with clinical outcome, showing that individuals who have C. difficile toxin present in their stool (ie, toxin-positive) experience higher rCDI and mortality rates compared with individuals who have toxigenic C. difficile bacteria, but no toxin, in their stool (ie, toxin-negative) [15, 16]. These findings suggest that toxigenic PCR and culture methods, which detect C. difficile bacteria regardless of toxin production, may not reflect true CDI.
Previous research has suggested that PCR-diagnosed individuals may be less likely to respond to CDI treatment compared with those diagnosed using a direct toxin-based test [17–19]. As both direct and indirect test methods were used to diagnose CDI in MODIFY I/II, it is possible that the dilution of treatment-responsive participants with non-treatment-responsive “false CDI” participants may have led to the underestimation of the therapeutic efficacy of bezlotoxumab. This post hoc analysis examined this hypothesis, with the aim to determine if the CDI diagnostic testing methodology used in MODIFY I/II had an impact on efficacy outcomes in participants receiving bezlotoxumab or placebo.
METHODS
Study Design
MODIFY I/II (NCT01241552/NCT01513239) were randomized, double-blind, placebo-controlled, multicenter, phase 3 trials conducted between November 1, 2011, and May 22, 2015, at 322 sites in 30 countries. Full trial details have previously been published [13]. Briefly, adults receiving a 10–14-day regimen of antibacterial drug treatment (metronidazole, vancomycin, or fidaxomicin) for primary CDI or rCDI were randomized to receive a single infusion of bezlotoxumab (10 mg/kg of body weight) or actoxumab (10 mg/kg; MODIFY I only) or bezlotoxumab plus actoxumab (10 mg/kg each) or placebo (0.9% saline). CDI was defined as ≥3 unformed bowel movements (types 5 to 7 on the Bristol Stool Scale [20] in 24 hours), with a positive stool test for toxigenic C. difficile.
In this post hoc analysis of pooled data from MODIFY I/II trials (including only participants who received bezlotoxumab or placebo), a number of end points were investigated through 12 weeks in 2 subgroups: participants diagnosed at baseline via an indirect method (toxin gene PCR [tgPCR]/toxigenic culture) and participants diagnosed at baseline via a direct toxin detection method (toxin EIA/CCA). Different populations were included in the diagnostic subgroups; the toxin EIA/CCA subgroup only included toxin-positive participants, whereas the tgPCR/toxigenic culture subgroup likely included both toxin-positive and toxin-negative participants. All permitted commercial test kits had a labeled specificity of ≥94% with the capacity to detect the presence of C. difficile toxin B (toxin EIA kits) or its cognate tcdB gene (PCR kits).
End Points
The main efficacy end points included initial clinical cure (ICC) and rCDI. ICC was defined as no diarrhea during the 2 consecutive days after completion of ≤16 calendar days of anti-CDI treatment. rCDI was defined as a new episode of diarrhea associated with a positive stool test for toxigenic C. difficile in participants who had achieved the ICC of the baseline CDI episode; the diagnostic methods used for testing the stool of recurrent diarrhea episodes were preferably the same as those used for trial eligibility.
Other efficacy end points included sustained cure (defined as clinical cure of the initial CDI episode and no rCDI through 12-week follow-up), diarrhea recurrence (a new episode of diarrhea in participants who had achieved ICC of the baseline CDI episode, regardless of whether a stool test for toxigenic C. difficile was positive, negative, or not performed), rehospitalization (the occurrence of 30-day all-cause and CDI-associated hospital readmissions), fecal microbiota transplant (FMT; the receipt of an FMT at any time during the study), and all-cause mortality (the occurrence of death within 30 and 90 days after randomization).
Statistical Analyses
The modified intent-to-treat (mITT) population was used for ICC estimations and included all randomized participants in the overall population of the MODIFY I/II trials who received the study infusion, had a positive baseline stool test for toxigenic C. difficile, and received anti-CDI treatment within 1 day of the study infusion. mITT participants who achieved ICC of the baseline CDI episode were included in rCDI and diarrhea recurrence estimations (clinical cure population). Rehospitalization was estimated in mITT participants who were hospitalized at the time of randomization. Mortality was estimated using the “all patients as treated” population, which included all randomized participants who received the study infusion.
Initial and sustained cure rates, as well as observed rCDI and diarrhea recurrence rates, are presented, along with rate differences between the bezlotoxumab and placebo groups and their 95% confidence intervals (CIs). The 95% CIs are based on the Miettinen and Nurminen method [21]. Other outcomes, including rehospitalization, FMT, and mortality during the 12-week follow-up period, are summarized descriptively using the frequency and percentage for each treatment group.
Assay Interference Experiment
An assay interference experiment was performed to determine if the reduced rate of rCDI observed in bezlotoxumab-treated participants diagnosed via toxin EIA/CCA compared with those diagnosed via tgPCR/toxigenic culture was a true result, rather than bezlotoxumab rendering diagnostic toxin EIA or CCA results falsely negative because of binding to free toxins present in fecal samples. Further detail is available in the Supplementary Data.
RESULTS
Participants
The integrated mITT population from MODIFY I/II consisted of 1554 participants, of whom 781 received bezlotoxumab and 773 received placebo. Baseline demographic and clinical characteristics were generally similar between treatment groups [13]. In total, 781 (50.3%) participants were diagnosed by tgPCR/toxigenic culture: 399 (out of 781, 51.1%) participants in the bezlotoxumab group and 382 (out of 773, 49.4%) participants in the placebo group. Overall, 773 (49.7%) participants were diagnosed by toxin EIA/CCA: 382 (out of 781, 48.9%) participants in the bezlotoxumab group and 391 (out of 773, 50.6%) participants in the placebo group (Table 1).
Baseline Demographics and Clinical Characteristics in the mITT Population by Treatment Group and Diagnostic Method
| . | Bezlotoxumab . | Placebo . | ||
|---|---|---|---|---|
| . | EIA + CCA,No.a (%) . | PCR + Culture,No.a (%) . | EIA + CCA,No.a (%) . | PCR + Culture,No.a (%) . |
| Participants in population | 382 | 399 | 391 | 382 |
| Inpatient | 299 (78.3) | 231 (57.9) | 307 (78.5) | 213 (55.8) |
| Female | 212 (55.5) | 230 (57.6) | 235 (60.1) | 214 (56.0) |
| Mean age (SD), y | 65.7 (16.8) | 58.2 (18.4) | 65.4 (17.9) | 61.5 (16.7) |
| Median | 69 | 60 | 68 | 63 |
| Range | 21–100 | 18–97 | 19–98 | 18–97 |
| Age ≥65 y | 226 (59.2) | 164 (41.1) | 230 (58.8) | 175 (45.8) |
| ≥1 CDI episodes in past 6 mo | 93 (24.3) | 123 (30.8) | 114 (29.2) | 105 (27.5) |
| ≥2 previous CDI episodes ever | 41 (10.9) | 59 (15.0) | 61 (15.8) | 65 (17.6) |
| Severe CDI (Zar score ≥ 2)b | 72 (18.8) | 50 (12.5) | 77 (19.7) | 48 (12.6) |
| Immunocompromisedc | 81 (21.2) | 97 (24.3) | 72 (18.4) | 81 (21.2) |
| Antibiotic used during ADT | 131 (34.3) | 115 (28.8) | 142 (36.3) | 134 (35.1) |
| Antibiotic used after ADT | 131 (34.3) | 115 (28.8) | 110 (28.1) | 114 (29.8) |
| At least 1 of the 5 predefined risk factorse | 304 (79.6) | 288 (72.2) | 313 (80.1) | 270 (70.7) |
| Charlson Comorbidity Index ≥3 | 170 (44.5) | 149 (37.3) | 157 (40.2) | 146 (38.2) |
| Albumin <2.5 g/dL | 63 (16.5) | 38 (9.5) | 58 (14.8) | 45 (11.8) |
| Renal impairmentf | 68 (17.8) | 55 (13.8) | 53 (13.6) | 57 (14.9) |
| Hepatic impairmentg | 30 (7.9) | 19 (4.8) | 24 (6.1) | 20 (5.2) |
| Antibiotic drug treatment for CDI | ||||
| Metronidazole | 191 (50.0) | 188 (47.1) | 193 (49.4) | 181 (47.4) |
| Vancomycin | 176 (46.1) | 196 (49.1) | 187 (47.8) | 186 (48.7) |
| Fidaxomicin | 15 (3.9) | 15 (3.8) | 11 (2.8) | 15 (3.9) |
| PCR ribotypeh | ||||
| Participants with a positive culture | 237 | 253 | 250 | 236 |
| 027, 078, or 244 strain | 58 (24.5) | 44 (17.4) | 71 (28.4) | 44 (18.6) |
| 027 strain | 54 (22.8) | 35 (13.8) | 64 (25.6) | 36 (15.3) |
| Regioni | ||||
| Africa | 0 (0.0) | 5 (1.3) | 0 (0.0) | 2 (0.5) |
| Asia-Pacific | 57 (14.9) | 22 (5.5) | 53 (13.6) | 24 (6.3) |
| Europe | 205 (53.7) | 108 (27.1) | 187 (47.8) | 106 (27.7) |
| Latin America | 21 (5.5) | 9 (2.3) | 26 (6.6) | 9 (2.4) |
| North America | 99 (25.9) | 255 (63.9) | 125 (32.0) | 241 (63.1) |
| . | Bezlotoxumab . | Placebo . | ||
|---|---|---|---|---|
| . | EIA + CCA,No.a (%) . | PCR + Culture,No.a (%) . | EIA + CCA,No.a (%) . | PCR + Culture,No.a (%) . |
| Participants in population | 382 | 399 | 391 | 382 |
| Inpatient | 299 (78.3) | 231 (57.9) | 307 (78.5) | 213 (55.8) |
| Female | 212 (55.5) | 230 (57.6) | 235 (60.1) | 214 (56.0) |
| Mean age (SD), y | 65.7 (16.8) | 58.2 (18.4) | 65.4 (17.9) | 61.5 (16.7) |
| Median | 69 | 60 | 68 | 63 |
| Range | 21–100 | 18–97 | 19–98 | 18–97 |
| Age ≥65 y | 226 (59.2) | 164 (41.1) | 230 (58.8) | 175 (45.8) |
| ≥1 CDI episodes in past 6 mo | 93 (24.3) | 123 (30.8) | 114 (29.2) | 105 (27.5) |
| ≥2 previous CDI episodes ever | 41 (10.9) | 59 (15.0) | 61 (15.8) | 65 (17.6) |
| Severe CDI (Zar score ≥ 2)b | 72 (18.8) | 50 (12.5) | 77 (19.7) | 48 (12.6) |
| Immunocompromisedc | 81 (21.2) | 97 (24.3) | 72 (18.4) | 81 (21.2) |
| Antibiotic used during ADT | 131 (34.3) | 115 (28.8) | 142 (36.3) | 134 (35.1) |
| Antibiotic used after ADT | 131 (34.3) | 115 (28.8) | 110 (28.1) | 114 (29.8) |
| At least 1 of the 5 predefined risk factorse | 304 (79.6) | 288 (72.2) | 313 (80.1) | 270 (70.7) |
| Charlson Comorbidity Index ≥3 | 170 (44.5) | 149 (37.3) | 157 (40.2) | 146 (38.2) |
| Albumin <2.5 g/dL | 63 (16.5) | 38 (9.5) | 58 (14.8) | 45 (11.8) |
| Renal impairmentf | 68 (17.8) | 55 (13.8) | 53 (13.6) | 57 (14.9) |
| Hepatic impairmentg | 30 (7.9) | 19 (4.8) | 24 (6.1) | 20 (5.2) |
| Antibiotic drug treatment for CDI | ||||
| Metronidazole | 191 (50.0) | 188 (47.1) | 193 (49.4) | 181 (47.4) |
| Vancomycin | 176 (46.1) | 196 (49.1) | 187 (47.8) | 186 (48.7) |
| Fidaxomicin | 15 (3.9) | 15 (3.8) | 11 (2.8) | 15 (3.9) |
| PCR ribotypeh | ||||
| Participants with a positive culture | 237 | 253 | 250 | 236 |
| 027, 078, or 244 strain | 58 (24.5) | 44 (17.4) | 71 (28.4) | 44 (18.6) |
| 027 strain | 54 (22.8) | 35 (13.8) | 64 (25.6) | 36 (15.3) |
| Regioni | ||||
| Africa | 0 (0.0) | 5 (1.3) | 0 (0.0) | 2 (0.5) |
| Asia-Pacific | 57 (14.9) | 22 (5.5) | 53 (13.6) | 24 (6.3) |
| Europe | 205 (53.7) | 108 (27.1) | 187 (47.8) | 106 (27.7) |
| Latin America | 21 (5.5) | 9 (2.3) | 26 (6.6) | 9 (2.4) |
| North America | 99 (25.9) | 255 (63.9) | 125 (32.0) | 241 (63.1) |
Abbreviations: ADT, antibacterial drug treatment; ALT, alanine aminotransferase; CDI, Clostridioides difficile infection; CCA, cell cytotoxicity assay; EIA, enzyme immunoassay; mITT, modified intent-to-treat; PCR, polymerase chain reaction; ULN, upper limit of normal; WBC, white blood cell.
aNo. represents the number of participants in the subgroup analysis population meeting the criteria for the end point.
bBased on the following: (1) age >60 years (1 point), (2) body temperature >38.3°C (>100°F; 1 point), (3) albumin level <2.5 g/dL (1 point), (4) peripheral WBC count >15 000 cells/mm3 within 48 hours (1 point), (5) endoscopic evidence of pseudomembranous colitis (2 points), and (6) treatment in an intensive care unit (2 points).
cDefined on the basis of a subject’s medical history or use of immunosuppressive therapy.
dSystemic antibiotic other than ADT given to treat CDI.
ePredefined risk factors include CDI history in the past 6 months, severe CDI at baseline (per Zar score), age ≥65 years, having a hypervirulent strain (027, 078, or 244 ribotypes) at baseline, and immunocompromised.
fRenal impairment defined as serum creatinine ≥1.5 mg/dL.
gHepatic impairment defined by 2 or more of the following: (a) albumin ≤3.1 g/dL, (b) ALT ≥2× ULN, (c) total bilirubin ≥1.3× ULN, or (d) mild, moderate, or severe liver disease (as reported on the Charlson Comorbidity Index).
hDenominator is participants in the mITT population with a positive culture.
iAfrica includes South Africa. Asia-Pacific includes Australia, Japan, Korea, New Zealand, and Taiwan. Latin America includes Argentina, Brazil, Chile, Colombia, and Mexico. Europe includes Austria, Belgium, Czech Republic, Denmark, Finland, France, Germany, Israel, Italy, Poland, Portugal, the Russian Federation, Spain, Sweden, Switzerland, Turkey, and the United Kingdom. North America includes Canada and the United States.
Baseline Demographics and Clinical Characteristics in the mITT Population by Treatment Group and Diagnostic Method
| . | Bezlotoxumab . | Placebo . | ||
|---|---|---|---|---|
| . | EIA + CCA,No.a (%) . | PCR + Culture,No.a (%) . | EIA + CCA,No.a (%) . | PCR + Culture,No.a (%) . |
| Participants in population | 382 | 399 | 391 | 382 |
| Inpatient | 299 (78.3) | 231 (57.9) | 307 (78.5) | 213 (55.8) |
| Female | 212 (55.5) | 230 (57.6) | 235 (60.1) | 214 (56.0) |
| Mean age (SD), y | 65.7 (16.8) | 58.2 (18.4) | 65.4 (17.9) | 61.5 (16.7) |
| Median | 69 | 60 | 68 | 63 |
| Range | 21–100 | 18–97 | 19–98 | 18–97 |
| Age ≥65 y | 226 (59.2) | 164 (41.1) | 230 (58.8) | 175 (45.8) |
| ≥1 CDI episodes in past 6 mo | 93 (24.3) | 123 (30.8) | 114 (29.2) | 105 (27.5) |
| ≥2 previous CDI episodes ever | 41 (10.9) | 59 (15.0) | 61 (15.8) | 65 (17.6) |
| Severe CDI (Zar score ≥ 2)b | 72 (18.8) | 50 (12.5) | 77 (19.7) | 48 (12.6) |
| Immunocompromisedc | 81 (21.2) | 97 (24.3) | 72 (18.4) | 81 (21.2) |
| Antibiotic used during ADT | 131 (34.3) | 115 (28.8) | 142 (36.3) | 134 (35.1) |
| Antibiotic used after ADT | 131 (34.3) | 115 (28.8) | 110 (28.1) | 114 (29.8) |
| At least 1 of the 5 predefined risk factorse | 304 (79.6) | 288 (72.2) | 313 (80.1) | 270 (70.7) |
| Charlson Comorbidity Index ≥3 | 170 (44.5) | 149 (37.3) | 157 (40.2) | 146 (38.2) |
| Albumin <2.5 g/dL | 63 (16.5) | 38 (9.5) | 58 (14.8) | 45 (11.8) |
| Renal impairmentf | 68 (17.8) | 55 (13.8) | 53 (13.6) | 57 (14.9) |
| Hepatic impairmentg | 30 (7.9) | 19 (4.8) | 24 (6.1) | 20 (5.2) |
| Antibiotic drug treatment for CDI | ||||
| Metronidazole | 191 (50.0) | 188 (47.1) | 193 (49.4) | 181 (47.4) |
| Vancomycin | 176 (46.1) | 196 (49.1) | 187 (47.8) | 186 (48.7) |
| Fidaxomicin | 15 (3.9) | 15 (3.8) | 11 (2.8) | 15 (3.9) |
| PCR ribotypeh | ||||
| Participants with a positive culture | 237 | 253 | 250 | 236 |
| 027, 078, or 244 strain | 58 (24.5) | 44 (17.4) | 71 (28.4) | 44 (18.6) |
| 027 strain | 54 (22.8) | 35 (13.8) | 64 (25.6) | 36 (15.3) |
| Regioni | ||||
| Africa | 0 (0.0) | 5 (1.3) | 0 (0.0) | 2 (0.5) |
| Asia-Pacific | 57 (14.9) | 22 (5.5) | 53 (13.6) | 24 (6.3) |
| Europe | 205 (53.7) | 108 (27.1) | 187 (47.8) | 106 (27.7) |
| Latin America | 21 (5.5) | 9 (2.3) | 26 (6.6) | 9 (2.4) |
| North America | 99 (25.9) | 255 (63.9) | 125 (32.0) | 241 (63.1) |
| . | Bezlotoxumab . | Placebo . | ||
|---|---|---|---|---|
| . | EIA + CCA,No.a (%) . | PCR + Culture,No.a (%) . | EIA + CCA,No.a (%) . | PCR + Culture,No.a (%) . |
| Participants in population | 382 | 399 | 391 | 382 |
| Inpatient | 299 (78.3) | 231 (57.9) | 307 (78.5) | 213 (55.8) |
| Female | 212 (55.5) | 230 (57.6) | 235 (60.1) | 214 (56.0) |
| Mean age (SD), y | 65.7 (16.8) | 58.2 (18.4) | 65.4 (17.9) | 61.5 (16.7) |
| Median | 69 | 60 | 68 | 63 |
| Range | 21–100 | 18–97 | 19–98 | 18–97 |
| Age ≥65 y | 226 (59.2) | 164 (41.1) | 230 (58.8) | 175 (45.8) |
| ≥1 CDI episodes in past 6 mo | 93 (24.3) | 123 (30.8) | 114 (29.2) | 105 (27.5) |
| ≥2 previous CDI episodes ever | 41 (10.9) | 59 (15.0) | 61 (15.8) | 65 (17.6) |
| Severe CDI (Zar score ≥ 2)b | 72 (18.8) | 50 (12.5) | 77 (19.7) | 48 (12.6) |
| Immunocompromisedc | 81 (21.2) | 97 (24.3) | 72 (18.4) | 81 (21.2) |
| Antibiotic used during ADT | 131 (34.3) | 115 (28.8) | 142 (36.3) | 134 (35.1) |
| Antibiotic used after ADT | 131 (34.3) | 115 (28.8) | 110 (28.1) | 114 (29.8) |
| At least 1 of the 5 predefined risk factorse | 304 (79.6) | 288 (72.2) | 313 (80.1) | 270 (70.7) |
| Charlson Comorbidity Index ≥3 | 170 (44.5) | 149 (37.3) | 157 (40.2) | 146 (38.2) |
| Albumin <2.5 g/dL | 63 (16.5) | 38 (9.5) | 58 (14.8) | 45 (11.8) |
| Renal impairmentf | 68 (17.8) | 55 (13.8) | 53 (13.6) | 57 (14.9) |
| Hepatic impairmentg | 30 (7.9) | 19 (4.8) | 24 (6.1) | 20 (5.2) |
| Antibiotic drug treatment for CDI | ||||
| Metronidazole | 191 (50.0) | 188 (47.1) | 193 (49.4) | 181 (47.4) |
| Vancomycin | 176 (46.1) | 196 (49.1) | 187 (47.8) | 186 (48.7) |
| Fidaxomicin | 15 (3.9) | 15 (3.8) | 11 (2.8) | 15 (3.9) |
| PCR ribotypeh | ||||
| Participants with a positive culture | 237 | 253 | 250 | 236 |
| 027, 078, or 244 strain | 58 (24.5) | 44 (17.4) | 71 (28.4) | 44 (18.6) |
| 027 strain | 54 (22.8) | 35 (13.8) | 64 (25.6) | 36 (15.3) |
| Regioni | ||||
| Africa | 0 (0.0) | 5 (1.3) | 0 (0.0) | 2 (0.5) |
| Asia-Pacific | 57 (14.9) | 22 (5.5) | 53 (13.6) | 24 (6.3) |
| Europe | 205 (53.7) | 108 (27.1) | 187 (47.8) | 106 (27.7) |
| Latin America | 21 (5.5) | 9 (2.3) | 26 (6.6) | 9 (2.4) |
| North America | 99 (25.9) | 255 (63.9) | 125 (32.0) | 241 (63.1) |
Abbreviations: ADT, antibacterial drug treatment; ALT, alanine aminotransferase; CDI, Clostridioides difficile infection; CCA, cell cytotoxicity assay; EIA, enzyme immunoassay; mITT, modified intent-to-treat; PCR, polymerase chain reaction; ULN, upper limit of normal; WBC, white blood cell.
aNo. represents the number of participants in the subgroup analysis population meeting the criteria for the end point.
bBased on the following: (1) age >60 years (1 point), (2) body temperature >38.3°C (>100°F; 1 point), (3) albumin level <2.5 g/dL (1 point), (4) peripheral WBC count >15 000 cells/mm3 within 48 hours (1 point), (5) endoscopic evidence of pseudomembranous colitis (2 points), and (6) treatment in an intensive care unit (2 points).
cDefined on the basis of a subject’s medical history or use of immunosuppressive therapy.
dSystemic antibiotic other than ADT given to treat CDI.
ePredefined risk factors include CDI history in the past 6 months, severe CDI at baseline (per Zar score), age ≥65 years, having a hypervirulent strain (027, 078, or 244 ribotypes) at baseline, and immunocompromised.
fRenal impairment defined as serum creatinine ≥1.5 mg/dL.
gHepatic impairment defined by 2 or more of the following: (a) albumin ≤3.1 g/dL, (b) ALT ≥2× ULN, (c) total bilirubin ≥1.3× ULN, or (d) mild, moderate, or severe liver disease (as reported on the Charlson Comorbidity Index).
hDenominator is participants in the mITT population with a positive culture.
iAfrica includes South Africa. Asia-Pacific includes Australia, Japan, Korea, New Zealand, and Taiwan. Latin America includes Argentina, Brazil, Chile, Colombia, and Mexico. Europe includes Austria, Belgium, Czech Republic, Denmark, Finland, France, Germany, Israel, Italy, Poland, Portugal, the Russian Federation, Spain, Sweden, Switzerland, Turkey, and the United Kingdom. North America includes Canada and the United States.
A toxin EIA kit was the most commonly used diagnostic test at baseline (48.7% of participants), followed by tgPCR (44.7%), toxigenic culture (5.6%), and CCA (1.1%) (Supplementary Table 1). Direct toxin detection methods (toxin EIA/CCA) were more common in Europe, whereas indirect toxin detection methods (tgPCR/toxigenic culture) were more common in North America (Table 1). Overall, the use of different test methods was balanced across treatment groups (Table 1).
In both treatment groups, a higher proportion of participants diagnosed via toxin EIA/CCA prematurely discontinued from the study (bezlotoxumab, 17.0%; placebo, 18.7%) vs tgPCR/toxigenic culture (bezlotoxumab, 11.5%; placebo, 13.9%). The most frequent reasons for discontinuation were death and participant withdrawal (Supplementary Table 2).
Compared with participants diagnosed via tgPCR/toxigenic culture, a higher proportion of participants diagnosed via toxin EIA/CCA were inpatients at the time of randomization, were older, and had severe CDI (Zar score ≥ 2) or ≥1 of 5 prespecified risk factors for rCDI (Table 1).
Initial Clinical Cure
ICC rates were slightly lower in participants diagnosed via tgPCR/toxigenic culture (bezlotoxumab, 78.4%; placebo, 77.7%) vs toxin EIA/CCA (bezlotoxumab, 81.7%; placebo, 82.9%), with a similar rate between treatment groups (Figure 1).
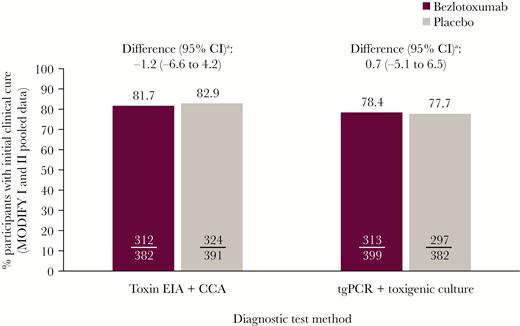
Proportion of participants with initial clinical cure (mITT population). aBased on the Miettinen and Nurminen method. Abbreviations: CCA, cell cytotoxicity assay; CI, confidence interval; EIA, enzyme immunoassay; mITT, modified intent-to-treat; tgPCR, toxin gene polymerase chain reaction.
CDI Recurrence
Compared with placebo, bezlotoxumab significantly reduced the rCDI rate regardless of diagnostic method, with an absolute difference of –15.4% (95% CI, –22.0% to –8.7%) and –9.7% (95% CI, –16.8% to –2.5%) in the toxin EIA/CCA and tgPCR/toxigenic culture subgroups, respectively (Figure 2). Among bezlotoxumab-treated participants, the rCDI rate tended to be lower in participants diagnosed by toxin EIA/CCA (17.6%) vs tgPCR/toxigenic culture (23.6%; absolute difference, –6.0%; 95% CI, –12.4% to 0.3%; relative difference –25.4%). In contrast, rCDI was ~33% in the placebo group regardless of diagnostic method (Figure 2). The relative reduction in rCDI rate for bezlotoxumab- vs placebo-treated participants was higher at 46.6% for toxin EIA/CCA vs 29.1% for tgPCR/toxigenic culture (Figure 3).
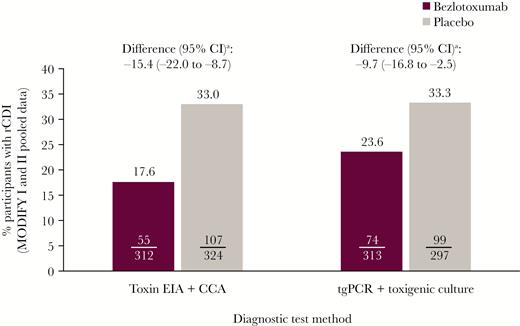
Proportion of participants with rCDI (clinical cure population). aBased on the Miettinen and Nurminen method. Abbreviations: CCA, cell cytotoxicity assay; CI, confidence interval; EIA, enzyme immunoassay; rCDI, recurrent Clostridioides difficile infection; tgPCR, toxin gene polymerase chain reaction.

The proportion of participants who experienced rCDI according to subgroup (clinical cure population; MODIFY I and II pooled data). A, Subgroup results for participants diagnosed by toxin EIA or CCA. B, Subgroup results for participants diagnosed by tgPCR or toxigenic culture. aNo. represents the number of participants in the subgroup analysis population meeting the criteria for the end point; n represents the number of participants within the subgroup. Abbreviations: CCA, cell cytotoxicity assay; CDI, Clostridioides difficile infection; EIA, enzyme immunoassay; rCDI, recurrent Clostridioides difficile infection; tgPCR, toxin gene polymerase chain reaction.
CDI Recurrence Stratified by Baseline Demographic and Clinical Characteristic Subgroups
Across the majority of baseline demographic and clinical characteristic subgroups in the treatment and placebo arms, rCDI rates were lower in participants diagnosed with toxin EIA/CCA (Figure 3A) vs tgPCR/toxigenic culture (Figure 3B). Absolute differences in rCDI between the bezlotoxumab and placebo groups were also larger in participants diagnosed by toxin EIA/CCA in these subgroups; however, the 95% CIs of the difference included 0 in some subgroups (Figure 3). In participants with primary CDI (ie, no CDI in the past 6 months), the absolute reduction in rCDI rate was greater in those diagnosed by toxin EIA/CCA (13.5%) compared with tgPCR/toxigenic culture (5.3%; 95% CIs included 0). A similar trend was observed for inpatients, metronidazole-treated participants, and European participants (Figure 3).
A sensitivity analysis was also conducted that investigated the proportion of participants who experienced diarrhea recurrence irrespective of an association with C. difficile. Here, a similar trend was observed, with lower diarrhea recurrence rates in the toxin EIA/CCA subgroup vs the tgPCR/toxigenic culture subgroup (Supplementary Table 3). Additionally, a higher proportion of participants diagnosed via toxin EIA/CCA achieved sustained cure vs tgPCR/toxigenic culture (Supplementary Table 4).
Assay Interference Experiment
Compared with control samples incubated without bezlotoxumab, incubation of fecal samples with the median observed stool concentration of bezlotoxumab (528.0 ng/mL) (Supplementary Data) did not change diagnostic toxin EIA or CCA results (Supplementary Table 5). Compared with control samples, incubation of samples with higher bezlotoxumab concentrations (10× median, 100× median) had no effect on diagnostic EIA results, but changed the diagnostic result from positive to negative in some samples tested by CCA (Supplementary Table 5).
Other Outcomes
In participants in both treatment groups who were inpatients at the time of randomization, the all-cause rehospitalization rate was higher in those diagnosed with tgPCR/toxigenic culture vs toxin EIA/CCA (Table 2). In contrast, in both treatment groups, there was a trend for higher CDI-associated rehospitalization rates in participants diagnosed with toxin EIA/CCA vs tgPCR/toxigenic culture (Table 2). Similarly, there was a trend for higher mortality in participants diagnosed with toxin EIA/CCA vs tgPCR/toxigenic culture in both treatment groups (Table 2). Compared with the bezlotoxumab group, a higher proportion of placebo-treated participants had an FMT procedure during the follow-up period, regardless of diagnostic method (Table 2).
Proportion of Participants Who Experienced Rehospitalization, FMT, or Mortality (MODIFY I and II Pooled Data)
| . | Bezlotoxumab . | Placebo . | ||
|---|---|---|---|---|
| . | Toxin EIA + CCA,a No./nb (%) . | tgPCR + Culture,a No./nb (%) . | Toxin EIA + CCA,a No./nb (%) . | tgPCR + Culture,a No./nb (%) . |
| Rehospitalizationc | ||||
| Any | 63/299 (21.1) | 60/231 (26.0) | 80/307 (26.1) | 60/213 (28.2) |
| Associated with CDId | 16/299 (5.4) | 11/231 (4.8) | 39/307 (12.7) | 19/213 (8.9) |
| Received an FMTe | 2/382 (0.5) | 5/399 (1.3) | 13/391 (3.3) | 10/382 (2.6) |
| Mortalityf | ||||
| 30-d mortality | 17/382 (4.5) | 10/404 (2.5) | 15/394 (3.8) | 12/387 (3.1) |
| 90-d mortality | 35/382 (9.2) | 19/404 (4.7) | 35/394 (8.9) | 24/387 (6.2) |
| . | Bezlotoxumab . | Placebo . | ||
|---|---|---|---|---|
| . | Toxin EIA + CCA,a No./nb (%) . | tgPCR + Culture,a No./nb (%) . | Toxin EIA + CCA,a No./nb (%) . | tgPCR + Culture,a No./nb (%) . |
| Rehospitalizationc | ||||
| Any | 63/299 (21.1) | 60/231 (26.0) | 80/307 (26.1) | 60/213 (28.2) |
| Associated with CDId | 16/299 (5.4) | 11/231 (4.8) | 39/307 (12.7) | 19/213 (8.9) |
| Received an FMTe | 2/382 (0.5) | 5/399 (1.3) | 13/391 (3.3) | 10/382 (2.6) |
| Mortalityf | ||||
| 30-d mortality | 17/382 (4.5) | 10/404 (2.5) | 15/394 (3.8) | 12/387 (3.1) |
| 90-d mortality | 35/382 (9.2) | 19/404 (4.7) | 35/394 (8.9) | 24/387 (6.2) |
Abbreviations: CCA, cell cytotoxicity assay; CDI, Clostridioides difficile infection; EIA, enzyme immunoassay; FMT, fecal microbiota transplant; mITT, modified intent-to-treat; rCDI, recurrent Clostridioides difficile infection; tgPCR, toxin gene polymerase chain reaction.
aDiagnostic subgroups are based on the test method used to diagnose the baseline CDI episode.
bNo. represents the number of participants in the subgroup analysis population meeting the criteria for the end point; n represents the number of participants within the subgroup.
cmITT population who were inpatients at the time of randomization.
dCDI-associated rehospitalization was defined as a 30-day readmission based on ≥1 of the following: readmission that occurred within 5 days after onset of a new CDI episode, onset of a new CDI episode during readmission, or the inclusion of terms synonymous with CDI, rCDI, or pseudomembranous colitis in the discharge diagnosis, as recorded on the trial case report form [22].
emITT population.
fAll participants as treated population.
Proportion of Participants Who Experienced Rehospitalization, FMT, or Mortality (MODIFY I and II Pooled Data)
| . | Bezlotoxumab . | Placebo . | ||
|---|---|---|---|---|
| . | Toxin EIA + CCA,a No./nb (%) . | tgPCR + Culture,a No./nb (%) . | Toxin EIA + CCA,a No./nb (%) . | tgPCR + Culture,a No./nb (%) . |
| Rehospitalizationc | ||||
| Any | 63/299 (21.1) | 60/231 (26.0) | 80/307 (26.1) | 60/213 (28.2) |
| Associated with CDId | 16/299 (5.4) | 11/231 (4.8) | 39/307 (12.7) | 19/213 (8.9) |
| Received an FMTe | 2/382 (0.5) | 5/399 (1.3) | 13/391 (3.3) | 10/382 (2.6) |
| Mortalityf | ||||
| 30-d mortality | 17/382 (4.5) | 10/404 (2.5) | 15/394 (3.8) | 12/387 (3.1) |
| 90-d mortality | 35/382 (9.2) | 19/404 (4.7) | 35/394 (8.9) | 24/387 (6.2) |
| . | Bezlotoxumab . | Placebo . | ||
|---|---|---|---|---|
| . | Toxin EIA + CCA,a No./nb (%) . | tgPCR + Culture,a No./nb (%) . | Toxin EIA + CCA,a No./nb (%) . | tgPCR + Culture,a No./nb (%) . |
| Rehospitalizationc | ||||
| Any | 63/299 (21.1) | 60/231 (26.0) | 80/307 (26.1) | 60/213 (28.2) |
| Associated with CDId | 16/299 (5.4) | 11/231 (4.8) | 39/307 (12.7) | 19/213 (8.9) |
| Received an FMTe | 2/382 (0.5) | 5/399 (1.3) | 13/391 (3.3) | 10/382 (2.6) |
| Mortalityf | ||||
| 30-d mortality | 17/382 (4.5) | 10/404 (2.5) | 15/394 (3.8) | 12/387 (3.1) |
| 90-d mortality | 35/382 (9.2) | 19/404 (4.7) | 35/394 (8.9) | 24/387 (6.2) |
Abbreviations: CCA, cell cytotoxicity assay; CDI, Clostridioides difficile infection; EIA, enzyme immunoassay; FMT, fecal microbiota transplant; mITT, modified intent-to-treat; rCDI, recurrent Clostridioides difficile infection; tgPCR, toxin gene polymerase chain reaction.
aDiagnostic subgroups are based on the test method used to diagnose the baseline CDI episode.
bNo. represents the number of participants in the subgroup analysis population meeting the criteria for the end point; n represents the number of participants within the subgroup.
cmITT population who were inpatients at the time of randomization.
dCDI-associated rehospitalization was defined as a 30-day readmission based on ≥1 of the following: readmission that occurred within 5 days after onset of a new CDI episode, onset of a new CDI episode during readmission, or the inclusion of terms synonymous with CDI, rCDI, or pseudomembranous colitis in the discharge diagnosis, as recorded on the trial case report form [22].
emITT population.
fAll participants as treated population.
DISCUSSION
CDI diagnosis is based on clinical presentation and laboratory testing, although the methodology for the latter remains controversial. Increasing evidence supports the higher predictive value of toxin detection rather than the presence of a strain with the capacity to produce toxin (ie, toxigenic culture or toxin gene detection) [14, 23]. As several different test methods were used to diagnose CDI in MODIFY I/II, it was important to establish if this had an effect on the measured efficacy of bezlotoxumab.
In these 2 trials, almost equal proportions of participants in the bezlotoxumab and placebo groups were diagnosed via tgPCR/toxigenic culture vs toxin EIA/CCA, which reflects the diagnostic variability at the time of trial design [14].
Our post hoc analysis demonstrates that bezlotoxumab was associated with clinically meaningful reductions in rCDI rates compared with placebo, regardless of the diagnostic method used; however, the magnitude of reduction of rCDI associated with bezlotoxumab was substantially higher among participants diagnosed by toxin EIA/CCA vs tgPCR/toxigenic culture. Notably, a similar trend in rCDI reduction was also observed across subgroup and sensitivity analyses. Furthermore, results from the assay interference experiment suggest that the trend in rCDI reduction is a true result rather than an artefact present due to falsely negative toxin EIA/CCA results, as exposure of fecal samples to physiological levels of bezlotoxumab generally had no effect on the results derived by either diagnostic test. As the bezlotoxumab stock had to be diluted to meet the fecal concentrations required, the change in diagnostic CCA results from positive to negative observed in some samples may have been due to a dilution effect.
ICC rates also tended to be slightly higher in participants diagnosed with toxin EIA/CCA compared with tgPCR/toxigenic culture, which was evident in both the bezlotoxumab and placebo groups. This is plausibly because participants diagnosed via toxin EIA/CCA were more likely to have CDI-related diarrhea, and thus to have a better response to CDI treatment compared with participants diagnosed by tgPCR/toxigenic culture (who may not have had true CDI) [16]. The larger reduction in rCDI could be partly explained by the difference in baseline characteristics, as there were more inpatients, older participants, and severe CDI cases in the toxin EIA/CCA subgroup vs the tgPCR/toxigenic culture subgroup at baseline; however, this is unlikely, as a lower reduction in rCDI in older participants with severe CDI would be expected.
In addition, a higher proportion of participants diagnosed via toxin EIA/CCA, vs tgPCR/toxigenic culture, prematurely discontinued from the study. There was also a trend for higher mortality rates in participants diagnosed by toxin EIA/CCA compared with tgPCR/toxigenic culture. Similarly, this could be partly influenced by the difference in baseline participant characteristics.
Our findings are consistent with previous post hoc clinical trial analyses, which have also suggested that CDI treatment is less efficacious in PCR-diagnosed individuals compared with those diagnosed using a toxin-based test [17–19]. Furthermore, our findings are consistent with recent data suggesting a correlation between the presence of fecal C. difficile toxins and true CDI. Planche and colleagues previously reported that mortality rates were significantly higher in individuals who were confirmed to be toxin-positive, compared with those who were toxin-negative, but had a positive toxigenic culture test result [15]. Similarly, in another study of hospitalized patients with suspected CDI, virtually all CDI-related complications and deaths occurred in toxin-positive individuals [16].
It has been speculated that exclusive reliance on diagnostic tests that only detect toxigenic C. difficile strains may lead to over-reporting of CDI cases. Indeed, a number of studies have shown that performing tgPCR instead of toxin EIA or CCA to diagnose CDI can lead to increases of ~50% in CDI rates [24–26]. In another study, the use of tgPCR vs toxin EIA was associated with inadequacies in the performance tool used to assess institutional CDI rates. This was due to a failure in the risk-adjustment approach, designed to correct for the use of different diagnostic test methods between institutions, which could lead to false reporting of high CDI rates in some institutions [27].
Notably, almost half of the participants enrolled in MODIFY I/II were not diagnosed using a toxin-based test; therefore, some of these participants may have had an alternate cause of diarrhea. This likely explains why bezlotoxumab was associated with a lower magnitude of reduction in rCDI rate in participants diagnosed by tgPCR/toxigenic culture.
Although recent treatment guidelines acknowledge the diagnostic concern regarding molecular tests that only detect the presence of C. difficile toxin genes, the choice of tgPCR and toxin-based testing is still included in their recommendations [28, 29]. This study provides important, therapy-based data on the most appropriate method of diagnosing true CDI. The results presented here support the fundamental importance of fecal C. difficile toxin detection in accurately determining if CDI is truly present and, therefore, identifying patients with treatment-responsive disease. This has multiple practical implications, notably regarding optimal patient management and design of clinical trials for new therapies.
The results presented here also suggest that the efficacy of bezlotoxumab in preventing rCDI may be higher than first reported. In the primary analysis of MODIFY I/II in the overall population, bezlotoxumab was associated with absolute rCDI rate reductions of 12.2% and 10.0% in the clinical cure and mITT populations, respectively, compared with placebo [13]. Here, we demonstrate that in the toxin EIA/CCA subgroup, bezlotoxumab was associated with an absolute reduction of 15.4% compared with placebo. Furthermore, the efficacy of bezlotoxumab at preventing rCDI in high-risk subgroups could also have been previously under-reported. Indeed, a previous analysis of MODIFY I/II showed that, compared with placebo, bezlotoxumab was associated with an absolute reduction of 15.9% in participants with ≥1 risk factor for CDI [30]. Here, we demonstrate that in participants with ≥1 risk factor who were diagnosed by toxin EIA/CCA, bezlotoxumab was associated with an absolute reduction of 18.5% compared with placebo. These results provide additional evidence to suggest that individuals with ≥1 risk factor for rCDI may benefit from bezlotoxumab, adding further clarification on which individuals may be suitable for bezlotoxumab treatment.
Study limitations include the fact that this was a post hoc analysis; the study was not designed to evaluate the impact of diagnostic method on the measured efficacy of bezlotoxumab. As such, the results can only be interpreted as trends rather than conclusive. In addition, multiple factors may have contributed to the observed difference in efficacy between diagnostic subgroups. Toxin-based testing was not performed in participants diagnosed via tgPCR/toxigenic culture; therefore, it is unknown if these participants had C. difficile toxins present in their stool. As rCDI episodes were confirmed using either direct C. difficile toxin detection methods (EIA/CCA) or indirect methods to determine toxin-producing ability (tgPCR/toxigenic culture), it is possible that some participants did not have true rCDI and thus had an alternative cause of diarrhea recurrence. In conclusion, the choice of diagnostic test is fundamentally important in making an accurate diagnosis of CDI, as demonstrated here in clinical trial design, to ensure that therapeutic efficacy is not underestimated.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Medical writing assistance, under the direction of the authors, was provided by Hannah Logan, PhD, of CMC AFFINITY, a division of McCann Health Medical Communications Ltd, Manchester, UK, in accordance with Good Publication Practice (GPP3) guidelines. This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey.
Financial support. This work was supported by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey.
Potential conflicts of interest. L.G., K.E., M.C.E., D.G., and M.B.D. are current employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, and may own stock and/or stock options. M.H.W. has received consulting fees from Actelion, Astellas, bioMerieux, MedImmune, MSD, Pfizer, Sanofi-Pasteur, Seres, Summit, Synthetic Biologics, and Valneva, lecture fees from Alere, Astellas, MSD, and Pfizer, and grant support from Actelion, Astellas, bioMerieux, Da Volterra, MSD, and Summit. G.R. is a consultant and scientific advisor to AstraZeneca, MSD, and Pfizer. E.R.D. has conducted research for MSD, Pfizer, Rebiotix, and Sanofi-Pasteur and is or has been a consultant to Daiichi, MSD, Pfizer, Rebiotix, Sanofi-Pasteur, and Summit. K.D. has received honoraria from Astellas Pharma Europe and Cepheid Inc. and grant support from Astellas Pharma Europe, bioMerieux, Pfizer, and Sanofi-Pasteur. C.B. has no disclosures. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. M.H.W., L.G., K.D., K.E., and M.B.D. were involved in the design of this analysis. G.R., E.R.D., L.G., C.B., K.E., D.G., and M.B.D. were involved in collecting the data. M.H.W., K.D., C.B., M.C.E., and M.B.D. were involved in the analysis of the data. M.H.W., G.R., E.R.D., D.G., and M.B.D. were involved in interpreting the results. M.H.W. was involved in the provision of study materials or participants. All authors were involved in drafting and revising this manuscript and provided final approval of the version to be published. All authors vouch for the accuracy of the content included in the final manuscript.
References





Comments