-
PDF
- Split View
-
Views
-
Cite
Cite
Achim J Kaasch, Winfried V Kern, Insa Joost, Martin Hellmich, Harald Seifert, Siegbert Rieg, Effect of Clinically Uninfected Orthopedic Implants and Pacemakers/AICDs in Low-Risk Staphylococcus aureus Bloodstream Infection on Crude Mortality Rate: A Post Hoc Analysis of a Large Cohort Study, Open Forum Infectious Diseases, Volume 6, Issue 5, May 2019, ofz170, https://doi.org/10.1093/ofid/ofz170
Close - Share Icon Share
Abstract
The standard treatment duration in low-risk Staphylococcus aureus bloodstream (SAB) is 14 days. However, it is unclear whether an extended course of antimicrobial therapy is necessary in patients with clinically uninfected prosthetic joints/osteosyntheses or pacemakers/automated implanted cardioverter-defibrillators (AICDs). Thus, we compared the duration of antimicrobial therapy and outcomes in patients with and those without clinically uninfected foreign bodies.
We conducted a post hoc analysis of data from the prospective Invasive Staphylococcus aureus Infection Cohort (INSTINCT) study. Adult low-risk patients who survived ≥4 days were assessed for duration of treatment, SAB-related events (attributable death, relapse, or new deep-seated infection), and survival.
Of the 1288 patients enrolled, 292 satisfied criteria for low-risk SAB. Forty-three patients (15%) had a clinically uninfected pacemaker/AICD or orthopedic implant. Patients with foreign bodies were significantly older (mean age, 72 vs 62 years for those without; P < .001; P = .9) and had a higher Charlson score (median, 3 vs 2; P = .06). The total duration of antimicrobial therapy (median, 18 vs 17 days, respectively; P = .7), all-cause mortality rate (16% vs 14%; P = .7), and prevalence of SAB-related events within 90 days were similar (2% vs 2%) in the 2 groups. At 1-year follow-up, SAB-related events were more frequent in patients with foreign bodies (7% vs 4% in those without; P = .4) (hazard ratio, 1.41; 95% confidence interval, .35–5.69; in a multivariable Cox model), but this difference was not statistically significant.
Low-risk patients with clinically uninfected foreign bodies received a similar duration of antimicrobial therapy without a significant impact on mortality rate. The observed higher hazard ratio of SAB-related events within 1 year necessitates additional studies before recommendations concerning treatment duration in this patient subgroup can be adapted or modified.
Staphylococcus aureus bloodstream (SAB) infection causes much disease and death worldwide, with an approximate incidence of 25 cases per 100 000 person-years in Europe [1, 2]. In about 80% of patients, a causative infective focus can be identified with catheter infection, skin and soft-tissue infection, infective endocarditis, pneumonia, and osteoarticular infection occurring most often [3]. Treatment can be difficult, especially because S. aureus has a propensity to cause relapse, local extension, and distant metastatic foci. Such a complicated course of SAB occurs in 2%–25% of patients [4–6], and intravenous antimicrobial therapy for ≥14 days is recommended to prevent these complications [7]. In certain clinical scenarios (eg, deep-seated foci, foreign body infection, and endocarditis), a longer treatment course of ≥4 weeks is recommended [7–9].
In the ongoing interventional SABATO study (Staphylococcus aureus bacteremia antibiotic treatment option clincaltrials.gov identifier: NCT01792804) we are currently testing whether switching to oral therapy after 7 days is as safe and effective as the standard intravenous 14-day regimen [10]. For the purpose of the trial, we defined a group of patients with SAB in whom we expected a low risk for a complicated clinical course. We reasoned that in these patients a 14-day course of intravenous therapy is likely to be sufficient. Exclusion criteria were a deep-seated infective focus, certain comorbid conditions (end-stage renal disease, severe liver disease, and injection drug use), presence of nonremovable foreign bodies or late removal, immunosuppression, and a delayed response to therapy. Some of these criteria—namely, infective focus, immunosuppression, and response to therapy—directly influence outcome. Less clear is the role of comorbid conditions and clinically uninfected foreign bodies.
Many patients with SAB carry foreign bodies, the most frequent being orthopedic implants (eg, prosthetic joints and osteosynthetic material) and pacemakers/automated implanted cardioverter-defibrillators (AICDs). These foreign bodies carry a significant risk of hematogenous infection. For example, in patients with SAB with prosthetic joints, the incidence of a prosthetic joint infection caused by hematogenous seeding (and not by direct inoculation or contiguous spread of infection) has been reported to be 34%–41% [11–13]. Whereas removal of infected foreign bodies is generally recommended [9], it is unclear, whether foreign bodies without signs of infection (called “clinically uninfected”) pose a risk to the patient and warrant device removal or a longer treatment schedule.
To evaluate our daily practice and to explore whether clinically uninfected foreign bodies pose a risk to patient outcome, we conducted a post hoc analysis of a large cohort study. Specifically, we tested whether the presence of orthopedic implants/osteosyntheses and pacemakers/AICDs are associated with longer treatment courses and/or an adverse outcome.
MATERIALS AND METHODS
Study Design and Setting
Patient data was analyzed post hoc from the prospective cohort study Invasive Staphylococcus aureus Infection Cohort (INSTINCT; registered as DRKS00005045) [14]. The study was conducted at 2 German tertiary care university hospitals in Cologne and Freiburg from 1 January 2006 to 31 December 2011. The study was approved by the institutional review board of each institution, and the ethical standards set by the Helsinki Declaration of 1975, as revised in 2004, were followed up. Written informed consent was obtained from all patients in 1 center and waived by the ethics committee in the other.
Study Population
Clinical data were collected from patients ≥18 years of age with ≥1 blood culture positive for S. aureus and with signs of infection. To select a low-risk group, the following exclusion criteria were applied: polymicrobial bacteremia, defined as isolation of a second significant bacterial pathogen from the blood culture; an episode of SAB in the previous 3 months; a deep-seated focus diagnosed within 14 days; ongoing septic shock or temperature >38.5°C on day 7 after onset; persistent bacteremia, defined as blood cultures positive for S. aureus >96 hours after the start of adequate antimicrobial therapy; presence of a prosthetic heart valve, vascular graft, ventriculoatrial shunt, or ventricular assist device; failure to remove an intravascular catheter present at onset within 96 hours; neutropenia ongoing on day 7; solid organ transplant or stem cell transplant; death within 14 days; end-stage renal disease; moderate to severe liver disease; an end-of-life pathway that prevented treatment or diagnostic procedures for SAB; and a life expectancy of <3 months due to comorbid conditions (McCabe score, “rapidly fatal”).
Data Sources and Collected Data
Data was prospectively collected by study personnel during patient visits, from patient charts, from other healthcare providers, and through telephone calls. The following variables were collected: baseline patient characteristics, S. aureus susceptibility testing results, duration of hospital stay, infective focus, diagnostic procedures (eg, echocardiography and other imaging studies), clinical signs of infective endocarditis, presence of prosthetic material (eg, prosthetic joint, osteosynthetic material, prosthetic heart valve, pacemaker, cardioverter, and vascular graft), results of follow-up blood cultures, comorbid conditions (eg, diabetes, immunosuppression, renal disease, and hemodialysis dependency), antimicrobial treatment, and attributable deaths. Patients were followed up for ≥12 months for relapse and deep-seated infection.
Definitions
The onset of bacteremia was defined as the time when the first positive blood culture was obtained. Patients were grouped as having community-acquired, healthcare-associated, or hospital-acquired SAB, according to the definitions by Friedman et al [15]. The burden of comorbid conditions was assessed with the Charlson comorbidity index [16], and disease severity at onset was determined using the acute physiology score portion of the Acute Physiological and Chronic Disease Health Evaluation II (APACHE II) score [17]. The infective focus was identified based on clinical signs, microbiological findings, and imaging results. Catheter-related SAB was defined as described elsewhere [18]. A deep-seated focus was defined as clinical signs and symptoms and/or imaging or microbiological proof of a deep-seated infection within 14 days. The following foci were considered deep seated: CNS infection, organ abscess, epidural abscess, infected deep-seated hematoma, infected thrombus, infective endocarditis, intraabdominal abscess, intramuscular abscess, intravascular implant infection, mycotic aneurysm, orthopedic implant infection, osteomyelitis, pacemaker/AICD electrode or pocket infection, peritonitis, pleural empyema, pneumonia, suppurative arthritis, and vertebral osteomyelitis. Echocardiography was considered if it was performed between 3 days before and 14 days after the first positive blood culture.
Pacemakers/AICDs and prosthetic joints/osteosyntheses were considered uninfected if there were no clinical signs (eg, new-onset local tenderness, pain, swelling, reddening, warmth, joint effusion, or any other signs of local inflammation) or imaging signs of local infection. Attributable deaths were defined as those with blood culture positive for S. aureus, persistent focus of staphylococcal infection, or persistent signs and symptoms of systemic infection at the time of death. Relapse was defined as a second occurrence of S. aureus bacteremia or isolation of S. aureus from a sterile body site after completion of a treatment schedule. Newly occurring skin and soft-tissue infection and catheter-related infection were not considered relapse. SAB-related events were defined as attributable death, relapse, or a new deep-seated infection with S. aureus.
Statistical Analysis
Data were recorded as numbers and percentages for qualitative variables and as means and quartiles (25th, 50th, and 75th percentiles) for quantitative data. Two-tailed P values were extracted by means of logistic regression using a univariable generalized linear model, and a differences were considered statistically significant at P < .05. Survival was described according to Kaplan-Meier estimates, supplemented by numbers of patients at risk and log-rank test results. A multivariable Cox regression model with prespecified variables was used to calculate hazard ratios. Statistical analysis was performed using R software, version 3.3.1 (R Foundation for Statistical Computing).
RESULTS
In the INSTINCT study, 1288 adult patients with SAB were enrolled between January 2006 and December 2013. Overall, 119 patients (9%) had a pacemaker/AICD, and 134 (10%) had an orthopedic implant or osteosynthetic material; 907 patients (70%) did not fulfill the criteria for low-risk SAB and were excluded (Figure 1). Among the excluded patients, 96 patients had a pacemaker/AICD, and 37 (41%) of them had a pacemaker/AICD infection. Also excluded were 110 patients (12%) with orthopedic implants or osteosynthetic material, of whom 31 (15%) had an infection of the orthopedic implant/osteosynthetic material. In 20 of these patients, hematogenous seeding to the implant was assumed.
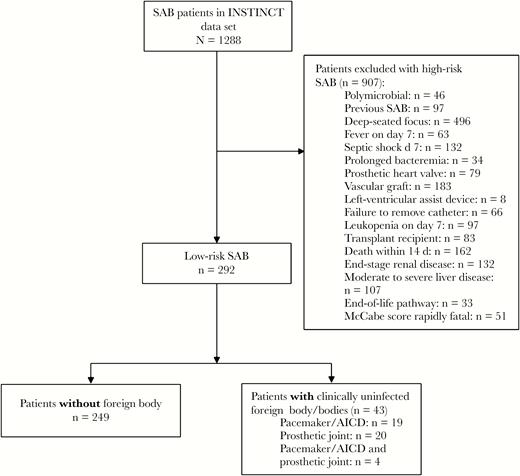
Analysis flow chart. Abbreviations: AICD, automated implanted cardioverter-defibrillator; INSTINCT, Invasive Staphylococcus aureus Infection Cohort; SAB, Staphylococcus aureus bloodstream.
We further analyzed 292 patients (23%) who were classified as having low-risk SAB. Low-risk SAB was mostly healthcare associated (272 patients [93%]). An infective focus was identified in 221 patients (76%); 149 (51%) had catheter-related infection, and 65 (22%) had skin soft-tissue infection (including surgical wound infection). A clinically uninfected foreign body was present in 43 patients (15%), a pacemaker/AICD and an orthopedic implant in 4 patients, a pacemaker/AICD in 23, a hip prosthesis in 15, foreign bodies in the spinal column in 4, knee implants in 3, a shoulder implant in 1, and an intramedullary femur nail and a plate osteosynthesis in 1.
Characteristics of patients with or without clinically uninfected foreign bodies are shown in Table 1. Low-risk patients with clinically uninfected foreign bodies were significantly older than those without foreign bodies (mean, 72 vs 62 years) and were more likely to undergo echocardiography (odds ratio [OR], 2.56; 95% confidence interval [CI], 1.30–5.30). Although the difference was not statistically significant, patients with foreign bodies had slightly more comorbid conditions, as expressed by the Charlson weighted comorbidity index (OR, 1.15; 95% CI, .99–1.35), but severity of illness at onset of infection, as measured by the acute physiology score, was similar in both groups (1.05; .98–1.12). The difference in Charlson weighted comorbidity index was most pronounced in patients with a pacemaker/AICD, who were predominantly male (Supplementary Table 1).
Characteristics of Low-Risk Patients With or Without Clinically Uninfected Foreign Bodies (Pacemaker/AICD or Orthopedic Implant)
| Characteristic . | Low-Risk Patients, No. (%)a . | . | . | OR (95% CI)b . | P Valuec . |
|---|---|---|---|---|---|
| . | Total (n = 292) . | Without Foreign Body (n = 249) . | With Foreign Body (n = 43) . | . | . |
| Age, mean; median (IQR) | 64; 67 (55–75) | 62; 65 (53–74) | 72; 75 (68–79) | 1.67 (1.28–2.25) per decade | <.001 |
| Male sex | 205 (70) | 173 (70) | 32 (74) | 1.28 (.63–2.78) | .5 |
| Study Center 1 | 178 (61) | 153 (61) | 25 (58) | 0.87 (.45–1.70) | .7 |
| MRSA | 30 (10) | 27 (11) | 3 (7) | 0.61 (.14–1.85) | .4 |
| Mode of acquisition | |||||
| Nosocomial | 231 (79) | 196 (79) | 35 (81) | Reference | … |
| Community acquired | |||||
| Healthcare associated | 41 (14) | 34 (14) | 7 (16) | 1.15 (.44–2.68) | .8 |
| Not healthcare associated | 20 (7) | 19 (8) | 1 (2) | 0.29 (.016–1.49) | .2 |
| Charlson score, mean; median (IQR) | 2.6; 2 (1–4) | 2.5; 2 (1–4) | 3.2; 3 (1–5) | 1.15 (.99–1.35) | .06 |
| APS score at onset, mean; median (IQR) | 5.9; 5 (3–8) | 5.8; 5 (3–8) | 6.8; 6 (4–10) | 1.05 (.98–1.12) | .2 |
| Focus | |||||
| Catheter relatedd | 149 (51) | 126 (51) | 23 (54) | Reference | … |
| SSTI, including surgical wounds | 65 (22) | 56 (23) | 9 (21) | 0.88 (.37–1.97) | .8 |
| Urogenital tract | 7 (2) | 6 (2) | 1 (2) | 0.91 (.05–5.70) | .9 |
| Not identified | 71 (24) | 61 (25) | 10 (23) | 0.90 (.39–1.96) | .8 |
| Echocardiography 14 days after positive blood culture | 148 (51) | 118 (47) | 30 (70) | 2.56 (1.30–5.30) | .008 |
| Duration of antimicrobial therapy, mean; median (IQR) | |||||
| In hospital: intravenous | 14.3; 14 (9–17) | 14.0; 14 (9–17) | 16.3; 14 (10–23) | 1.03 (.99–1.07) | .09 |
| In hospital: intravenous + oral | 15.1; 14 (10–18) | 14.8; 14 (10–17) | 16.8; 14 (11–24) | 1.02 (.99–1.06) | .2 |
| Total: in hospital and after discharge | 25.8; 17 (13–29) | 26.2; 17 (13–31) | 23.7; 18 (14–28) | 0.99 (.96–1.00) | .5 |
| Receiving >5 d of antimicrobial combination therapy | 47 (16) | 36 (15) | 11 (26) | 2.03 (.91–4.3) | .07 |
| Length of hospital stay, mean; median (IQR) | 18.4; 15 (10–24) | 18.3; 14 (9–23) | 19.1; 17 (11–25 | 1.00 (.98–1.02) | .7 |
| Characteristic . | Low-Risk Patients, No. (%)a . | . | . | OR (95% CI)b . | P Valuec . |
|---|---|---|---|---|---|
| . | Total (n = 292) . | Without Foreign Body (n = 249) . | With Foreign Body (n = 43) . | . | . |
| Age, mean; median (IQR) | 64; 67 (55–75) | 62; 65 (53–74) | 72; 75 (68–79) | 1.67 (1.28–2.25) per decade | <.001 |
| Male sex | 205 (70) | 173 (70) | 32 (74) | 1.28 (.63–2.78) | .5 |
| Study Center 1 | 178 (61) | 153 (61) | 25 (58) | 0.87 (.45–1.70) | .7 |
| MRSA | 30 (10) | 27 (11) | 3 (7) | 0.61 (.14–1.85) | .4 |
| Mode of acquisition | |||||
| Nosocomial | 231 (79) | 196 (79) | 35 (81) | Reference | … |
| Community acquired | |||||
| Healthcare associated | 41 (14) | 34 (14) | 7 (16) | 1.15 (.44–2.68) | .8 |
| Not healthcare associated | 20 (7) | 19 (8) | 1 (2) | 0.29 (.016–1.49) | .2 |
| Charlson score, mean; median (IQR) | 2.6; 2 (1–4) | 2.5; 2 (1–4) | 3.2; 3 (1–5) | 1.15 (.99–1.35) | .06 |
| APS score at onset, mean; median (IQR) | 5.9; 5 (3–8) | 5.8; 5 (3–8) | 6.8; 6 (4–10) | 1.05 (.98–1.12) | .2 |
| Focus | |||||
| Catheter relatedd | 149 (51) | 126 (51) | 23 (54) | Reference | … |
| SSTI, including surgical wounds | 65 (22) | 56 (23) | 9 (21) | 0.88 (.37–1.97) | .8 |
| Urogenital tract | 7 (2) | 6 (2) | 1 (2) | 0.91 (.05–5.70) | .9 |
| Not identified | 71 (24) | 61 (25) | 10 (23) | 0.90 (.39–1.96) | .8 |
| Echocardiography 14 days after positive blood culture | 148 (51) | 118 (47) | 30 (70) | 2.56 (1.30–5.30) | .008 |
| Duration of antimicrobial therapy, mean; median (IQR) | |||||
| In hospital: intravenous | 14.3; 14 (9–17) | 14.0; 14 (9–17) | 16.3; 14 (10–23) | 1.03 (.99–1.07) | .09 |
| In hospital: intravenous + oral | 15.1; 14 (10–18) | 14.8; 14 (10–17) | 16.8; 14 (11–24) | 1.02 (.99–1.06) | .2 |
| Total: in hospital and after discharge | 25.8; 17 (13–29) | 26.2; 17 (13–31) | 23.7; 18 (14–28) | 0.99 (.96–1.00) | .5 |
| Receiving >5 d of antimicrobial combination therapy | 47 (16) | 36 (15) | 11 (26) | 2.03 (.91–4.3) | .07 |
| Length of hospital stay, mean; median (IQR) | 18.4; 15 (10–24) | 18.3; 14 (9–23) | 19.1; 17 (11–25 | 1.00 (.98–1.02) | .7 |
Abbreviations: APS, acute physiology score; CI, confidence interval; IQR, interquartile range; MRSA, methicillin-resistant Staphylococcus aureus; OR, odds ratio; SSTI, skin and soft-tissue infection.
aData represent no. (%) of patients unless otherwise specified.
bORs represent the risk of carrying a foreign body.
cP values based on Fisher exact or Student t tests.
dIncluding peripheral venous catheters (n = 78), central venous catheters (n = 48), short-term central catheters for hemodialysis (n = 13), peripheral arterial catheters (n = 5), port catheters (n = 4), and implanted catheter (n = 1).
Characteristics of Low-Risk Patients With or Without Clinically Uninfected Foreign Bodies (Pacemaker/AICD or Orthopedic Implant)
| Characteristic . | Low-Risk Patients, No. (%)a . | . | . | OR (95% CI)b . | P Valuec . |
|---|---|---|---|---|---|
| . | Total (n = 292) . | Without Foreign Body (n = 249) . | With Foreign Body (n = 43) . | . | . |
| Age, mean; median (IQR) | 64; 67 (55–75) | 62; 65 (53–74) | 72; 75 (68–79) | 1.67 (1.28–2.25) per decade | <.001 |
| Male sex | 205 (70) | 173 (70) | 32 (74) | 1.28 (.63–2.78) | .5 |
| Study Center 1 | 178 (61) | 153 (61) | 25 (58) | 0.87 (.45–1.70) | .7 |
| MRSA | 30 (10) | 27 (11) | 3 (7) | 0.61 (.14–1.85) | .4 |
| Mode of acquisition | |||||
| Nosocomial | 231 (79) | 196 (79) | 35 (81) | Reference | … |
| Community acquired | |||||
| Healthcare associated | 41 (14) | 34 (14) | 7 (16) | 1.15 (.44–2.68) | .8 |
| Not healthcare associated | 20 (7) | 19 (8) | 1 (2) | 0.29 (.016–1.49) | .2 |
| Charlson score, mean; median (IQR) | 2.6; 2 (1–4) | 2.5; 2 (1–4) | 3.2; 3 (1–5) | 1.15 (.99–1.35) | .06 |
| APS score at onset, mean; median (IQR) | 5.9; 5 (3–8) | 5.8; 5 (3–8) | 6.8; 6 (4–10) | 1.05 (.98–1.12) | .2 |
| Focus | |||||
| Catheter relatedd | 149 (51) | 126 (51) | 23 (54) | Reference | … |
| SSTI, including surgical wounds | 65 (22) | 56 (23) | 9 (21) | 0.88 (.37–1.97) | .8 |
| Urogenital tract | 7 (2) | 6 (2) | 1 (2) | 0.91 (.05–5.70) | .9 |
| Not identified | 71 (24) | 61 (25) | 10 (23) | 0.90 (.39–1.96) | .8 |
| Echocardiography 14 days after positive blood culture | 148 (51) | 118 (47) | 30 (70) | 2.56 (1.30–5.30) | .008 |
| Duration of antimicrobial therapy, mean; median (IQR) | |||||
| In hospital: intravenous | 14.3; 14 (9–17) | 14.0; 14 (9–17) | 16.3; 14 (10–23) | 1.03 (.99–1.07) | .09 |
| In hospital: intravenous + oral | 15.1; 14 (10–18) | 14.8; 14 (10–17) | 16.8; 14 (11–24) | 1.02 (.99–1.06) | .2 |
| Total: in hospital and after discharge | 25.8; 17 (13–29) | 26.2; 17 (13–31) | 23.7; 18 (14–28) | 0.99 (.96–1.00) | .5 |
| Receiving >5 d of antimicrobial combination therapy | 47 (16) | 36 (15) | 11 (26) | 2.03 (.91–4.3) | .07 |
| Length of hospital stay, mean; median (IQR) | 18.4; 15 (10–24) | 18.3; 14 (9–23) | 19.1; 17 (11–25 | 1.00 (.98–1.02) | .7 |
| Characteristic . | Low-Risk Patients, No. (%)a . | . | . | OR (95% CI)b . | P Valuec . |
|---|---|---|---|---|---|
| . | Total (n = 292) . | Without Foreign Body (n = 249) . | With Foreign Body (n = 43) . | . | . |
| Age, mean; median (IQR) | 64; 67 (55–75) | 62; 65 (53–74) | 72; 75 (68–79) | 1.67 (1.28–2.25) per decade | <.001 |
| Male sex | 205 (70) | 173 (70) | 32 (74) | 1.28 (.63–2.78) | .5 |
| Study Center 1 | 178 (61) | 153 (61) | 25 (58) | 0.87 (.45–1.70) | .7 |
| MRSA | 30 (10) | 27 (11) | 3 (7) | 0.61 (.14–1.85) | .4 |
| Mode of acquisition | |||||
| Nosocomial | 231 (79) | 196 (79) | 35 (81) | Reference | … |
| Community acquired | |||||
| Healthcare associated | 41 (14) | 34 (14) | 7 (16) | 1.15 (.44–2.68) | .8 |
| Not healthcare associated | 20 (7) | 19 (8) | 1 (2) | 0.29 (.016–1.49) | .2 |
| Charlson score, mean; median (IQR) | 2.6; 2 (1–4) | 2.5; 2 (1–4) | 3.2; 3 (1–5) | 1.15 (.99–1.35) | .06 |
| APS score at onset, mean; median (IQR) | 5.9; 5 (3–8) | 5.8; 5 (3–8) | 6.8; 6 (4–10) | 1.05 (.98–1.12) | .2 |
| Focus | |||||
| Catheter relatedd | 149 (51) | 126 (51) | 23 (54) | Reference | … |
| SSTI, including surgical wounds | 65 (22) | 56 (23) | 9 (21) | 0.88 (.37–1.97) | .8 |
| Urogenital tract | 7 (2) | 6 (2) | 1 (2) | 0.91 (.05–5.70) | .9 |
| Not identified | 71 (24) | 61 (25) | 10 (23) | 0.90 (.39–1.96) | .8 |
| Echocardiography 14 days after positive blood culture | 148 (51) | 118 (47) | 30 (70) | 2.56 (1.30–5.30) | .008 |
| Duration of antimicrobial therapy, mean; median (IQR) | |||||
| In hospital: intravenous | 14.3; 14 (9–17) | 14.0; 14 (9–17) | 16.3; 14 (10–23) | 1.03 (.99–1.07) | .09 |
| In hospital: intravenous + oral | 15.1; 14 (10–18) | 14.8; 14 (10–17) | 16.8; 14 (11–24) | 1.02 (.99–1.06) | .2 |
| Total: in hospital and after discharge | 25.8; 17 (13–29) | 26.2; 17 (13–31) | 23.7; 18 (14–28) | 0.99 (.96–1.00) | .5 |
| Receiving >5 d of antimicrobial combination therapy | 47 (16) | 36 (15) | 11 (26) | 2.03 (.91–4.3) | .07 |
| Length of hospital stay, mean; median (IQR) | 18.4; 15 (10–24) | 18.3; 14 (9–23) | 19.1; 17 (11–25 | 1.00 (.98–1.02) | .7 |
Abbreviations: APS, acute physiology score; CI, confidence interval; IQR, interquartile range; MRSA, methicillin-resistant Staphylococcus aureus; OR, odds ratio; SSTI, skin and soft-tissue infection.
aData represent no. (%) of patients unless otherwise specified.
bORs represent the risk of carrying a foreign body.
cP values based on Fisher exact or Student t tests.
dIncluding peripheral venous catheters (n = 78), central venous catheters (n = 48), short-term central catheters for hemodialysis (n = 13), peripheral arterial catheters (n = 5), port catheters (n = 4), and implanted catheter (n = 1).
The duration of intravenous and oral antimicrobial therapy during hospital admission was similar in both groups (median, 14 days in both). Moreover, there was no difference between the 2 groups in the total duration of antimicrobial therapy (median, 18 days in the foreign body group vs 17 days in those without foreign bodies; the means [23.7 vs 26.2 days] reflect longer durations in a few outliers). Biofilm-active combination antimicrobial therapy for ≥5 days was more frequent in the group with foreign bodies (26% vs 15%, respectively; P = .07). Patients with pacemaker/AICDs were less likely to receive combination therapy than those with orthopedic implants/osteosyntheses (OR, 0.44; 95% CI, .08–1.99) (Supplementary Table 1).
There was a trend toward a longer hospital stay in patients with foreign bodies than in those without (median, 17 vs 14 days; mean, 19.1 vs 18.3 days). All-cause mortality rates at 30 and 90 days were similar in the groups (5% vs 2%, respectively, at 30 days, and 16% vs 14% at 90 days) (Table 1) and Kaplan-Meier estimates of mortality rate did not differ significantly during the 1-year observation period (Figure 2A; P = .7, log-rank test). When considering the type of foreign body, Kaplan-Meier estimates showed a lower survival rate in patients with a pacemaker/AICD (Figure 2B; P = .02).
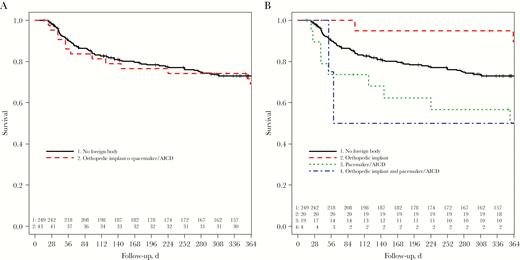
One-year survival among low-risk patients who survived the first 14 days (Kaplan-Meier plot), stratified by presence of a clinically uninfected foreign body (log-rank test, P = .7) (A) and type of clinically uninfected foreign body (log-rank test, P = .02) (B). Abbreviation: AICD, automated implanted cardioverter-defibrillator.
We observed a similar rate of SAB-related events (attributable death, relapse or new deep-seated infection) within 90 days in the 2 groups (2% in both), but in the full 1-year follow-up period the rate was higher in the foreign body group, though not significantly higher (7% vs 4%; OR, 2.0; 95% CI, .43–7.04) (Table 2 and Figure 3A). Cox regression analysis provided estimates for outcomes (Table 2). The hazard ratio for experiencing a SAB-related event within 1 year was 1.91 (95% CI, .52–7.05; P = .3). In a multivariable Cox-model that adjusted for age, sex, comorbid conditions, severity at onset, and the focus of infection, the hazard ratio was reduced to 1.41 (.35–5.69; P = .6).
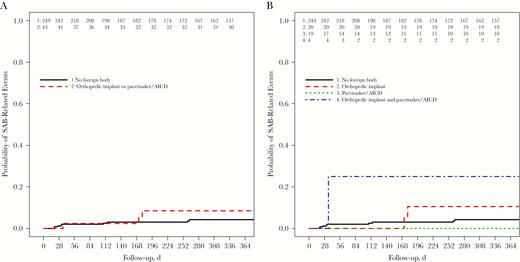
Cumulative probability of Staphylococcus aureus bloodstream (SAB)–related events (attributable death, relapse, or new deep-seated infection) plotted by presence of a foreign body (log-rank test, P = .32) (A) and type of foreign body (log-rank test, P = .06) (B). Abbreviation: AICD, automated implanted cardioverter-defibrillator.
Outcome in Low-Risk Patients With or Without Clinically Uninfected Foreign Bodies
| Outcome . | Low-Risk Patients, No. (%) . | . | . | Univariable HRa (95% CI) . | P Value . | Multivariable HRa (95% CI) . | P Value . |
|---|---|---|---|---|---|---|---|
| . | Total (n = 292) . | Without Foreign Body (n = 249) . | With Foreign Body (n = 43) . | . | . | . | . |
| Mortality rate | |||||||
| At 30 d | 7 (2) | 5 (2) | 2 (5) | 2.35 (.46–12.11) | .3 | 1.74 (.3–10.11) | .5 |
| At 90 d | 42 (14) | 35 (14) | 7 (16) | 1.17 (.52–2.6) | .7 | 0.84 (.37–1.91) | .7 |
| At 1 y | 77 (26) | 64 (26) | 13 (30) | 1.15 (.63–2.1) | .6 | 0.81 (.44–1.49) | .5 |
| Attributable mortality rate | 3 (1) | 3 (1) | 0 (0) | NA (0 to infinity) | NA | NA (0 to infinity) | NA |
| Relapse or new deep-seated infection | 11 (4) | 8 (3) | 3 (7) | 2.14 (.57–8.07 | .3 | 1.7 (.41–6.97) | .5 |
| SAB-related eventb | |||||||
| Within 1 y | 12 (4) | 9 (4) | 3 (7) | 1.91 (.52–7.05) | .3 | 1.41 (.35–5.69) | .6 |
| Within 90 d | 6 (2) | 5 (2) | 1 (2) | 1.15 (.13–9.87) | .9 | 0.45 (.04–4.54) | .5 |
| Outcome . | Low-Risk Patients, No. (%) . | . | . | Univariable HRa (95% CI) . | P Value . | Multivariable HRa (95% CI) . | P Value . |
|---|---|---|---|---|---|---|---|
| . | Total (n = 292) . | Without Foreign Body (n = 249) . | With Foreign Body (n = 43) . | . | . | . | . |
| Mortality rate | |||||||
| At 30 d | 7 (2) | 5 (2) | 2 (5) | 2.35 (.46–12.11) | .3 | 1.74 (.3–10.11) | .5 |
| At 90 d | 42 (14) | 35 (14) | 7 (16) | 1.17 (.52–2.6) | .7 | 0.84 (.37–1.91) | .7 |
| At 1 y | 77 (26) | 64 (26) | 13 (30) | 1.15 (.63–2.1) | .6 | 0.81 (.44–1.49) | .5 |
| Attributable mortality rate | 3 (1) | 3 (1) | 0 (0) | NA (0 to infinity) | NA | NA (0 to infinity) | NA |
| Relapse or new deep-seated infection | 11 (4) | 8 (3) | 3 (7) | 2.14 (.57–8.07 | .3 | 1.7 (.41–6.97) | .5 |
| SAB-related eventb | |||||||
| Within 1 y | 12 (4) | 9 (4) | 3 (7) | 1.91 (.52–7.05) | .3 | 1.41 (.35–5.69) | .6 |
| Within 90 d | 6 (2) | 5 (2) | 1 (2) | 1.15 (.13–9.87) | .9 | 0.45 (.04–4.54) | .5 |
Abbreviations: CI, confidence interval; HR, hazard ratio; NA, not applicable; SAB, Staphylococcus aureus bloodstream.
aHRs were calculated from a Cox model. Variables added to the model were age, sex, Charlson score, acute physiology score, and the infective focus
bAttributable death, relapse or new deep-seated S. aureus infection
Outcome in Low-Risk Patients With or Without Clinically Uninfected Foreign Bodies
| Outcome . | Low-Risk Patients, No. (%) . | . | . | Univariable HRa (95% CI) . | P Value . | Multivariable HRa (95% CI) . | P Value . |
|---|---|---|---|---|---|---|---|
| . | Total (n = 292) . | Without Foreign Body (n = 249) . | With Foreign Body (n = 43) . | . | . | . | . |
| Mortality rate | |||||||
| At 30 d | 7 (2) | 5 (2) | 2 (5) | 2.35 (.46–12.11) | .3 | 1.74 (.3–10.11) | .5 |
| At 90 d | 42 (14) | 35 (14) | 7 (16) | 1.17 (.52–2.6) | .7 | 0.84 (.37–1.91) | .7 |
| At 1 y | 77 (26) | 64 (26) | 13 (30) | 1.15 (.63–2.1) | .6 | 0.81 (.44–1.49) | .5 |
| Attributable mortality rate | 3 (1) | 3 (1) | 0 (0) | NA (0 to infinity) | NA | NA (0 to infinity) | NA |
| Relapse or new deep-seated infection | 11 (4) | 8 (3) | 3 (7) | 2.14 (.57–8.07 | .3 | 1.7 (.41–6.97) | .5 |
| SAB-related eventb | |||||||
| Within 1 y | 12 (4) | 9 (4) | 3 (7) | 1.91 (.52–7.05) | .3 | 1.41 (.35–5.69) | .6 |
| Within 90 d | 6 (2) | 5 (2) | 1 (2) | 1.15 (.13–9.87) | .9 | 0.45 (.04–4.54) | .5 |
| Outcome . | Low-Risk Patients, No. (%) . | . | . | Univariable HRa (95% CI) . | P Value . | Multivariable HRa (95% CI) . | P Value . |
|---|---|---|---|---|---|---|---|
| . | Total (n = 292) . | Without Foreign Body (n = 249) . | With Foreign Body (n = 43) . | . | . | . | . |
| Mortality rate | |||||||
| At 30 d | 7 (2) | 5 (2) | 2 (5) | 2.35 (.46–12.11) | .3 | 1.74 (.3–10.11) | .5 |
| At 90 d | 42 (14) | 35 (14) | 7 (16) | 1.17 (.52–2.6) | .7 | 0.84 (.37–1.91) | .7 |
| At 1 y | 77 (26) | 64 (26) | 13 (30) | 1.15 (.63–2.1) | .6 | 0.81 (.44–1.49) | .5 |
| Attributable mortality rate | 3 (1) | 3 (1) | 0 (0) | NA (0 to infinity) | NA | NA (0 to infinity) | NA |
| Relapse or new deep-seated infection | 11 (4) | 8 (3) | 3 (7) | 2.14 (.57–8.07 | .3 | 1.7 (.41–6.97) | .5 |
| SAB-related eventb | |||||||
| Within 1 y | 12 (4) | 9 (4) | 3 (7) | 1.91 (.52–7.05) | .3 | 1.41 (.35–5.69) | .6 |
| Within 90 d | 6 (2) | 5 (2) | 1 (2) | 1.15 (.13–9.87) | .9 | 0.45 (.04–4.54) | .5 |
Abbreviations: CI, confidence interval; HR, hazard ratio; NA, not applicable; SAB, Staphylococcus aureus bloodstream.
aHRs were calculated from a Cox model. Variables added to the model were age, sex, Charlson score, acute physiology score, and the infective focus
bAttributable death, relapse or new deep-seated S. aureus infection
Three of the patients with clinically uninfected foreign bodies had a relapse of infection. Two of them had hip replacements and 1 had both a pacemaker and a prosthetic stabilization of a dens fracture after an accidental trauma. The latter patient received 17 days of antibiotic therapy and had an early relapse of bacteremia on day 35, with septic pulmonary emboli that were attributed to pacemaker-related endocarditis. Another patient was treated for 9 days with appropriate intravenous antimicrobials and was then discharged. A relapse of bacteremia of unknown source occurred after 172 days in the setting of an urothelial carcinoma and an indwelling double-J catheter. The third patient received 32 days of appropriate intravenous antimicrobial treatment, and at day 178 this patient had an infection of a retained hip prosthesis. In the initial episode, the hip prosthesis was classified as uninfected on the basis of a negative microbiological culture and negative histological findings in a biopsy specimen, disregarding a positive S. aureus polymerase chain reaction result from that specimen. Two more patients had recurring bacteremia that was considered not a relapse but a new superficial infection (1 catheter-related infection and 1 superficial skin infection [furuncle]). Both patients were in the group without a foreign body.
DISCUSSION
In this study we sought to describe current treatment patterns and outcome in patients with low-risk SAB and clinically uninfected orthopedic implants or pacemaker/AICDs from a large study cohort. Patients with and those without clinically uninfected foreign bodies received similar courses of antimicrobial treatment and showed comparable short-term outcome measures. However, we observed a nonsignificant trend toward a higher rate of SAB-related events within 1 year in patients with clinically uninfected devices.
The key question in clinical practice is how to recognize and define low-risk patients. We defined low-risk patients as those having a low risk for a complicated clinical course, that is, absence of a deep-seated infection and low risk of subsequent dissemination. Previously, we and others found that positive follow-up blood cultures predict deep foci, in particular infective endocarditis [19–21] and that an early removal of an intravenous catheter present at infection onset is crucial [4, 21, 22]. In addition, we excluded patients with severe immunosuppression, end-stage renal disease, or moderate to severe liver disease, following our definition from the SABATO study [10]. We purposely did not restrict the analysis to low-risk foci (eg, catheter-related infection and skin-soft-tissue infection). Thus, we included patients in whom an infective focus could not be identified within 14 days in an extensive diagnostic workup, despite the generally poor prognosis of this patient group [3].
We further restricted our analysis to patients with pacemakers/AICDs or prosthetic joints/osteosyntheses. Other types of foreign bodies (eg, vascular grafts and prosthetic heart valves) were excluded, because their replacement is usually more complicated and associated with a higher risk. This is important to note, because our findings should not be extrapolated to these foreign bodies.
In our study centers, patients with SAB are routinely seen by an infectious disease physician or clinical microbiologist at the bedside. This thorough evaluation may have played an important role in patient stratification and the diagnostic workup. For example, 50% of all patients and 79% of those with foreign bodies received echocardiography within 14 days to rule out infective endocarditis.
In this study, all-cause mortality rates were in the range expected for low-risk foci [23] and did not differ between the groups. This was somewhat surprising, because patients with foreign bodies were on average 10 years older and had more comorbid conditions. We therefore expected a higher mortality rate in this group. To measure events that could be prevented by extended antimicrobial therapy, we defined SAB-related events as attributable death, relapse, or new deep-seated S. aureus infection. Interestingly, even in this low-risk group of patients with SAB, there was a significant risk for SAB-related events within 1 year (4% in those without and 7% in those with a foreign body), a finding that underscores the significant morbidity risk imposed by SAB. This observed difference between the groups was not statistically significant (hazard ratio 1.91; 95% CI, .52–7.05; P = .3), and with adjustment for age, sex, comorbid conditions, severity at onset, and infective focus in a multivariable Cox model, the hazard ratio was reduced (1.41; 95% CI, .35–5.69; P = .6). A retrospective inspection of the 3 patients with relapse and foreign bodies showed that there was a misclassification in at least 1 patient (with a disregarded polymerase chain reaction result).
Generally, 14 days of antimicrobial therapy is recommended in patients with uncomplicated SAB [24]. A recent trial confirmed this duration but highlighted the need for a careful evaluation regarding metastatic infection [25]. The INSTINCT protocol did not set any rules for the duration of treatment, but owing to our treatment algorithms and local antimicrobial policies, we had expected longer treatment duration and a higher rate of combination therapy in patients with foreign bodies [26]. To our surprise, the median duration of treatment did not differ between the groups, and the slightly longer mean treatment duration in patients with foreign bodies was due to patients with orthopedic implants. Furthermore, biofilm-active antimicrobial combination therapy was only moderately more common in patients with foreign bodies.
The strength of the study is its diligent patient workup and long follow-up but there are also limitations: First, this is a post hoc analysis of a prospective study and although the study was designed with similar research questions in mind, it has not been focused on this particular research question. Second, there is the possibility of underreporting for data referring to time points after the hospital stay. We took particular care in obtaining information from patients, general practitioners, and hospital discharge letters, but we cannot completely exclude underreporting of antimicrobial prescriptions and SAB-related events. Third, in a post hoc analysis, there is a danger in using knowledge that would not be available in the study at a given time point (eg, results of diagnostics) and thereby wrongly excluding patients from the low-risk group. However, we are confident in our results, because time stamps for diagnostic procedures and clinical assessments were available in our database. Fourth, the small sample size reduces statistical power, especially in the analysis of subgroups, and thus it is unclear whether true differences in SAB-related events remained undetected.
In conclusion, data from our large prospective cohort of patients with SAB show that in a thoroughly selected subgroup of patients, SAB-related complications are well below 10%. In these low-risk patients, the presence of clinically uninfected prosthetic joints or pacemakers/AICD does not increase the mortality rate and longer antimicrobial treatment than in patients without a foreign body may not be necessary. However, additional confirmatory studies are needed for firm conclusions regarding treatment duration, and it cannot be overstressed that a diligent diagnostic workup is required to correctly identify low-risk patients.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The following individuals collected clinical data and/or provided administrative support: Marc-Fabian Juzek-Küpper, Christian Theilacker, and Gabriele Peyerl-Hoffmann (Division of Infectious Diseases, Medical Center, University of Freiburg); Katharina Achilles, Christian Bernasch, Christina Dörbecker, Andreas Langhorst, Stephan Neumann, and Georg Peppinghaus (Institute for Medical Microbiology, Immunology and Hygiene, University of Cologne); Hanna Birkholz (Clinical Trials Center, University of Cologne); and Andreas Wendel (Heinrich-Heine University Düsseldorf).
Disclaimer. The funding organizations had no role in the design of the study, data collection, or data analysis.
Financial support. This work was supported by the Deutsche Forschungsgemeinschaft (grant KA 3104/1-1 to A. J. K.), the Paul-Ehrlich Gesellschaft für Chemotherapie (to W. V. K. and H. S.), and the Bundesministerium für Bildung und Forschung (BMBF ; grants 01KI1017 to A. J. K. and 01KN1106 to the Clinical Trial Center Cologne).
Potential conflicts of interest. A. J. K. has received payments for lectures from BD Biosciences, bioMérieux, Merck Sharp & Dohme (MSD), Limbach Gruppe, and ViiV Healthcare and travel support from Janssen-Cilag. W. V. K. reports personal fees from Gilead Sciences and grants from ViiV Healthcare, Gilead Sciences, Pfizer, Cellectis, the German Centre for Infection Research, BMBF, and the Federal State of Baden-Württemberg. H. S. has received grants or research support from the BMBF, Germany, the German Centre for Infection Research, Cubist, Tetraphase, Entasis, and Novartis and personal fees from Basilea Pharmaceuticals, Roche Pharma, Gilead, Tetraphase, MSD, Novartis, Genentech, Entasis, Shionogi. S. R. has received personal fees from Pfizer, Limbach Gruppe, and MSD and travel support from Astellas and MSD. I. J. and M. H. report no conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References





Comments