-
PDF
- Split View
-
Views
-
Cite
Cite
Kathleen M Ward, Oluwaseun Falade-Nwulia, Juhi Moon, Catherine G Sutcliffe, Sherilyn Brinkley, Taryn Haselhuhn, Stephanie Katz, Kayla Herne, Lilian Arteaga, Shruti H Mehta, Carl Latkin, Robert K Brooner, Mark S Sulkowski, A Randomized Controlled Trial of Cash Incentives or Peer Support to Increase HCV Treatment for Persons With HIV Who Use Drugs: The CHAMPS Study, Open Forum Infectious Diseases, Volume 6, Issue 4, April 2019, ofz166, https://doi.org/10.1093/ofid/ofz166
Close - Share Icon Share
Abstract
Despite access to direct-acting antivirals, barriers to a hepatitis C virus (HCV) cure persist, especially among persons living with human immunodeficiency virus (HIV) (PLWH) who use drugs. Interventions such as peer mentors or cash incentives may improve the care continuum.
The CHAMPS (Chronic HepAtitis C Management to ImProve OutcomeS) study randomized 144 PLWH, recruited from an outpatient clinic, with substance use disorders into three treatment groups: usual care (UC) (n = 36), UC plus cash incentives (n = 54), and UC plus peer mentors (n = 54) to evaluate HCV treatment uptake and cure. All participants received 12-weeks of ledipasvir/sofosbuvir (LDV/SOF). Trained peer mentors had well-controlled HIV and HCV. Cash incentives were contingent on visit attendance (maximum $220). The primary endpoint was HCV treatment initiation; secondary endpoints included sustained virologic response (SVR) and HCV reinfection.
The majority of participants were male (61%), Black (93%), and unemployed (85%). Depression and active drug and alcohol use were common. Overall, 110 of 144 (76%) participants initiated LDV/SOF. Although treatment initiation rates were higher in PLWH randomized to peers (83%, 45 of 54) or cash (76%, 41 of 54) compared to UC (67%, 24 of 36), these differences were not statistically significant (P = .11). Most PLWH who initiated treatment achieved SVR (100 of 110, 91%). LDV/SOF was well tolerated; peers and cash had no effect on drug and alcohol use during therapy. One individual from the cash cohort experienced HCV reinfection.
After removal of system barriers, one-third of PLWH in UC did not initiate HCV treatment. Among those who initiated, SVR rates were high. Research involving PLWH who use drugs should focus on overcoming barriers to treatment initiation.
The registration data for the trial are in the ClinicalTrials.gov database, number NCT02402218.
In the United States, approximately 300 000 people are coinfected with human immunodeficiency virus (HIV) and hepatitis C virus (HCV). The prevalence of HCV is especially high among people living with HIV (PLWH) who inject drugs (PWID), of whom 50% to 90% are coinfected [1–4]. Compared to persons without HIV, those with HIV/HCV coinfection have a higher risk of progressive liver disease, hepatocellular carcinoma, and liver-related death, and, in many settings, HCV disease remains a leading cause of morbidity and mortality [5, 6]. Accordingly, curative HCV treatment is recommended for all persons with coinfection [3, 4]. In the interferon era, sustained virologic response (SVR) rates were lower in persons with HIV compared to those without HIV coinfection [7, 8]. However, treatment with HCV direct-acting antivirals (DAAs) has been safe, tolerable, and highly effective in this population, both in clinical trials and real-world settings [9–12].
Based on these successes, PLWH are a population in which HCV micro-elimination may be achievable [13, 14]. The basis for this optimism is that, compared to persons with HCV alone, PLWH are more likely to be in care and have greater access to treatment [13]. However, in the United States, less than 50% of PLWH have HIV suppression, with lower rates of linkage to HIV care and treatment observed in PWID [15]. This observation suggests that HCV elimination may be challenging for some PLWH due to ongoing barriers to care, such as drug and alcohol use disorders, comorbid medical/psychiatric illness, and stigma [16, 17]. In HIV care, novel strategies to improve linkage and retention such as patient navigators, peer mentors, and contingent financial incentives have been extensively studied [18]. Unlike HIV, HCV can be cured with a brief duration of DAAs and long-term retention is less important. Although interventions to improve the HCV care continuum may be more effective than in HIV infection, less is known about their usefulness.
The CHAMPS (Chronic HepAtitis C Management to ImProve OutcomeS) study aimed to test the hypothesis that, compared to usual care, novel interventions of cash incentives and peer mentors would increase the rate of HCV treatment initiation and cure in a population of persons with HIV who use drugs (PWUD).
METHODS
Study Population
We identified 194 persons with HIV/HCV coinfection receiving HIV care at the Johns Hopkins Moore Clinic for HIV Care who had not engaged in co-located HCV care within 8 months of entry. Eligible patients had chronic HCV genotype 1 infection and were HCV treatment-naïve. Other inclusion criteria included the following: Age ≥18 years, CD4 count >100 mm3, estimated glomerular filtration rate ≥30 mL/min/1.73 m2, no evidence of hepatocellular carcinoma or decompensated liver disease, and life expectancy greater than 2 years.
HCV Treatment Regimen
To remove barriers introduced by payers, 12 weeks of once-daily LDV/SOF (Gilead Sciences, Foster City, California) was provided. LDV/SOF was dispensed in bottles of 28 pills at treatment weeks 0, 4, and 8. Medication adherence was assessed by self-report and pill count.
Study Interventions
Following written informed consent and screening, participants were randomized (2:3:3) to (1) usual care (UC), (2) UC plus peer mentors (peer), and (3) UC plus contingent cash incentives (cash). Before enrollment, a randomization sequence was generated in blocks of 16 using STATA version 12 (StataCorp LP, College Station, Texas) and uploaded to the randomization module in REDCap (https://www.project-redcap.org/). Study coordinators and participants were not blind to intervention assignment.
Usual Care
All participants were linked to an HCV provider and treated according to the standard clinic protocol, which involved clinical visits and calls delivered by a nurse-led multidisciplinary team that included pharmacists and case managers [10]. HIV clinicians assessed potential drug interactions with LDV/SOF and, if applicable, managed antiretroviral therapy (ART) modification. In addition to clinical visits, all participants had study-specific visits for which they received remuneration ($10–$30/visit) (Supplemental Figure 1).
Peer Mentor Support
Participants randomized to the peer group had structured interactions with trained peer mentors (n = 5) who had been successfully treated for HIV and HCV. Peers provided written informed consent and completed 10 hours of training that emphasized privacy and confidentiality. Mentors had a face-to-face meeting with mentees to understand barriers to HCV cure. Peers used mobile phones to contact mentees before, during, and after HCV treatment. Mentors met monthly with the study team to review mentee interactions and were compensated.
Contingent Cash Incentive
Participants received incentives designed to reinforce visit-attendance behaviors and were not based on pill count or HCV RNA response. Participants received $10 for the HCV initiation visit followed by incentives that increased by $5 for attendance every 2 weeks during treatment (6 reinforced visits) and $50 for attendance at the post-treatment week 12 visit. If a participant missed a visit, the incentive dropped to $10, and the escalation scale was re-initiated. The maximum total cash incentive was $220.
Assessments
Laboratory monitoring, including quantitative HCV RNA (COBAS TaqMan HCV Test v2.0; Roche Molecular Systems Inc., Branchburg, New Jersey), was performed at treatment weeks 0, 4, 8, 12, and post-treatment weeks 6 and 12. Adverse effects and medication adherence were assessed at each visit. CD4 cell count and HIV RNA levels were measured at screening and as clinically indicated. Liver elastography (FibroScan 502 Touch, Echosens North America, Waltham, Massachusetts) was performed before HCV treatment and at post-treatment week 12. Drug and alcohol use was assessed at each study visit by questionnaires, including the 10-question Alcohol Use Disorders Identification Test (AUDIT) [19]. At study entry and treatment week 6, drug use was measured by urine toxicology and alcohol use was measured by whole blood levels of phosphatidylethanol (PEth) (United States Drug Testing Laboratories, Des Plaines, Illinois) [20].
Study Outcomes
The primary study endpoint was LDV/SOF initiation within 8 weeks of randomization (extended to 12 weeks if changes in ART were recommended). Participants who did not initiate were followed at 24 weeks after non-initiation. Secondary endpoints were SVR, HCV relapse, and HCV reinfection. SVR was defined as an undetectable HCV RNA at 12 or more weeks after stopping LDV/SOF. Participants who discontinued early were followed until their intended post-treatment week 12 visit. Participants with undetectable HCV RNA at the end of treatment and detectable HCV RNA at post-treatment weeks 6 or 12 were assessed for HCV relapse versus reinfection by exposure history and virus characteristics.
Statistical Analysis
Intention-to-treat analyses were performed. For the liver elastography, participants were classified as either having no or mild stiffness (≤8 kPa), moderate stiffness (8.1–11.9 kPa), or severe stiffness/cirrhosis (>12.0 kPa) [21]. For alcohol use, PEth values ≥50 ng/mL were considered moderate to heavy use, and AUDIT scores ≥8 for men and ≥4 for women were categorized as hazardous drinking [20, 22, 23]. Undetectable HIV and HCV RNA were defined as ≤50 copies/mL and ≤15 IU/mL, respectively. For the primary and secondary outcomes, the proportion of participants with the outcome in each group was compared using a chi-square test. For the secondary outcome of SVR, the proportion achieving SVR was calculated among all enrolled participants and in the subset who initiated HCV treatment. Univariable associations between the outcome and covariates of interest were also explored using log-binomial regression. Relative risks and 95% confidence intervals are reported. A post-hoc analysis was performed to evaluate a potential interaction between the study interventions and employment status. Adverse events, serious adverse events, and changes in the frequency of drug and alcohol use before and during treatment were compared using chi-square tests to evaluate the safety of cash incentives. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina).
Sample Size
At the time of study design (2013), the sample size was based on an estimated HCV treatment initiation rate of 50% in the UC group (based on a 33% rate observed during the interferon era) and 80% in the intervention groups, a significance level of 0.05, a desired ratio of participants in the intervention group compared to UC group of 3 to 2, and power of 80% to detect this difference between groups. The minimum number of participants needed was 47 participants in each intervention group and 32 participants in the UC group. The study was not powered to detect differences between the peer and cash groups.
The study was approved by the Johns Hopkins University School of Medicine Institutional Review Board and conducted in accordance with provisions of the Declaration of Helsinki and Good Clinical Practice guidelines. All study participants, including peer mentors, provided written informed consent.
RESULTS
Study Population
From a population of 194 patients receiving care for HIV but not HCV, 144 were enrolled and randomized between August 2015 and October 2016 to receive LDV/SOF in 1 of 3 care groups: (1) usual care (n = 36), (2) UC plus peers (n = 54), and (3) UC plus cash (n = 54). Of the 50 persons not enrolled, most (n = 26) could not be contacted, 13 declined participation, 6 did not meet criteria, and 5 did not attend screening visits (Figure 1). At enrollment, the characteristics of participants were similar in each group (Table 1). The majority of participants were male (61%), Black (93%), and unemployed (85%). All participants were infected with HCV genotype 1 (subtype 1a, 78%) and 12% had cirrhosis; the majority were prescribed ART (97%) and most (80%) had HIV RNA ≤50 copies/mL. Medical and psychiatric comorbid conditions were common; 61% of participants had active depression. By urine drug test, cocaine or heroin was detected in 63 persons (46%); of whom, only 36 (57%) reported use within 30 days of entry. Methadone was detected in 38 participants (28%). Moderate to heavy alcohol use within 14 days of entry was detected by blood PEth (≥50 ng/mL) in 47 participants (33%).
| . | Usual Care (N = 36) (n,%) . | Peer Mentor (N = 54) (n,%) . | Cash Incentive (N = 54) (n,%) . | Total (N = 144) (n,%) . |
|---|---|---|---|---|
| Age, median years (IQR) | 55.8 (51.8, 60.2) | 55.1 (50.5, 59.3) | 54.0 (48.8, 59.2) | 54.9 (50.6, 59.3) |
| Male | 22 (61) | 35 (65) | 31 (57) | 88 (61) |
| Black | 33 (92) | 52 (96) | 48 (89) | 133 (93) |
| Unemployed | 30 (84) | 47 (87) | 45 (83) | 122 (85) |
| Never married | 19 (53) | 27 (50) | 26 (48) | 72 (50) |
| Self-report cocaine or heroin use within 30 days | 12 (33) | 12 (22) | 12 (22) | 36 (25) |
| Urine toxicology positive for cocaine or heroin | 13 (42) | 23 (45) | 27 (50) | 63 (46) |
| Self-report risky alcohol use, AUDIT ≥8 for men, ≥4 for women | 9 (25) | 13 (24) | 16 (30) | 38 (26) |
| Moderate to heavy alcohol use, PEth ≥50 ng/mL | 15 (42) | 19 (35) | 13 (24) | 47 (33) |
| Depression, activea | 20 (56) | 34 (63) | 34 (63) | 88 (61) |
| Low emotional supportb | 11 (31) | 24 (44) | 21 (39) | 56 (39) |
| No recent primary care visit, past 12 months | 0 | 4 (8) | 1 (2) | 5 (4) |
| On antiretroviral therapy | 35 (97) | 52 (96) | 52 (96) | 139 (97) |
| Undetectable HIV RNA, ≤50 copies/mL | 30 (83) | 43 (80) | 42 (78) | 115 (80) |
| Median HIV RNA if ≥50 copies/mL, copies/mL (range) | 20 767 (251, 101 942) | 645 (55, 357 083) | 15 903 (52, 114 561) | 5702 (52, 357 083) |
| CD4 Count, cell/mm3 (IQR) | 453 (325, 760) | 581 (387, 851) | 509 (343, 756) | 530 (340, 797) |
| HCV genotype 1a | 29 (81) | 40 (74) | 43 (80) | 112 (78) |
| Median HCV RNA, IU/mL (range) | 2 730 000 (472 000, 5 740 000) | 4 790 000 (1 680 000, 10 300 000) | 2 730 000 (624 000, 8 350 000) | 2 975 000 (869 000, 8 825 000) |
| ALT, U/Lc (range) | 39 (26, 60) | 33 (20, 46) | 29 (21, 44) | 33 (21, 50) |
| AST, U/Ld (range) | 45 (34, 60) | 39 (27, 59) | 36 (30, 59) | 40 (31, 59) |
| Cirrhosis by elastography, ≥12 kPa | 4 (11) | 5 (10) | 7 (14) | 16 (12) |
| Liver disease stage by elastography, median kPa (IQR) | 7.8 (5.4, 9.9) (1 unsuccessful) | 6.8 (5.2, 9.1) (2 unsuccessful) | 6.7 (5.1, 8.6) (4 unsuccessful) | 6.9 (5.2, 9.0) (7 unsuccessful) |
| HIV med contraindicatione | 10 (32) | 11 (22) | 7 (15) | 28 (22) |
| . | Usual Care (N = 36) (n,%) . | Peer Mentor (N = 54) (n,%) . | Cash Incentive (N = 54) (n,%) . | Total (N = 144) (n,%) . |
|---|---|---|---|---|
| Age, median years (IQR) | 55.8 (51.8, 60.2) | 55.1 (50.5, 59.3) | 54.0 (48.8, 59.2) | 54.9 (50.6, 59.3) |
| Male | 22 (61) | 35 (65) | 31 (57) | 88 (61) |
| Black | 33 (92) | 52 (96) | 48 (89) | 133 (93) |
| Unemployed | 30 (84) | 47 (87) | 45 (83) | 122 (85) |
| Never married | 19 (53) | 27 (50) | 26 (48) | 72 (50) |
| Self-report cocaine or heroin use within 30 days | 12 (33) | 12 (22) | 12 (22) | 36 (25) |
| Urine toxicology positive for cocaine or heroin | 13 (42) | 23 (45) | 27 (50) | 63 (46) |
| Self-report risky alcohol use, AUDIT ≥8 for men, ≥4 for women | 9 (25) | 13 (24) | 16 (30) | 38 (26) |
| Moderate to heavy alcohol use, PEth ≥50 ng/mL | 15 (42) | 19 (35) | 13 (24) | 47 (33) |
| Depression, activea | 20 (56) | 34 (63) | 34 (63) | 88 (61) |
| Low emotional supportb | 11 (31) | 24 (44) | 21 (39) | 56 (39) |
| No recent primary care visit, past 12 months | 0 | 4 (8) | 1 (2) | 5 (4) |
| On antiretroviral therapy | 35 (97) | 52 (96) | 52 (96) | 139 (97) |
| Undetectable HIV RNA, ≤50 copies/mL | 30 (83) | 43 (80) | 42 (78) | 115 (80) |
| Median HIV RNA if ≥50 copies/mL, copies/mL (range) | 20 767 (251, 101 942) | 645 (55, 357 083) | 15 903 (52, 114 561) | 5702 (52, 357 083) |
| CD4 Count, cell/mm3 (IQR) | 453 (325, 760) | 581 (387, 851) | 509 (343, 756) | 530 (340, 797) |
| HCV genotype 1a | 29 (81) | 40 (74) | 43 (80) | 112 (78) |
| Median HCV RNA, IU/mL (range) | 2 730 000 (472 000, 5 740 000) | 4 790 000 (1 680 000, 10 300 000) | 2 730 000 (624 000, 8 350 000) | 2 975 000 (869 000, 8 825 000) |
| ALT, U/Lc (range) | 39 (26, 60) | 33 (20, 46) | 29 (21, 44) | 33 (21, 50) |
| AST, U/Ld (range) | 45 (34, 60) | 39 (27, 59) | 36 (30, 59) | 40 (31, 59) |
| Cirrhosis by elastography, ≥12 kPa | 4 (11) | 5 (10) | 7 (14) | 16 (12) |
| Liver disease stage by elastography, median kPa (IQR) | 7.8 (5.4, 9.9) (1 unsuccessful) | 6.8 (5.2, 9.1) (2 unsuccessful) | 6.7 (5.1, 8.6) (4 unsuccessful) | 6.9 (5.2, 9.0) (7 unsuccessful) |
| HIV med contraindicatione | 10 (32) | 11 (22) | 7 (15) | 28 (22) |
aCenters for Depression Epidemiology Scale (CES-D); active depression defined as score ≥16.
bPatient-reported Outcomes Measurement Information System Short Form v2.0, Emotional Support 8a; low support defined as short form score <33.
cn = 143; missing 1 from peer mentor.
dn = 139; missing 2 from peer mentor, 3 from cash incentive.
eMedication change was necessary before starting direct-acting antivirals. Does not include 15 participants never evaluated by an HCV clinician (5 from usual care, 4 from peer mentor, 6 from cash incentive).
Abbreviations: AUDIT, alcohol use disorders identification test; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range; PEth, Phosphatidylethanol.
| . | Usual Care (N = 36) (n,%) . | Peer Mentor (N = 54) (n,%) . | Cash Incentive (N = 54) (n,%) . | Total (N = 144) (n,%) . |
|---|---|---|---|---|
| Age, median years (IQR) | 55.8 (51.8, 60.2) | 55.1 (50.5, 59.3) | 54.0 (48.8, 59.2) | 54.9 (50.6, 59.3) |
| Male | 22 (61) | 35 (65) | 31 (57) | 88 (61) |
| Black | 33 (92) | 52 (96) | 48 (89) | 133 (93) |
| Unemployed | 30 (84) | 47 (87) | 45 (83) | 122 (85) |
| Never married | 19 (53) | 27 (50) | 26 (48) | 72 (50) |
| Self-report cocaine or heroin use within 30 days | 12 (33) | 12 (22) | 12 (22) | 36 (25) |
| Urine toxicology positive for cocaine or heroin | 13 (42) | 23 (45) | 27 (50) | 63 (46) |
| Self-report risky alcohol use, AUDIT ≥8 for men, ≥4 for women | 9 (25) | 13 (24) | 16 (30) | 38 (26) |
| Moderate to heavy alcohol use, PEth ≥50 ng/mL | 15 (42) | 19 (35) | 13 (24) | 47 (33) |
| Depression, activea | 20 (56) | 34 (63) | 34 (63) | 88 (61) |
| Low emotional supportb | 11 (31) | 24 (44) | 21 (39) | 56 (39) |
| No recent primary care visit, past 12 months | 0 | 4 (8) | 1 (2) | 5 (4) |
| On antiretroviral therapy | 35 (97) | 52 (96) | 52 (96) | 139 (97) |
| Undetectable HIV RNA, ≤50 copies/mL | 30 (83) | 43 (80) | 42 (78) | 115 (80) |
| Median HIV RNA if ≥50 copies/mL, copies/mL (range) | 20 767 (251, 101 942) | 645 (55, 357 083) | 15 903 (52, 114 561) | 5702 (52, 357 083) |
| CD4 Count, cell/mm3 (IQR) | 453 (325, 760) | 581 (387, 851) | 509 (343, 756) | 530 (340, 797) |
| HCV genotype 1a | 29 (81) | 40 (74) | 43 (80) | 112 (78) |
| Median HCV RNA, IU/mL (range) | 2 730 000 (472 000, 5 740 000) | 4 790 000 (1 680 000, 10 300 000) | 2 730 000 (624 000, 8 350 000) | 2 975 000 (869 000, 8 825 000) |
| ALT, U/Lc (range) | 39 (26, 60) | 33 (20, 46) | 29 (21, 44) | 33 (21, 50) |
| AST, U/Ld (range) | 45 (34, 60) | 39 (27, 59) | 36 (30, 59) | 40 (31, 59) |
| Cirrhosis by elastography, ≥12 kPa | 4 (11) | 5 (10) | 7 (14) | 16 (12) |
| Liver disease stage by elastography, median kPa (IQR) | 7.8 (5.4, 9.9) (1 unsuccessful) | 6.8 (5.2, 9.1) (2 unsuccessful) | 6.7 (5.1, 8.6) (4 unsuccessful) | 6.9 (5.2, 9.0) (7 unsuccessful) |
| HIV med contraindicatione | 10 (32) | 11 (22) | 7 (15) | 28 (22) |
| . | Usual Care (N = 36) (n,%) . | Peer Mentor (N = 54) (n,%) . | Cash Incentive (N = 54) (n,%) . | Total (N = 144) (n,%) . |
|---|---|---|---|---|
| Age, median years (IQR) | 55.8 (51.8, 60.2) | 55.1 (50.5, 59.3) | 54.0 (48.8, 59.2) | 54.9 (50.6, 59.3) |
| Male | 22 (61) | 35 (65) | 31 (57) | 88 (61) |
| Black | 33 (92) | 52 (96) | 48 (89) | 133 (93) |
| Unemployed | 30 (84) | 47 (87) | 45 (83) | 122 (85) |
| Never married | 19 (53) | 27 (50) | 26 (48) | 72 (50) |
| Self-report cocaine or heroin use within 30 days | 12 (33) | 12 (22) | 12 (22) | 36 (25) |
| Urine toxicology positive for cocaine or heroin | 13 (42) | 23 (45) | 27 (50) | 63 (46) |
| Self-report risky alcohol use, AUDIT ≥8 for men, ≥4 for women | 9 (25) | 13 (24) | 16 (30) | 38 (26) |
| Moderate to heavy alcohol use, PEth ≥50 ng/mL | 15 (42) | 19 (35) | 13 (24) | 47 (33) |
| Depression, activea | 20 (56) | 34 (63) | 34 (63) | 88 (61) |
| Low emotional supportb | 11 (31) | 24 (44) | 21 (39) | 56 (39) |
| No recent primary care visit, past 12 months | 0 | 4 (8) | 1 (2) | 5 (4) |
| On antiretroviral therapy | 35 (97) | 52 (96) | 52 (96) | 139 (97) |
| Undetectable HIV RNA, ≤50 copies/mL | 30 (83) | 43 (80) | 42 (78) | 115 (80) |
| Median HIV RNA if ≥50 copies/mL, copies/mL (range) | 20 767 (251, 101 942) | 645 (55, 357 083) | 15 903 (52, 114 561) | 5702 (52, 357 083) |
| CD4 Count, cell/mm3 (IQR) | 453 (325, 760) | 581 (387, 851) | 509 (343, 756) | 530 (340, 797) |
| HCV genotype 1a | 29 (81) | 40 (74) | 43 (80) | 112 (78) |
| Median HCV RNA, IU/mL (range) | 2 730 000 (472 000, 5 740 000) | 4 790 000 (1 680 000, 10 300 000) | 2 730 000 (624 000, 8 350 000) | 2 975 000 (869 000, 8 825 000) |
| ALT, U/Lc (range) | 39 (26, 60) | 33 (20, 46) | 29 (21, 44) | 33 (21, 50) |
| AST, U/Ld (range) | 45 (34, 60) | 39 (27, 59) | 36 (30, 59) | 40 (31, 59) |
| Cirrhosis by elastography, ≥12 kPa | 4 (11) | 5 (10) | 7 (14) | 16 (12) |
| Liver disease stage by elastography, median kPa (IQR) | 7.8 (5.4, 9.9) (1 unsuccessful) | 6.8 (5.2, 9.1) (2 unsuccessful) | 6.7 (5.1, 8.6) (4 unsuccessful) | 6.9 (5.2, 9.0) (7 unsuccessful) |
| HIV med contraindicatione | 10 (32) | 11 (22) | 7 (15) | 28 (22) |
aCenters for Depression Epidemiology Scale (CES-D); active depression defined as score ≥16.
bPatient-reported Outcomes Measurement Information System Short Form v2.0, Emotional Support 8a; low support defined as short form score <33.
cn = 143; missing 1 from peer mentor.
dn = 139; missing 2 from peer mentor, 3 from cash incentive.
eMedication change was necessary before starting direct-acting antivirals. Does not include 15 participants never evaluated by an HCV clinician (5 from usual care, 4 from peer mentor, 6 from cash incentive).
Abbreviations: AUDIT, alcohol use disorders identification test; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range; PEth, Phosphatidylethanol.
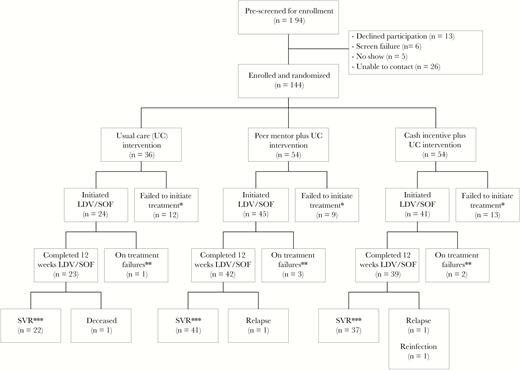
CONSORT Flow Diagram.
Abbreviations: CONSORT, consolidated standards of reporting trials; LDV/SOF, ledipasvir/sofosbuvir; SVR, sustained virologic response; UC, usual care.
*Participant failed to initiate treatment within 8 or 12 weeks of randomization (12 weeks if changes in antiretroviral therapy were required prior to direct-acting antivirals).
**Three participants discontinued treatment due to adverse events (nausea, peer; tinnitus, peer; insomnia, incentive). One participant from each group (3 total) discontinued treatment early due to non-adherence to pharmacy visits.
***Sustained virologic response defined as an undetectable HCV RNA level at 12 or more weeks after stopping ledipasvir/sofosbuvir. No participants were lost to follow-up.
HCV Treatment Initiation (Primary Endpoint)
Overall, 110 of 144 (76%) participants initiated LDV/SOF. The initiation rate was higher in persons randomized to peers (83%, 45 of 54) or cash (76%, 41 of 54) compared to usual care (67%, 24 of 36); however, these differences did not reach statistical significance (P = .11) (Figure 2a, Figure 3a). The proportion of participants initiating LDV/SOF did not vary by age, sex, race, depression status, liver disease stage, HIV status, or active drug and alcohol use; however, employment and prompt attendance to the initial clinician visit were associated with higher rates of treatment initiation. Persons who attended the clinician visit as scheduled were significantly more likely to proceed with treatment (86 of 100, 86%) than those who missed or rescheduled the visit 1 or more times (≥3 missed, 1 of 9 participants, 11%) (P < .0001). Employed persons were more likely to initiate treatment (20 of 22, 91%) than unemployed persons (90 of 122, 74%) (P = .08) (Supplemental Figure 2a). In a post-hoc analysis, there was a significant interaction between the peer mentor intervention and employment status (P-value for interaction = .03). Among participants not employed, those assigned to peers were significantly more likely to initiate treatment compared to the usual care group (83% vs. 60%, P = .02) (Figure 2b).
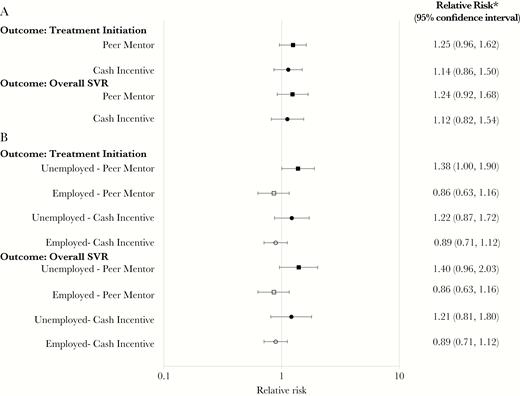
HCV Treatment Initiation and SVR by Intervention Group and Employment Status. (A) HCV Treatment Initiation and SVR Among All Participants Randomized by Intervention Group. (B) Post-Hoc Analysis of Employment Status as a Predictor of HCV Treatment Initiation and SVR.
Abbreviations: HCV, hepatitis C virus; SVR, sustained virologic response.
*Reference group for relative risk: usual care.
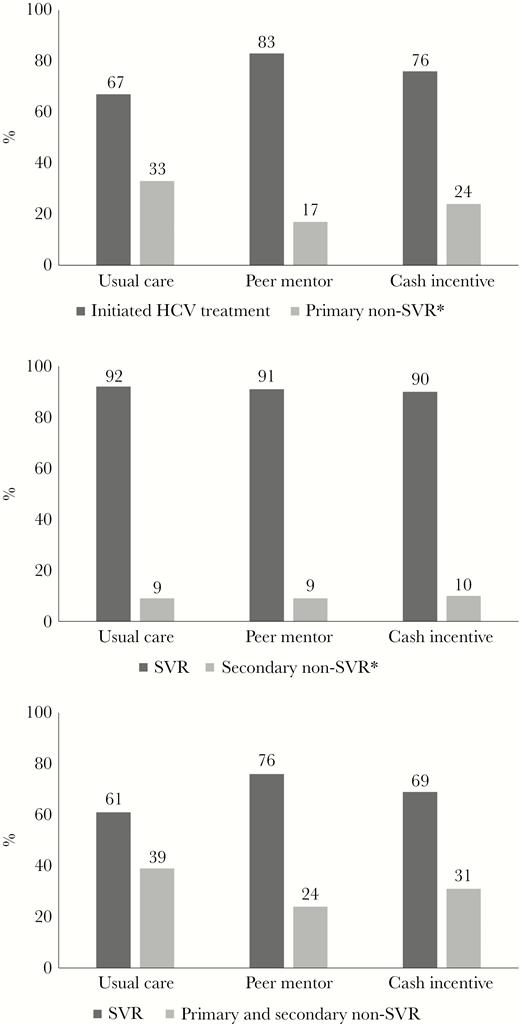
HCV Treatment Initiation and SVR Overall and by Treatment Initiation Status. (A) HCV Treatment Initiation Across Intervention Groups (Primary Endpoint). (B) SVR Among Participants who Initiated HCV Treatment (Secondary Endpoint). (C) SVR Among All Participants Randomized (Secondary Endpoint).
Abbreviations: HCV, hepatitis C virus; SVR, sustained virologic response.
(A)*Primary non-SVR includes participants that failed to initiate treatment within 8 or 12 weeks of randomization (12 weeks if changes in antiretroviral therapy were required prior to direct-acting antivirals).
(B)*Secondary non-SVR includes 10 participants without SVR, 1 participant (usual care) died during follow-up (last HCV RNA not detected), 2 participants (1 peer mentor and 1 cash incentive) had post-treatment HCV relapse, 1 participant (cash incentive) with HCV genotype 1b at entry was reinfected with HCV genotype 1a between post-treatment weeks 4 and 12 in the setting of injection drug use, and 6 participants stopped LDV/SOF after less than 7 weeks (1 usual care, 3 peer mentor, and 2 cash incentive).
Sustained Virologic Response
Among the 110 participants who started LDV/SOF, 100 (91%) participants achieved SVR. SVR rates did not vary by study intervention (usual care, 92%; peers, 91%; cash, 90%; Figure 3b). Of the 10 participants without SVR, 1 participant (UC) died during follow-up (last HCV RNA not detected), 2 participants (1 peer and 1 cash) had HCV relapse, 1 participant (cash) was reinfected (switch from subtype 1b to 1a in the setting of active injection drug use), and 6 participants stopped LDV/SOF after less than 7 weeks (1 UC, 3 peer, and 2 cash) (Figure 1). Of these 6 participants with early discontinuation, 3 did not follow-up after initiation (non-adherent) and 3 reported adverse effects, none of which were severe. Among participants randomized to incentives, the median cash incentive received was $190 (range $0–$220). Among all 144 participants enrolled, noninitiation of LDV/SOF accounted for 77% of non-SVR outcomes (34 of 44 persons without SVR). The overall SVR rate for all enrolled persons was 69% (100 of 144). The proportion of all enrolled participants with HCV cure was not significantly higher in the peer (76%, 41 of 54) and cash (69%, 37 of 54) groups compared to the usual care group (61%, 22 of 36) (P = .22) (Figure 2a, Figure 3c). Similar to HCV treatment initiation, employment and prompt attendance to the initial clinician visit were associated with higher SVR rates. Persons who attended the clinician visit as scheduled were significantly more likely to achieve SVR (81 of 100 participants, 81%) than those who missed or rescheduled the visit 1 or more times (≥3 missed, 1 of 9 participants, 11%) (P < .0001). Employed persons were more likely to achieve SVR (20 of 22 participants, 91%) than unemployed persons (80 of 122 participants, 66%) (P = .01) (Supplemental Figure 2b). In the post-hoc analysis, there was a significant interaction between the peer mentor intervention and employment status (P-value for interaction = .05) (Figure 2b).
Safety and Tolerability
Treatment was well tolerated; the most common adverse events observed were a headache (24%), fatigue (17%), nausea (9%), diarrhea (6%), and insomnia (5%). The rate of adverse events was similar in each group (Table 2). The frequency of drug and alcohol use during treatment was similar to the rates of use at entry and did not differ by intervention group (Supplemental Table 1). No HIV-related infections or adverse events were reported and no change in HIV suppression was observed during the study period (Supplemental Table 2). Serious adverse events were reported in 13 of the 110 (12%) participants who initiated treatment; none were related to LDV/SOF. Three participants discontinued LDV/SOF due to adverse events (1 each with nausea, tinnitus, and insomnia). One participant (UC) became pregnant and stopped treatment after taking 8 weeks of LDV/SOF over a 12-week interval; she achieved SVR, and her baby was born healthy with no detectable HCV RNA. One death (unknown cause) was observed at post-treatment week 8 (UC).
| . | Usual Care (N = 24) (n,%) . | Peer Mentor (N = 45) (n,%) . | Cash Incentive (N = 41) (n,%) . | Total (N = 110) (n,%) . |
|---|---|---|---|---|
| Adverse events at any visit | 13 (54) | 24 (53) | 20 (49) | 57 (52) |
| Headache | 5 (21) | 9 (20) | 12 (29) | 26 (24) |
| Fatigue | 6 (25) | 7 (16) | 6 (15) | 19 (17) |
| Nausea | 2 (8) | 4 (9) | 4 (10) | 10 (9) |
| Diarrhea | 1 (4) | 3 (7) | 3 (7) | 7 (6) |
| Insomnia | 0 | 0 | 6 (15) | 6 (5) |
| Serious adverse eventsa | 3 (13) | 6 (13) | 4 (10) | 13 (12) |
| Discontinued <12 weeksb | 1 (4) | 3 (7) | 2 (5) | 6 (5) |
| <4 weeks | 1 (4) | 2 (4) | 2 (5) | 5 (5) |
| ≥4 weeks | 0 | 1 (2) | 0 | 1 (<1) |
| Deathc | 1 (4) | 0 | 0 | 1 (<1) |
| Pregnancyd | 1 (4) | 0 | 0 | 1 (<1) |
| . | Usual Care (N = 24) (n,%) . | Peer Mentor (N = 45) (n,%) . | Cash Incentive (N = 41) (n,%) . | Total (N = 110) (n,%) . |
|---|---|---|---|---|
| Adverse events at any visit | 13 (54) | 24 (53) | 20 (49) | 57 (52) |
| Headache | 5 (21) | 9 (20) | 12 (29) | 26 (24) |
| Fatigue | 6 (25) | 7 (16) | 6 (15) | 19 (17) |
| Nausea | 2 (8) | 4 (9) | 4 (10) | 10 (9) |
| Diarrhea | 1 (4) | 3 (7) | 3 (7) | 7 (6) |
| Insomnia | 0 | 0 | 6 (15) | 6 (5) |
| Serious adverse eventsa | 3 (13) | 6 (13) | 4 (10) | 13 (12) |
| Discontinued <12 weeksb | 1 (4) | 3 (7) | 2 (5) | 6 (5) |
| <4 weeks | 1 (4) | 2 (4) | 2 (5) | 5 (5) |
| ≥4 weeks | 0 | 1 (2) | 0 | 1 (<1) |
| Deathc | 1 (4) | 0 | 0 | 1 (<1) |
| Pregnancyd | 1 (4) | 0 | 0 | 1 (<1) |
aThe most common serious adverse events during hospitalization were emphysema (5), pneumonia (3), back pain (3), pancreatitis (2), otitis media with effusion (2), syncope (2), and acute kidney injury (2).
bThree participants discontinued treatment due to adverse events (nausea, peer; tinnitus, peer; insomnia, incentive). The remaining 3 participants discontinued treatment early due to non-adherence to clinic and pharmacy visits.
cOne death was observed at post-treatment week 8. The participant tolerated treatment well reporting only nausea at week 1 of treatment that resolved and no serious adverse events or hospitalizations during treatment. Cause of death could not be determined.
dPregnancy occurred in 1 participant at treatment week 10. Participant achieved sustained virologic response; her baby was not hepatitis C virus-infected and was reported to be healthy.
| . | Usual Care (N = 24) (n,%) . | Peer Mentor (N = 45) (n,%) . | Cash Incentive (N = 41) (n,%) . | Total (N = 110) (n,%) . |
|---|---|---|---|---|
| Adverse events at any visit | 13 (54) | 24 (53) | 20 (49) | 57 (52) |
| Headache | 5 (21) | 9 (20) | 12 (29) | 26 (24) |
| Fatigue | 6 (25) | 7 (16) | 6 (15) | 19 (17) |
| Nausea | 2 (8) | 4 (9) | 4 (10) | 10 (9) |
| Diarrhea | 1 (4) | 3 (7) | 3 (7) | 7 (6) |
| Insomnia | 0 | 0 | 6 (15) | 6 (5) |
| Serious adverse eventsa | 3 (13) | 6 (13) | 4 (10) | 13 (12) |
| Discontinued <12 weeksb | 1 (4) | 3 (7) | 2 (5) | 6 (5) |
| <4 weeks | 1 (4) | 2 (4) | 2 (5) | 5 (5) |
| ≥4 weeks | 0 | 1 (2) | 0 | 1 (<1) |
| Deathc | 1 (4) | 0 | 0 | 1 (<1) |
| Pregnancyd | 1 (4) | 0 | 0 | 1 (<1) |
| . | Usual Care (N = 24) (n,%) . | Peer Mentor (N = 45) (n,%) . | Cash Incentive (N = 41) (n,%) . | Total (N = 110) (n,%) . |
|---|---|---|---|---|
| Adverse events at any visit | 13 (54) | 24 (53) | 20 (49) | 57 (52) |
| Headache | 5 (21) | 9 (20) | 12 (29) | 26 (24) |
| Fatigue | 6 (25) | 7 (16) | 6 (15) | 19 (17) |
| Nausea | 2 (8) | 4 (9) | 4 (10) | 10 (9) |
| Diarrhea | 1 (4) | 3 (7) | 3 (7) | 7 (6) |
| Insomnia | 0 | 0 | 6 (15) | 6 (5) |
| Serious adverse eventsa | 3 (13) | 6 (13) | 4 (10) | 13 (12) |
| Discontinued <12 weeksb | 1 (4) | 3 (7) | 2 (5) | 6 (5) |
| <4 weeks | 1 (4) | 2 (4) | 2 (5) | 5 (5) |
| ≥4 weeks | 0 | 1 (2) | 0 | 1 (<1) |
| Deathc | 1 (4) | 0 | 0 | 1 (<1) |
| Pregnancyd | 1 (4) | 0 | 0 | 1 (<1) |
aThe most common serious adverse events during hospitalization were emphysema (5), pneumonia (3), back pain (3), pancreatitis (2), otitis media with effusion (2), syncope (2), and acute kidney injury (2).
bThree participants discontinued treatment due to adverse events (nausea, peer; tinnitus, peer; insomnia, incentive). The remaining 3 participants discontinued treatment early due to non-adherence to clinic and pharmacy visits.
cOne death was observed at post-treatment week 8. The participant tolerated treatment well reporting only nausea at week 1 of treatment that resolved and no serious adverse events or hospitalizations during treatment. Cause of death could not be determined.
dPregnancy occurred in 1 participant at treatment week 10. Participant achieved sustained virologic response; her baby was not hepatitis C virus-infected and was reported to be healthy.
DISCUSSION
With the advent of highly effective HCV DAA therapy, the World Health Organization has established goals for HCV elimination by 2030 [24]. In this context, PLWH have been identified as a population among whom HCV micro-elimination may be achievable [14]. Compared to persons with HCV alone, PLWH are more often engaged in care, screened for HCV infection and have access to HCV DAAs, leading to high rates of HCV identification and cure [13, 25, 26]. Indeed, investigators in the Netherlands and Switzerland have reported that more than 75% of persons with HIV/HCV coinfection followed in their cohorts have achieved SVR [11, 12].
However, these studies also demonstrate lower rates of HCV treatment uptake and cure among HIV-infected PWID compared to men who have sex with men (MSM); for example, Boerekamps and colleagues reported that the SVR rate was 57% and 83% among PWID and MSM, respectively. As such, strategies to eliminate HCV among PLWH will need to focus on overcoming barriers to HCV treatment among persons who use drugs (PWUD) [27]. In this context, our findings provide important insight about the potential for peer mentors and contingent financial incentive programs to facilitate HCV treatment and cure among HIV-infected PWUD.
In the CHAMPS study, we removed system-based barriers to SVR by providing rapid access to DAAs and expert clinicians; despite this, only 69% of the enrolled population of PLWH achieved HCV cure (SVR). Noninitiation of HCV treatment accounted for most of the persons with non-SVR (77%) in our study, and we observed consistent, high SVR rates (90%) among participants who started treatment. We observed that 33% of participants randomized to usual care did not start HCV treatment, which is remarkably similar to the rate of HCV noninitiation reported by Boerekamps and coworkers among HIV-infected PWID in the Netherlands (35%, 79 of 225 PWID) [12]. These observations underscore the notion that HCV treatment initiation is the critical step for HCV cure in PLWH who use drugs and should be the focus of programs designed to achieve HCV micro-elimination in PLWH.
Although rates of HCV treatment initiation were higher among PLWH assigned to peer mentors (83%) and cash incentives (76%) compared to those receiving usual care (67%), these differences did not reach statistical significance based on the pre-defined data analysis plan. These observations may be, in part, explained by the enhanced usual care in our clinic that was delivered by a well-resourced, experienced care team using an innovative nurse-led protocol that was initiated after the study was designed. In settings in which such resources are not available, interventions like peer mentors may have a greater impact on HCV treatment outcomes, particularly in care settings with less experience working with PLWH who use drugs, like primary care clinics. In a post-hoc subgroup analysis of the CHAMPS study population, we found that peer mentors were more effective for under-employed persons for whom the barriers to treatment may be different than from working individuals. This population is likely to benefit from strategies that simplify the approach to HCV treatment by allowing for greater flexibility with less monitoring and visits. Taken together, our observations suggest that interventions to improve the HCV care continuum in PLWH who use drugs should be tailored to the individual’s unique set of barriers to HCV treatment.
Another potential explanation for our findings is the use of peer mentors rather than navigators. The beneficial effect of peer-based interventions have been observed in other disease states [28]. For example, among Black men with poorly controlled diabetes, improvements in control of blood glucose levels was greater in those randomized to peer mentors than those assigned to financial incentives [29]. Similarly, in a randomized trial of racial or ethnic minorities living with HIV and behavioral health co-morbidities, Cabral and coworkers found peer intervention was associated with improved retention in primary HIV care [18]. In New York City, Ford and coworkers demonstrated the successful implementation of a standardized patient navigation protocol in the Check Hep C program at 4 sites; however, only 55% of 235 persons eligible for HCV treatment initiated therapy, which underscores the importance of this step in the care continuum [30]. Although peer mentors in our study were not specifically tasked with helping participants navigate the health care system, qualitative interviews with the CHAMPS mentors suggest this was one of the most valuable services that they provided mentees, and it should be prioritized in future research.
Compared to peer intervention, the potential of contingent financial incentives to improve health outcomes in PLWH and HCV is more complex. For PLWH, financial incentives have effectively increased rates of HIV suppression in some, but not all, settings. For example, El-Sadr et al observed that, among PLWH already engaged in HIV care, financial incentives resulted in higher rates of HIV suppression and clinic attendance compared to usual care. However, among persons newly diagnosed with HIV, financial incentives were not associated with increased linkage to HIV care [31]. In the setting of substance use disorder treatment, contingency management interventions have led to increased attendance at counseling sessions, improved psychiatric care, and decreased substance use [32]. Hybrid strategies that combine peers and incentives at critical steps in the care continuum, such as treatment initiation and medication refills, may be a useful approach worthy of additional research [33].
Among persons who initiated HCV treatment, the SVR rate was high, and rates of treatment adherence, completion, and SVR did not differ between groups. This finding was unexpected; we hypothesized that peer support and cash incentives would enhance treatment adherence and persistence. The lack of an effect on these measures may reflect the intrinsic motivation of those persons who initiated HCV treatment and the effectiveness of the nurse-led HCV care model established in the HIV practice. Our data also demonstrate high SVR rates among participants with active drug and alcohol use during HCV treatment. This finding is consistent with other studies of PWUD treated with DAAs and extends the observation to persons who are actively drinking [34, 35]. These findings are consistent with the recommendation that all PLWH should be considered for curative HCV treatment, and, although treatment persistence and adherence is critical, strategies to increase HCV treatment initiation are likely to result in greater declines in HCV prevalence in populations of PLWH.
CONCLUSIONS
Our study has several potential limitations. First, we studied persons engaged in HIV care for whom HCV cure may be more readily achieved. Second, although the study was conducted at a single center with adequate resources and trained staff and may not be generalizable to other settings, we would expect the interventions to have greater impact in settings in which usual care is not enhanced. Third, the cash incentives may have been too low to reinforce behavioral changes. The amount used was selected based on its effectiveness in substance use disorders treatment; nonetheless, larger incentives for HCV treatment initiation should be investigated. Fourth, we imposed a finite period for HCV treatment initiation; however, when we evaluated treatment uptake over 6 months, there was limited evidence of DAA uptake among untreated participants (4 of 34). Finally, our intervention focused on HCV outcomes among PLWH who use drugs and did not assess interventions to improve HIV suppression and reduce harm due to active substance use disorders.
After providing access to DAAs and skilled clinicians, we found that one-third of PLWH did not initiate HCV treatment with usual care. Failure to initiate treatment accounted for more than 75% of the persons who did not achieve SVR, and most patients who started HCV DAAs were cured, including those with active drug and alcohol use. Overall, our findings support the view that access to HCV DAAs may not be sufficient to eliminate HCV in PLWH with substance use disorders and demonstrate the need for strategies to increase HCV treatment initiation, which is a critical step in the HCV care continuum. Additional research is necessary to better define the role of peers and incentives in HCV elimination programs.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
This work was supported by the National Institutes of Health (grant numbers R01DA16065, R37DA013806, U01DA036935, K24DA034621, and K23DA041294). This work was also made possible by the Johns Hopkins Institute for Clinical and Translational Research and the Center for Clinical Data Analytics (funded in part by grant number UL1 TR001079) and the Johns Hopkins Center for AIDS Research (grant number P30AI094189).
Study medication was provided by Gilead Sciences (Foster City, CA).
Disclosures. K.M.W., O.F.N., J.M., C.G.S., T.H., S.K., K.H., L.A., S.H.M., C.L., and R.K.B. report nothing to disclose. S.B. reports consulting agreements with Gilead, AbbVie, and Merck. M.S.S. reports consulting arrangements with Gilead, AbbVie, and Merck and research grants to his institution from AbbVie, AssemblyBio, Gilead, Proteus Digital Health, and the National Institutes of Health (grant numbers R01DA16065 and K24DA034621).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.





Comments