-
PDF
- Split View
-
Views
-
Cite
Cite
Guillaume Butler-Laporte, Cedric P Yansouni, Katryn Paquette, Alexander Lawandi, Sarah N Stabler, Murtaza Akhter, Adam C Davidson, Marko Gavric, Rehman Jinah, Zahid Saeed, Koray Demir, Sassan Sangsari, Kelly Huang, Amirali Mahpour, Chris Shamatutu, Chelsea Caya, Jean-Marc Troquet, Greg Clark, Titus Wong, Todd C Lee, Robert Stenstrom, David Sweet, Matthew P Cheng, FABLED investigators, Real-world Time to Positivity of 2 Widely Used Commercial Blood Culture Systems in Patients With Severe Manifestations of Sepsis: An Analysis of the FABLED Study, Open Forum Infectious Diseases, Volume 7, Issue 9, September 2020, ofaa371, https://doi.org/10.1093/ofid/ofaa371
Close - Share Icon Share
Abstract
Of all microbiological tests performed, blood cultures have the most impact on patient care. Timely results are essential, especially in the management of sepsis. While there are multiple available blood culture systems on the market, they have never been compared in a prospective study in a critically ill population.
We performed an analysis of the FABLED study cohort to compare culture results and time to positivity (TTP) of 2 widely used blood culture systems: BacT/Alert and BACTEC. In this multisite prospective study, patients with severe manifestations of sepsis had cultures drawn before antibiotics using systematic enrollment criteria and blood drawing methodology allowing for minimization of pre-analytical biases.
We enrolled 315 patients; 144 had blood cultures (47 positive) with BacT/Alert and 171 with BACTEC (53 positive). Patients whose blood cultures were processed using the BacT/Alert system were younger (median, 64 vs 70 years; P = .003), had a higher proportion of HIV (9.03% vs 1.75%; P = .008) and a lower qSOFA (P = .003). There were no statistically significant differences in the most commonly identified bacterial species. TTP was shorter for BACTEC (median [interquartile range {IQR}], 12.5 [10–14] hours) compared with BacT/Alert (median [IQR], 17 [14–21] hours; P < .0001).
In this large prospective multi-centre study comparing the two blood culture systems among patients with severe manifestations of sepsis, and using a rigorous pre-analytical methodology, the BACTEC system yielded positive culture results 4.5 hours earlier than BacT/Alert. These results apply to commonly isolated bacteria. However, our study design did not allow direct comparison of TTP for unusual pathogens nor of clinical sensitivity between systems. More research is needed to determine the clinical implications of this finding.
Identification of bloodstream infections is the cornerstone of any microbiology laboratory, as these are associated with high mortality without appropriate treatment [1]. The processing of positive blood cultures has undergone important changes in recent years, as improvements in bacterial identification [2] and susceptibility testing [3] have significantly decreased turnaround time. For example, Matrix assisted laser desorption ionization - time of flight technology has decreased time to species identification by up to 1 day [2]. In this context, blood culture incubation time now represents the largest delay in obtaining a result.
Since the 1991 Food and Drug Administration (FDA) review criteria for assessment of blood culture systems, a sufficient criterion for clinical use is to show substantial equivalence with a previously FDA-cleared blood culture system [4]. As a result, manufacturers of the 2 most used systems, BacT/Alert (Biomérieux, Marcy-l’Étoile, France) and BACTEC (Becton, Dickinson and Company, Franklin Lakes, NJ, USA), have often utilized this pathway and shown equivalence to a product from the same brand’s previous-generation culture system. Few recent studies have directly compared the 2 systems. Previous studies used artificially seeded blood cultures or could not adjust for patient-level factors [5, 6]. There remains a paucity of data comparing the 2 systems’ performances in a clinical setting, especially in the critically ill, where timely diagnosis is crucial.
In a prospective multicenter study, we previously reported on the yield of blood cultures pre- and postantibiotics among 325 patients presenting with severe manifestations of sepsis [7]. These patients were defined by prespecified criteria, and a standardized blood specimen collection method was used. To increase the generalizability of those results, participating sites used their routine blood culture protocols and incubation systems. In this analysis, we set out to compare the time to positivity of the BacT/Alert and BACTEC blood culture systems among a septic patient population with an increased probability of bloodstream infection while controlling for pre-analytical factors.
METHODS
Participants
The inclusion and exclusion criteria of the FABLED study have previously been published [7]. Briefly, patients were included if they presented to the emergency department with severe manifestations of sepsis and had 2 sets of blood cultures drawn before the start of antibiotics. Patients required the following markers of disease severity: either a systolic blood pressure <90 mmHg or serum lactate level ≥4 mmol/L. If patients met the study criteria, additional sets of blood cultures were obtained within 240 minutes after the initiation of antimicrobial therapy.
For the purposes of this analysis, the 2 pairs of sites that were part of the same health center and used the same microbiology laboratory were considered single centers. One site was excluded from the analysis, as it was the only 1 to use the PHOENIX system (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) and had a lower proportion of patients, preventing further statistical analysis.
Blood Culture Collection and Incubation
Before starting antibiotics, a set of blood cultures was defined as 1 aerobic and 1 anaerobic culture vial from a single venipuncture site. Each set was performed at a different venipuncture site. For 1 of the participating sites, the second set was defined as a single aerobic vial, per institutional policy. Following initiation of antibiotics, 2 additional sets of blood cultures were drawn at 4 of the participating institutions. For the other site, a single additional set of cultures was performed based on their ethics board requirements to obtain the minimum amount of blood possible, per current recommendations on blood culture volumes (Supplementary Table 1) [8]. Blood culture collection was otherwise performed as per the manufacturer’s recommendations, including blood volume per bottle. Bottles were processed per local standard operating procedures at accredited microbiology laboratories. All sites used blood culture bottles containing antibiotic binding resins. Apart from St. Paul’s Hospital, automated incubation systems remained the same throughout the study period (Supplementary Table 1).
Analysis
We compared time to positivity (TTP) between BacT/Alert and BACTEC blood culture systems. Contaminants were defined as low-virulence skin flora recovered from a single set of blood cultures when other sets were negative and were all reviewed by 2 infectious diseases and medical microbiology specialists (M.P.C. and C.P.Y.) blinded to the blood culture system. TTP was defined as the time (in hours) from blood draw to a positive result flagged by the automated culture system, as reported in the Laboratory Information System. In cases of polymicrobial infections, the same time to positivity was assigned to all organisms, as it is impossible to determine which organism was the trigger for the positive alarm. Patient characteristics are presented by culture system. Baseline characteristics, as well as and pre- and postantibiotic blood culture results, were compared using Pearson chi-square or Mann-Whitney U test, as appropriate. Overall TTP was compared using Mann-Whitney U as the primary analysis. A secondary analysis on TTP stratified by gram stain was performed. Our analysis was restricted to bacterial isolates and did not consider fungal pathogens.
As a further sensitivity analysis, we performed a multivariable Cox proportional hazard regression analysis on the primary analysis by adding covariates in our model that were statistically significant (P ≤ .05) in the univariable analysis and by adding the most frequent bacterial species as “dummy variables” (defined as those with a count of 3 or more in each blood culture system). Finally, as the proportional hazards assumption is difficult to test for, we also employed multivariable accelerated failure time models with the same covariates as above using Weibull, exponential, log-normal, logistic, and log-logistic distributions. All analyses were performed using the base R statistical package (3.5.1) and the survival package (2.42–3).
Ethics
The study was approved by the research ethics board of each participating institution.
RESULTS
We enrolled 325 patients, of whom 315 had cultures taken on either the BACTEC (n = 171, 54.3%) or BacT/Alert (n = 144, 45.7%) system. Patient characteristics are presented in Table 1. Notably, patients whose blood cultures were processed using the BacT/Alert system were younger (median age, of 64 vs 70 years; P = .003) and had a higher proportion of HIV infection (9.03% vs 1.75%; P = .008). BACTEC patients had a higher qSOFA (P = .003) score, with 23.4% of enrolled patients having a qSOFA of 3 (vs 11.8% in BacT Alert) and 43.3% having a qSOFA of 2 (vs 39.6% in BacT Alert).
| Characteristic, No. (%) . | System . | . | P Valuec . |
|---|---|---|---|
| . | BacT/Alert (n = 144) . | BACTEC (n = 171) . | . |
| Age, median (IQR), y | 64 (51–74.3) | 70 (58–84) | .003 |
| Male sex | 90 (62.5) | 110 (64.3) | .814 |
| Charlson comorbidity index, median (IQR) | 1 (1–3) | 1 (1–3) | .760 |
| HIV | 13 (9.03) | 3 (1.75) | .004 |
| qSOFA, median (IQR) | 2 (1–2) | 2 (1–2) | .003 |
| qSOFA = 0 | 15 (10.4) | 15 (8.77) | n/a |
| qSOFA = 1 | 55 (38.2) | 42 (24.6) | n/a |
| qSOFA = 2 | 57 (39.6) | 74 (43.2) | n/a |
| qSOFA = 3 | 17 (11.8) | 40 (23.4) | n/a |
| Systolic blood pressure, median (IQR) | 89 (83–119) | 99 (82–126) | .081 |
| Lactate, median (IQR), mmol/L | 4.2 (2.70–5.28) | 4.45 (2.98–5.80) | .244 |
| Sources of infection (nonexclusive categories) | |||
| Respiratory | 50 (34.7) | 56 (32.7) | .721 |
| Genitourinary | 20 (13.9) | 38 (22.2) | .060 |
| Gastrointestinal | 25 (17.4) | 28 (16.4) | .880 |
| Skin and soft tissue | 19 (13.2) | 21 (12.3) | .866 |
| Endovascular | 5 (3.47) | 3 (1.75) | .477 |
| Central nervous system | 1 (0.69) | 2 (1.17) | 1 |
| Unknown | 26 (18.1) | 23 (13.5) | .278 |
| Positive blood cultures (excluding contaminants) | 47 (32.3) | 53 (31.0) | .808 |
| Gram-positive | 30 (20.8) | 24 (14.0) | .134 |
| Staphylococcus aureus | 12 (8.33) | 4 (2.34) | .020 |
| Streptococcus pneumoniae | 7 (4.86) | 5 (2.92) | .393 |
| Other Streptococcus spp. | 3 (2.08) | 8 (4.68) | .237 |
| Viridans group streptococci | 4 (2.78) | 3 (1.75) | .706 |
| Enterococcus spp. | 1 (0.69) | 2 (1.17) | 1 |
| Actinomyces spp. | 0 (0) | 1 (0.58) | 1 |
| Clostridium perfringens | 1 (0.69) | 0 (0) | .457 |
| Gemella morbillorum | 1 (0.69) | 0 (0) | .457 |
| Staphylococcus epidermidis | 1 (0.69) | 0 (0) | .457 |
| Gram-negative | 20 (13.9) | 37 (21.6) | .080 |
| Escherichia coli | 8 (5.56) | 18 (10.5) | .150 |
| Klebsiella spp. (except aerogenes) | 6 (4.17) | 9 (5.26) | .793 |
| Proteus mirabilis | 1 (0.69) | 3 (1.75) | .628 |
| Bacteroides fragilis | 1 (0.69) | 2 (1.17) | 1 |
| Klebsiella aerogenes | 1 (0.69) | 2 (1.17) | 1 |
| Haemophilus influenzae | 0 (0) | 2 (1.17) | .502 |
| Morganella morganii | 1 (0.69) | 1 (0.58) | 1 |
| Butyricimonas virosa | 1 (0.69) | 0 (0) | .457 |
| Salmonella enteritidis | 1 (0.69) | 0 (0) | .457 |
| Candida spp. | 2 (1.39) | 0 (0) | .208 |
| No growth (including contaminants) | 97 (67.4) | 118 (69.0) | .808 |
| Polymicrobial culturea | 6 (4.17) | 8 (4.68) | 1 |
| Contaminantb | 6 (4.17) | 9 (5.26) | .793 |
| Characteristic, No. (%) . | System . | . | P Valuec . |
|---|---|---|---|
| . | BacT/Alert (n = 144) . | BACTEC (n = 171) . | . |
| Age, median (IQR), y | 64 (51–74.3) | 70 (58–84) | .003 |
| Male sex | 90 (62.5) | 110 (64.3) | .814 |
| Charlson comorbidity index, median (IQR) | 1 (1–3) | 1 (1–3) | .760 |
| HIV | 13 (9.03) | 3 (1.75) | .004 |
| qSOFA, median (IQR) | 2 (1–2) | 2 (1–2) | .003 |
| qSOFA = 0 | 15 (10.4) | 15 (8.77) | n/a |
| qSOFA = 1 | 55 (38.2) | 42 (24.6) | n/a |
| qSOFA = 2 | 57 (39.6) | 74 (43.2) | n/a |
| qSOFA = 3 | 17 (11.8) | 40 (23.4) | n/a |
| Systolic blood pressure, median (IQR) | 89 (83–119) | 99 (82–126) | .081 |
| Lactate, median (IQR), mmol/L | 4.2 (2.70–5.28) | 4.45 (2.98–5.80) | .244 |
| Sources of infection (nonexclusive categories) | |||
| Respiratory | 50 (34.7) | 56 (32.7) | .721 |
| Genitourinary | 20 (13.9) | 38 (22.2) | .060 |
| Gastrointestinal | 25 (17.4) | 28 (16.4) | .880 |
| Skin and soft tissue | 19 (13.2) | 21 (12.3) | .866 |
| Endovascular | 5 (3.47) | 3 (1.75) | .477 |
| Central nervous system | 1 (0.69) | 2 (1.17) | 1 |
| Unknown | 26 (18.1) | 23 (13.5) | .278 |
| Positive blood cultures (excluding contaminants) | 47 (32.3) | 53 (31.0) | .808 |
| Gram-positive | 30 (20.8) | 24 (14.0) | .134 |
| Staphylococcus aureus | 12 (8.33) | 4 (2.34) | .020 |
| Streptococcus pneumoniae | 7 (4.86) | 5 (2.92) | .393 |
| Other Streptococcus spp. | 3 (2.08) | 8 (4.68) | .237 |
| Viridans group streptococci | 4 (2.78) | 3 (1.75) | .706 |
| Enterococcus spp. | 1 (0.69) | 2 (1.17) | 1 |
| Actinomyces spp. | 0 (0) | 1 (0.58) | 1 |
| Clostridium perfringens | 1 (0.69) | 0 (0) | .457 |
| Gemella morbillorum | 1 (0.69) | 0 (0) | .457 |
| Staphylococcus epidermidis | 1 (0.69) | 0 (0) | .457 |
| Gram-negative | 20 (13.9) | 37 (21.6) | .080 |
| Escherichia coli | 8 (5.56) | 18 (10.5) | .150 |
| Klebsiella spp. (except aerogenes) | 6 (4.17) | 9 (5.26) | .793 |
| Proteus mirabilis | 1 (0.69) | 3 (1.75) | .628 |
| Bacteroides fragilis | 1 (0.69) | 2 (1.17) | 1 |
| Klebsiella aerogenes | 1 (0.69) | 2 (1.17) | 1 |
| Haemophilus influenzae | 0 (0) | 2 (1.17) | .502 |
| Morganella morganii | 1 (0.69) | 1 (0.58) | 1 |
| Butyricimonas virosa | 1 (0.69) | 0 (0) | .457 |
| Salmonella enteritidis | 1 (0.69) | 0 (0) | .457 |
| Candida spp. | 2 (1.39) | 0 (0) | .208 |
| No growth (including contaminants) | 97 (67.4) | 118 (69.0) | .808 |
| Polymicrobial culturea | 6 (4.17) | 8 (4.68) | 1 |
| Contaminantb | 6 (4.17) | 9 (5.26) | .793 |
Abbreviation: IQR, interquartile range.
aBlood cultures that included multiple species.
bIncluding contaminants recovered from polymicrobial cultures that included a noncontaminant species.
cP values are not corrected for multiple comparisons.
| Characteristic, No. (%) . | System . | . | P Valuec . |
|---|---|---|---|
| . | BacT/Alert (n = 144) . | BACTEC (n = 171) . | . |
| Age, median (IQR), y | 64 (51–74.3) | 70 (58–84) | .003 |
| Male sex | 90 (62.5) | 110 (64.3) | .814 |
| Charlson comorbidity index, median (IQR) | 1 (1–3) | 1 (1–3) | .760 |
| HIV | 13 (9.03) | 3 (1.75) | .004 |
| qSOFA, median (IQR) | 2 (1–2) | 2 (1–2) | .003 |
| qSOFA = 0 | 15 (10.4) | 15 (8.77) | n/a |
| qSOFA = 1 | 55 (38.2) | 42 (24.6) | n/a |
| qSOFA = 2 | 57 (39.6) | 74 (43.2) | n/a |
| qSOFA = 3 | 17 (11.8) | 40 (23.4) | n/a |
| Systolic blood pressure, median (IQR) | 89 (83–119) | 99 (82–126) | .081 |
| Lactate, median (IQR), mmol/L | 4.2 (2.70–5.28) | 4.45 (2.98–5.80) | .244 |
| Sources of infection (nonexclusive categories) | |||
| Respiratory | 50 (34.7) | 56 (32.7) | .721 |
| Genitourinary | 20 (13.9) | 38 (22.2) | .060 |
| Gastrointestinal | 25 (17.4) | 28 (16.4) | .880 |
| Skin and soft tissue | 19 (13.2) | 21 (12.3) | .866 |
| Endovascular | 5 (3.47) | 3 (1.75) | .477 |
| Central nervous system | 1 (0.69) | 2 (1.17) | 1 |
| Unknown | 26 (18.1) | 23 (13.5) | .278 |
| Positive blood cultures (excluding contaminants) | 47 (32.3) | 53 (31.0) | .808 |
| Gram-positive | 30 (20.8) | 24 (14.0) | .134 |
| Staphylococcus aureus | 12 (8.33) | 4 (2.34) | .020 |
| Streptococcus pneumoniae | 7 (4.86) | 5 (2.92) | .393 |
| Other Streptococcus spp. | 3 (2.08) | 8 (4.68) | .237 |
| Viridans group streptococci | 4 (2.78) | 3 (1.75) | .706 |
| Enterococcus spp. | 1 (0.69) | 2 (1.17) | 1 |
| Actinomyces spp. | 0 (0) | 1 (0.58) | 1 |
| Clostridium perfringens | 1 (0.69) | 0 (0) | .457 |
| Gemella morbillorum | 1 (0.69) | 0 (0) | .457 |
| Staphylococcus epidermidis | 1 (0.69) | 0 (0) | .457 |
| Gram-negative | 20 (13.9) | 37 (21.6) | .080 |
| Escherichia coli | 8 (5.56) | 18 (10.5) | .150 |
| Klebsiella spp. (except aerogenes) | 6 (4.17) | 9 (5.26) | .793 |
| Proteus mirabilis | 1 (0.69) | 3 (1.75) | .628 |
| Bacteroides fragilis | 1 (0.69) | 2 (1.17) | 1 |
| Klebsiella aerogenes | 1 (0.69) | 2 (1.17) | 1 |
| Haemophilus influenzae | 0 (0) | 2 (1.17) | .502 |
| Morganella morganii | 1 (0.69) | 1 (0.58) | 1 |
| Butyricimonas virosa | 1 (0.69) | 0 (0) | .457 |
| Salmonella enteritidis | 1 (0.69) | 0 (0) | .457 |
| Candida spp. | 2 (1.39) | 0 (0) | .208 |
| No growth (including contaminants) | 97 (67.4) | 118 (69.0) | .808 |
| Polymicrobial culturea | 6 (4.17) | 8 (4.68) | 1 |
| Contaminantb | 6 (4.17) | 9 (5.26) | .793 |
| Characteristic, No. (%) . | System . | . | P Valuec . |
|---|---|---|---|
| . | BacT/Alert (n = 144) . | BACTEC (n = 171) . | . |
| Age, median (IQR), y | 64 (51–74.3) | 70 (58–84) | .003 |
| Male sex | 90 (62.5) | 110 (64.3) | .814 |
| Charlson comorbidity index, median (IQR) | 1 (1–3) | 1 (1–3) | .760 |
| HIV | 13 (9.03) | 3 (1.75) | .004 |
| qSOFA, median (IQR) | 2 (1–2) | 2 (1–2) | .003 |
| qSOFA = 0 | 15 (10.4) | 15 (8.77) | n/a |
| qSOFA = 1 | 55 (38.2) | 42 (24.6) | n/a |
| qSOFA = 2 | 57 (39.6) | 74 (43.2) | n/a |
| qSOFA = 3 | 17 (11.8) | 40 (23.4) | n/a |
| Systolic blood pressure, median (IQR) | 89 (83–119) | 99 (82–126) | .081 |
| Lactate, median (IQR), mmol/L | 4.2 (2.70–5.28) | 4.45 (2.98–5.80) | .244 |
| Sources of infection (nonexclusive categories) | |||
| Respiratory | 50 (34.7) | 56 (32.7) | .721 |
| Genitourinary | 20 (13.9) | 38 (22.2) | .060 |
| Gastrointestinal | 25 (17.4) | 28 (16.4) | .880 |
| Skin and soft tissue | 19 (13.2) | 21 (12.3) | .866 |
| Endovascular | 5 (3.47) | 3 (1.75) | .477 |
| Central nervous system | 1 (0.69) | 2 (1.17) | 1 |
| Unknown | 26 (18.1) | 23 (13.5) | .278 |
| Positive blood cultures (excluding contaminants) | 47 (32.3) | 53 (31.0) | .808 |
| Gram-positive | 30 (20.8) | 24 (14.0) | .134 |
| Staphylococcus aureus | 12 (8.33) | 4 (2.34) | .020 |
| Streptococcus pneumoniae | 7 (4.86) | 5 (2.92) | .393 |
| Other Streptococcus spp. | 3 (2.08) | 8 (4.68) | .237 |
| Viridans group streptococci | 4 (2.78) | 3 (1.75) | .706 |
| Enterococcus spp. | 1 (0.69) | 2 (1.17) | 1 |
| Actinomyces spp. | 0 (0) | 1 (0.58) | 1 |
| Clostridium perfringens | 1 (0.69) | 0 (0) | .457 |
| Gemella morbillorum | 1 (0.69) | 0 (0) | .457 |
| Staphylococcus epidermidis | 1 (0.69) | 0 (0) | .457 |
| Gram-negative | 20 (13.9) | 37 (21.6) | .080 |
| Escherichia coli | 8 (5.56) | 18 (10.5) | .150 |
| Klebsiella spp. (except aerogenes) | 6 (4.17) | 9 (5.26) | .793 |
| Proteus mirabilis | 1 (0.69) | 3 (1.75) | .628 |
| Bacteroides fragilis | 1 (0.69) | 2 (1.17) | 1 |
| Klebsiella aerogenes | 1 (0.69) | 2 (1.17) | 1 |
| Haemophilus influenzae | 0 (0) | 2 (1.17) | .502 |
| Morganella morganii | 1 (0.69) | 1 (0.58) | 1 |
| Butyricimonas virosa | 1 (0.69) | 0 (0) | .457 |
| Salmonella enteritidis | 1 (0.69) | 0 (0) | .457 |
| Candida spp. | 2 (1.39) | 0 (0) | .208 |
| No growth (including contaminants) | 97 (67.4) | 118 (69.0) | .808 |
| Polymicrobial culturea | 6 (4.17) | 8 (4.68) | 1 |
| Contaminantb | 6 (4.17) | 9 (5.26) | .793 |
Abbreviation: IQR, interquartile range.
aBlood cultures that included multiple species.
bIncluding contaminants recovered from polymicrobial cultures that included a noncontaminant species.
cP values are not corrected for multiple comparisons.
There were no statistically significant differences in the most commonly identified bacterial species before or after antibiotics (Table 1; Supplementary Table 2). There was a total of 13 polymicrobial cultures, of which 1 was due to S. epidermidis contaminating a culture of S. dysgalactiae and 1 was B. cereus contaminating a culture of S. aureus. Of the remaining 11 polymycrobial cultures, all involved at least 1 bacterium commonly found in the gastrointestinal tract. This includes 1 culture that grew both B. fragilis and Actinomyces spp. and another that grew C. perfringens, S. enteritidis, and G. morbillorum.
Before initiating antibiotics, TTP was recorded for 98 of 100 patients. TTP was unavailable for 1 culture growing S. aureus and 1 culture growing B. virosa. TTP (Figure 1) was shorter for BACTEC (median [interquartile range {IQR}], 12.5 [10–14] hours) compared with BacT/Alert (median [IQR], 17 [14–21] hours; P < .0001). For gram-positive organisms, TTP for BacT/Alert (IQR) was 18 (15–20) hours vs 13 (9–13.5) hours for BACTEC (median difference, 5 hours; P = .0004). For gram-negative organisms, TTP for BacT/Alert (IQR) was 14.5 (13–17.8) hours vs 12 (10.3–14) hours for BACTEC (median difference, 2.5 hours; P = .002). The Cox proportional hazard model still showed a statistically significant TTP difference in favor of BACTEC (hazard ratio, 3.49; 95% CI, 2.06–5.92) (Supplementary Table 3). Likewise, 4 of the accelerated failure time models showed a statistically significant difference in favor of BACTEC (P < 4.3 × 10-6). Only the exponentially distributed accelerated failure time model did not show a statistically significant difference between blood culture systems. After initiating antibiotics, both systems performed similarly in terms of TTP and types of organisms recovered (Table 2; Supplementary Figure 1).
Time to Positivity for Both Automated Blood Culture Systems, Divided by Gram-Negative and Gram-Positive Organisms
| Median Time to Positivity (IQR) of Blood Cultures Pre-antibiotics . | . | . | . |
|---|---|---|---|
| Organisms | System | P Value | |
| BacT/Alert | BACTEC | ||
| Gram-positive organisms | 18 (15–20) | 13 (9–13.5) | .0004 |
| Gram-negative organisms | 14.5 (13–17.8) | 12 (10.3–14) | .002 |
| Median Time to Positivity (IQR) of Blood Cultures Postantibiotics | |||
| Organisms | System | P Value | |
| BacT/Alert | BACTEC | ||
| Gram-positive organisms | 17 (12–22) | 16 (14.5–20.5) | .901 |
| Gram-negative organisms | 18 (14.3–24) | 17.5 (11–25.3) | .603 |
| Median Time to Positivity (IQR) of Blood Cultures Pre-antibiotics . | . | . | . |
|---|---|---|---|
| Organisms | System | P Value | |
| BacT/Alert | BACTEC | ||
| Gram-positive organisms | 18 (15–20) | 13 (9–13.5) | .0004 |
| Gram-negative organisms | 14.5 (13–17.8) | 12 (10.3–14) | .002 |
| Median Time to Positivity (IQR) of Blood Cultures Postantibiotics | |||
| Organisms | System | P Value | |
| BacT/Alert | BACTEC | ||
| Gram-positive organisms | 17 (12–22) | 16 (14.5–20.5) | .901 |
| Gram-negative organisms | 18 (14.3–24) | 17.5 (11–25.3) | .603 |
Time to Positivity for Both Automated Blood Culture Systems, Divided by Gram-Negative and Gram-Positive Organisms
| Median Time to Positivity (IQR) of Blood Cultures Pre-antibiotics . | . | . | . |
|---|---|---|---|
| Organisms | System | P Value | |
| BacT/Alert | BACTEC | ||
| Gram-positive organisms | 18 (15–20) | 13 (9–13.5) | .0004 |
| Gram-negative organisms | 14.5 (13–17.8) | 12 (10.3–14) | .002 |
| Median Time to Positivity (IQR) of Blood Cultures Postantibiotics | |||
| Organisms | System | P Value | |
| BacT/Alert | BACTEC | ||
| Gram-positive organisms | 17 (12–22) | 16 (14.5–20.5) | .901 |
| Gram-negative organisms | 18 (14.3–24) | 17.5 (11–25.3) | .603 |
| Median Time to Positivity (IQR) of Blood Cultures Pre-antibiotics . | . | . | . |
|---|---|---|---|
| Organisms | System | P Value | |
| BacT/Alert | BACTEC | ||
| Gram-positive organisms | 18 (15–20) | 13 (9–13.5) | .0004 |
| Gram-negative organisms | 14.5 (13–17.8) | 12 (10.3–14) | .002 |
| Median Time to Positivity (IQR) of Blood Cultures Postantibiotics | |||
| Organisms | System | P Value | |
| BacT/Alert | BACTEC | ||
| Gram-positive organisms | 17 (12–22) | 16 (14.5–20.5) | .901 |
| Gram-negative organisms | 18 (14.3–24) | 17.5 (11–25.3) | .603 |
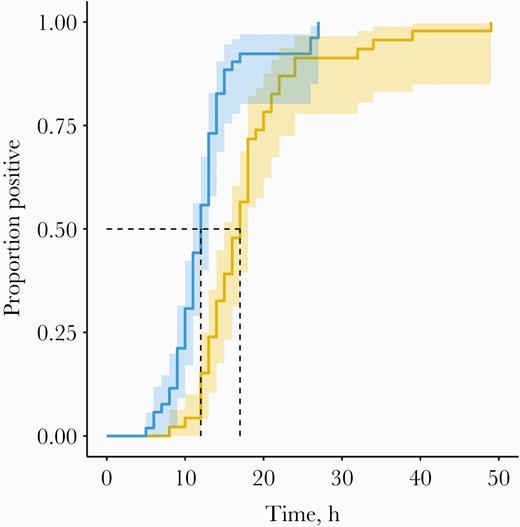
Pre-antibiotic cumulative event time to positivity curve, with 95% confidence intervals. Blue: BACTEC. Orange: BacT/Alert.
DISCUSSION
In this study, we prospectively collected patient-level data on potential confounders of TTP and controlled for them in the analysis. Such patient-level variables and pre-analytic considerations could have been sources of bias in existing data comparing these 2 automated blood culture systems, which are widely used in North America. We observed that both systems had a comparable proportion of positive blood cultures in a homogeneous patient population, yet TTP was shorter for the BACTEC system for the organisms recovered from study patients. Recognizing differences in laboratory workflow and reporting systems, the practical impact of this difference will likely vary between laboratories. However, in environments where a 4-hour delay in blood culture positivity may be amplified into a longer downstream delay, our results suggest that time to positivity could be taken into account when selecting a blood culture system. Further, in patients with severe manifestations of sepsis, prompt microbial identification and susceptibility testing could result in a shorter time to appropriate treatment and improved clinical outcomes, as suggested by recent guidelines on sepsis [9].
Further, the TTP difference between the blood culture systems is minimized after antibiotic administration. One explanation for this lies in the composition of the 2 different blood culture bottle medias. While both contain antibiotic neutralization resins, if the BACTEC system and media were less effective at the neutralization of the antibiotics, then the overall load of viable bacteria within the culture would be reduced and therefore take longer to achieve a detectable inoculum. While this seems plausible and the stability in the TTPs of the gram-positive organisms with the BacT/Alert is consistent, we also saw a significant increase in the system’s TTPs for gram-negative organisms after administration of antibiotics. Consequently, we are unable to draw conclusions on the issue of postantibiotic TTP. However, its importance should not be understated. Given the drive for early antibiotic administration in patients with severe sepsis and septic shock, further studies to investigate the potential consequences on TTP after antibiotics between the 2 blood culture systems are needed.
Although our study methodology was novel, other groups have previously suggested a 4.5-hour shorter TTP with the BACTEC system when compared with BacT/Alert [6]. However, unlike our study, they did not account for patient level. As both culture systems are proprietary, it is unclear which of their components explains the difference, necessitating further research into the optimization of automated blood culture systems.
Our study has several limitations. First, the impact of this difference on patient mortality or other clinical outcomes could not be assessed. However, as there appear to be differences in the performance of 2 of the major blood culture systems, our study has implications for the interpretation of studies that have used TTP as a diagnostic tool or to compare laboratory methods [10, 11]. Second, 1 of the study sites using BacT/Alert obtained 1 less anaerobic blood culture bottle than the sites using BACTEC. This may have influenced results since many facultative anaerobes can grow more quickly in an anaerobic environment. However, even when adjusting for this through sensitivity analysis, the difference between BACTEC and BacT/Alert remained statistically significant. Further, there is controversy as to the need for a fourth culture bottle [12], and we believe it is unlikely to have altered our results. Third, although we performed statistical adjustment, we were unable to account for all differences in pre-analytic factors due to intrinsic differences between institutions. Fourth, as BACTEC and BacT/Alert are commercial brand names, it is reasonable to assume that both systems have evolved since they were first commercialized, and our comparative study may not apply to all generations of these systems. As the majority of blood cultures in the BacT/Alert group were with the 3D system, this analysis does not reliably convey what would happen with the Virtuo system [13]. Fifth, although the rates of culture positivity were similar across sites and systems, our study design did not allow us to directly compare the systems’ clinical sensitivity for the recovery of pathogens and did not allow us to completely control for bacterial species. These factors may obviously affect outcomes independently of TTP. Finally, while nurses, physicians, and phlebotomists were instructed on the correct amount of blood to be drawn per culture bottle, specimens were not rejected if the volume was too small, and we did not weigh each bottle. However, in this study we do not believe there were systemic biases in adequacy of collection associated with 1 or the other culture system.
CONCLUSIONS
In a real-world clinical setting among patients with severe manifestations of sepsis, we show that the median BACTEC time to positivity is 4.5 hours shorter than that of BacT/Alert for commonly isolated bacteria.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors want to thank all study participants.
Financial support. Vancouver Coastal Health, St. Paul’s Hospital Foundation Emergency Department Support Fund, the Fonds de recherche Sante - Québec, and the Maricopa Medical Foundation.
Potential conflicts of interest. M. P. C. reports grants from McGill Interdisciplinary Initiative in Infection and Immunity, grants from Canadian Institutes of Health Research, during the conduct of the study; personal fees from GEn1E Lifesciences (as a member of the Scientific Advisory Board), outside the submitted work. All other authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. G.B.L., C.P.Y., T.C.L., and M.P.C. designed this study. C.P.Y., K.P., A.L., S.N.S., M.A., A.C.D., M.G., R.J., Z.S., K.D., S.S., K.H., A.M., C.S., C.C., J.M.T., G.C., T.W., R.S., D.S., and M.P.C. conceived the FABLED study and implemented patient enrollment in their respective institutions. G.B.L., T.C.L., and M.P.C. analyzed the data. G.B.L. and M.P.C. drafted the original manuscript. All authors contributed to the original manuscript editing process. G.B.L., A.L., T.C.L., and M.P.C. wrote the reviews and the updated manuscript.
Availability of data. Researchers with a reasonable analysis plan will be able to access the data through the corresponding author.
Patient consent. The FABLED study and this analysis were approved by every participating institution’s ethics review board: Maricopa Integrated Health Center research ethics board, McGill University Health Centre research ethics board, Saint-Paul’s Hospital research ethics board, Surrey Memorial Hospital research ethics board, and Vancouver Coastal Health research ethics board. Written informed consent was obtained from every participant enrolled.





Comments