-
PDF
- Split View
-
Views
-
Cite
Cite
J S Davis, M Young, C Marshall, J Tate-Baker, M Madison, S Sharma, C Silva, T Jones, J Davies, Minimal Compared With Standard Monitoring During Sofosbuvir-Based Hepatitis C Treatment: A Randomized Controlled Trial, Open Forum Infectious Diseases, Volume 7, Issue 2, February 2020, ofaa022, https://doi.org/10.1093/ofid/ofaa022
Close - Share Icon Share
Abstract
Oral direct-acting antiviral agents (DAAs) for hepatitis C virus (HCV) became government subsidized in Australia in March 2016, bringing the interferon era to a close. The ideal monitoring schedule for patients receiving DAAs is unclear.
This study is a randomized controlled trial comparing standard with minimal monitoring in adults receiving sofosbuvir-based therapy for HCV genotypes 1 or 3. Exclusion criteria were cirrhosis or predicted poor adherence. Standard monitoring included blood tests and face-to-face clinic visits at treatment weeks 4 and 12 and 12 weeks after treatment completion. Minimal monitoring included a phone call at weeks 4 and 12 and one set of blood tests plus a clinic visit 12 weeks after treatment completion. The coprimary outcomes were as follows: (1) proportion of participants with sustained virological response; (2) staff time spent on patient support; and (3) patient satisfaction on a 10-point Likert scale.
Thirty-six patients were randomized to standard monitoring and 38 to minimal monitoring. Sustained virological response at 12 weeks after the end of treatment was documented in 32 of 36 (89%) in the standard versus 37 of 38 (97%) in the minimal monitoring group. Staff time was nonsignificantly longer in the standard group (median 69 [interquartile range {IQR}, 54–80] versus 52 [IQR, 40–75] minutes). Patient satisfaction scores were not different (mean 9.8 of 10 standard versus 9.6 of 10 minimal group). There was no difference in adverse events or unplanned hospital visits; mean per-patient blood test costs were higher in the standard monitoring group ($432 versus $123, P < .001).
On-treatment monitoring with blood tests and clinic visits may not be necessary during sofosbuvir-based HCV treatment in selected patients.
The World Health Organization launched the first global health strategy on viral hepatitis in 2016 with ambitious targets of achieving an 80% reduction in the incidence of hepatitis C virus (HCV) infection and a 65% reduction in HCV-related mortality by 2030 [1]. This is feasible because the advent of direct-acting antiviral (DAA) therapy means that, in contrast to earlier interferon-based regimens, almost all people are now suitable for treatment and cure rates are over 90%. However, for the WHO targets to be achievable, simplified models of care are essential to enable broader access to treatment. Evidence to support the safety, acceptability, and noninferiority of less frequent monitoring during treatment is crucial to support the rapid development and scale up of innovative models of care.
Sofosbuvir, daclatasvir, and ledipasvir—DAA drugs for chronic HCV infection—became government subsidized in Australia in March 2016, bringing the interferon era to a close. These treatments have minimal side effects, require no injections, and require only 12 weeks of oral treatment in most people. In multiple phase 3 trials and in postmarketing data from the United States and Europe, sofosbuvir-based combination therapy is very safe, with low rates of adverse events [2–6]. This is a major contrast to interferon-based regimens, in which frequent clinical reviews and blood tests are required to monitor both safety and efficacy during treatment. Most services providing interferon-based HCV treatment use specialist support nurses, and treatment monitoring for interferon-based therapy involves blood tests and face-to-face clinic visits every 2–4 weeks for 24–48 weeks.
At the time that DAA oral regimens became publicly funded in Australia, there were no clear guidelines for pre-, on-, and posttreatment monitoring of these regimens. Monitoring in the phase 3 clinical trials for ledipasvir-sofosbuvir for genotype 1 and sofosbuvir plus daclatasvir for genotype 3 HCV infection occurred at least every 2 weeks in those receiving 12-week courses of therapy [2, 6]. The commercial product information provides no guidance regarding recommended monitoring on treatment, and many clinicians involved in the treatment of chronic HCV infection were concerned that decreasing the amount of on-treatment monitoring could lead to a decrease in adherence and thus an increase in failure rates. The Australian consensus recommendations for the management of HCV infection published in April 2016 suggested face-to-face clinic visits and blood tests at baseline, treatment weeks 4 and 12, and 12 weeks after completion of treatment [7].
We initiated a pilot randomized trial comparing minimal with standard monitoring in uncomplicated patients treated with sofosbuvir-based therapy for HCV, with the intention of running a subsequent larger multisite trial, the design of which was informed by this trial.
In September 2018, before publication of the present study, the Australian consensus HCV statement was updated to recommend minimal monitoring for most patients treated for HCV with DAA-based regimens (www.hepcguidelines.org.au). Hence, the planned subsequent trial was rendered unfeasible due to a lack of equipoise. We present the findings of the pilot trial here.
METHODS
Setting and Participants
Between December 2016 and December 2017, we enrolled patients from the outpatient liver clinics of 2 Australian teaching hospitals: Hospital A is a 750-bed tertiary referral hospital in New South Wales, and Hospital B is a 450-bed teaching hospital in Australia’s Northern Territory. The study was prospectively granted approval by the Human Research Ethics Committees for each of the 2 sites (approval numbers 16/04/20/4.01 and 2016–2570). The study was registered with the Australia and New Zealand Clinical Trials Registry (ACTRN12617000935336). Written informed consent was gained from all participants.
Eligible patients were over 18 years of age at the time of randomization, had confirmed chronic hepatitis C infection with either genotype 1 or 3, and were planned to commence therapy with 8–12 weeks of sofosbuvir/ledipasvir or sofosbuvir/daclatasvir without ribavirin. Patients were excluded if they had any of the following: (1) both genotype 1 chronic HCV with cirrhosis and were treatment experienced; (2) genotype 3 chronic HCV with cirrhosis; (3) Childs-Pugh class B or C cirrhosis; (4) hepatitis B coinfection defined as hepatitis B surface antigen detected in blood—those with isolated hepatitis B core antibody were not excluded; or (5) comorbidities requiring special monitoring or a high risk of poor adherence, both of which were determined by the treating physician.
Allocation and Nature of Intervention
In this open-label, parallel group, randomized controlled pilot trial participants were randomly assigned in a 1:1 ratio using permuted blocks of variable size to either “standard” or “minimal” monitoring as defined in Table 1, stratified by genotype (1 versus 3). Randomization was performed by study staff at the time of enrollment of each patient, using “sealedenvelope” a web-based randomization tool (www.sealedenvelope.com).
| Study time point . | Standard . | Minimal . |
|---|---|---|
| Start of treatment | • Face-to-face clinic appointment • FBC, EUC, LFTsa • HCV viral loada • Drug dispensingb | • Face-to-face clinic appointment • Drug dispensing |
| Treatment week 4 | • Face-to-face clinic appointment • FBC, EUC, LFTs • HCV viral load • Drug dispensingb | • Phone call • Drug dispensing |
| End of treatment (week 12) | • Face-to-face clinic appointment • FBC, EUC, LFTs • HCV qualitative PCR | • Phone call |
| 12 weeks after end of treatment (week 24) | • Face-to-face clinic appointment • HCV qualitative PCR • FBC, EUC, LFTs | • Face-to-face clinic appointment • HCV qualitative PCR • FBC, EUC, LFTs |
| Study time point . | Standard . | Minimal . |
|---|---|---|
| Start of treatment | • Face-to-face clinic appointment • FBC, EUC, LFTsa • HCV viral loada • Drug dispensingb | • Face-to-face clinic appointment • Drug dispensing |
| Treatment week 4 | • Face-to-face clinic appointment • FBC, EUC, LFTs • HCV viral load • Drug dispensingb | • Phone call • Drug dispensing |
| End of treatment (week 12) | • Face-to-face clinic appointment • FBC, EUC, LFTs • HCV qualitative PCR | • Phone call |
| 12 weeks after end of treatment (week 24) | • Face-to-face clinic appointment • HCV qualitative PCR • FBC, EUC, LFTs | • Face-to-face clinic appointment • HCV qualitative PCR • FBC, EUC, LFTs |
Abbreviations: EUC, electrolytes urea and creatinine; FBC, full blood count; HCV, hepatitis c virus; LFT, liver enzymes, albumin and bilirubin; PCR, polymerase chain reaction.
aBlood tests at start of treatment if not done within 4 weeks before treatment start date.
bDrugs were dispensed by the commercial pharmacy of the patient’s choice; the patient did not have to attend the hospital to collect medications. Drugs were also dispensed at week 8, although this is not shown in Table 1.
| Study time point . | Standard . | Minimal . |
|---|---|---|
| Start of treatment | • Face-to-face clinic appointment • FBC, EUC, LFTsa • HCV viral loada • Drug dispensingb | • Face-to-face clinic appointment • Drug dispensing |
| Treatment week 4 | • Face-to-face clinic appointment • FBC, EUC, LFTs • HCV viral load • Drug dispensingb | • Phone call • Drug dispensing |
| End of treatment (week 12) | • Face-to-face clinic appointment • FBC, EUC, LFTs • HCV qualitative PCR | • Phone call |
| 12 weeks after end of treatment (week 24) | • Face-to-face clinic appointment • HCV qualitative PCR • FBC, EUC, LFTs | • Face-to-face clinic appointment • HCV qualitative PCR • FBC, EUC, LFTs |
| Study time point . | Standard . | Minimal . |
|---|---|---|
| Start of treatment | • Face-to-face clinic appointment • FBC, EUC, LFTsa • HCV viral loada • Drug dispensingb | • Face-to-face clinic appointment • Drug dispensing |
| Treatment week 4 | • Face-to-face clinic appointment • FBC, EUC, LFTs • HCV viral load • Drug dispensingb | • Phone call • Drug dispensing |
| End of treatment (week 12) | • Face-to-face clinic appointment • FBC, EUC, LFTs • HCV qualitative PCR | • Phone call |
| 12 weeks after end of treatment (week 24) | • Face-to-face clinic appointment • HCV qualitative PCR • FBC, EUC, LFTs | • Face-to-face clinic appointment • HCV qualitative PCR • FBC, EUC, LFTs |
Abbreviations: EUC, electrolytes urea and creatinine; FBC, full blood count; HCV, hepatitis c virus; LFT, liver enzymes, albumin and bilirubin; PCR, polymerase chain reaction.
aBlood tests at start of treatment if not done within 4 weeks before treatment start date.
bDrugs were dispensed by the commercial pharmacy of the patient’s choice; the patient did not have to attend the hospital to collect medications. Drugs were also dispensed at week 8, although this is not shown in Table 1.
Baseline assessment was not part of the randomized intervention; the standard baseline assessment consisted of 1–2 face-to-face visits, medical history and physical examination, a panel of blood tests (full blood count; urea, electrolytes and creatinine [UEC]; liver enzymes, albumin and bilirubin [LFTs]; serology for human immunodeficiency virus and hepatitis B virus and HCV genotype), estimation of hepatic fibrosis using transient elastography (FibroScan; Echosens, Paris, France), a check for drug-drug interactions, and provision of verbal and written information to the patient about HCV and its treatment. Monitoring allocation was not blinded. Baseline blood tests were repeated in those in the standard arm if they had not been done within the 4 weeks before treatment commencement. In the minimal arm, baseline blood tests were not repeated if they had been done within the prior 12 months.
Participants in both arms were given phone contact details for the viral hepatitis service in case of any problems or queries and were told to visit their general practitioner (GP) or the hospital emergency department (ED) if needed during the treatment.
Outcome Measures
The 3 coprimary outcome measures were as follows: (1) the proportion of patients achieving a sustained virological response at 12 weeks after the end of treatment (SVR12); (2) the total staff time required including nursing, medical, social work, and face to face, phone, SMS, email and letters; and (3) patient satisfaction using a 10-point Likert scale Figure S1. Secondary outcomes were as follows: (1) attributable adverse events; (2) attributable serious adverse events; (3) unplanned admissions including GP and ED visits; (4) unplanned investigations; (5) total cost of blood tests; and (6) self-reported medication adherence.
The cost of blood tests was estimated using the Australian Medicare Benefits Schedule (www.mbsonline.gov.au), which is a conservative estimate of the true cost of a blood test. All blood tests done on each patient between the date of randomization and 12 weeks after the end of treatment were counted, whether or not they were consistent with the allocated monitoring strategy, and regardless of whether they were ordered by the local hospital or an outside doctor.
Data Management
Data were recorded on paper case report forms and entered into a purpose-built database for storage (EpiData entry, https://www.epidata.dk). Central monitoring was used to check any missing values or outliers with site investigators. No assumptions were made about missing data.
Sample Size Determination and Statistical Analysis
Because this was a pilot randomized control trial, the sample size was determined by what was achievable at the 2 sites during the study period. We estimated that 200 patients would present to participating sites per year, 30% of whom would fulfill eligibility criteria and consent to participate, resulting in the potential for a minimum of 60 patients over a 12-month period. We considered this number would be sufficient to assess feasibility of and refine assumptions for a larger subsequent trial.
Continuous variables were summarized using mean (standard deviation) and compared using Student’s t test if normally distributed and summarized with median (interquartile range) and compared using Mann-Whitney U test if not. Categorical variables were compared using χ 2 tests. A P value of <.05 was considered significant. All analyses were performed using Stata version 15 (StataCorp, College Station, TX). Data were analyzed using intention-to-treat principles (patients were analyzed in the group to which they were allocated, regardless of the monitoring they actually received).
RESULTS
Seventy-four individuals were enrolled into the study, 36 of whom were randomized to standard monitoring and 38 were randomized to minimal monitoring (Figure 1). Baseline characteristics are presented in Table 2. Approximately two thirds of patients had genotype 1 infection (and were thus treated with sofosbuvir/ledipasvir) and none had cirrhosis. Comorbidities were minimal, approximately 60% of patients were male, and average age was approximately 50 years old. The 2 groups were well matched in baseline characteristics.
| Characteristics . | Standard Monitoring n = 36 . | Minimal Monitoring n = 38 . |
|---|---|---|
| Male (n, %) | 23 (64%) | 22 (58%) |
| Agea | 52.0 (11.3) | 49.7 (10.0) |
| HCV genotype 1 | 24 (67%) | 26 (68%) |
| HCV genotype 3 | 12 (33%) | 12 (32%) |
| Treatment naive | 33 (92%) | 37 (97%) |
| Previous interferon-based treatment | 3 (8%) | 1 (3%) |
| Cirrhosis | 0 | 0 |
| Liver stiffness scorea | 6.2 (1.8) | 5.8 (1.7) |
| Obesity (BMI >30) | 2 (6%) | 2 (5%) |
| Depression/anxiety | 6 (17%) | 5 (13%) |
| Diabetes mellitus | 0 | 1 (3%) |
| Hazardous alcohol use | 1 (3%) | 2 (5%) |
| Current IVDU | 0 | 1 (3%) |
| Baseline ALTb | 56 (46–100) | 62 (46–78) |
| Baseline creatinineb | 75 (67–83) | 76 (67–81) |
| Baseline platelet countb | 221 (186–263) | 225 (201–268) |
| Characteristics . | Standard Monitoring n = 36 . | Minimal Monitoring n = 38 . |
|---|---|---|
| Male (n, %) | 23 (64%) | 22 (58%) |
| Agea | 52.0 (11.3) | 49.7 (10.0) |
| HCV genotype 1 | 24 (67%) | 26 (68%) |
| HCV genotype 3 | 12 (33%) | 12 (32%) |
| Treatment naive | 33 (92%) | 37 (97%) |
| Previous interferon-based treatment | 3 (8%) | 1 (3%) |
| Cirrhosis | 0 | 0 |
| Liver stiffness scorea | 6.2 (1.8) | 5.8 (1.7) |
| Obesity (BMI >30) | 2 (6%) | 2 (5%) |
| Depression/anxiety | 6 (17%) | 5 (13%) |
| Diabetes mellitus | 0 | 1 (3%) |
| Hazardous alcohol use | 1 (3%) | 2 (5%) |
| Current IVDU | 0 | 1 (3%) |
| Baseline ALTb | 56 (46–100) | 62 (46–78) |
| Baseline creatinineb | 75 (67–83) | 76 (67–81) |
| Baseline platelet countb | 221 (186–263) | 225 (201–268) |
Abbreviations: ALT, alanine aminotransferase; BMI, body mass index; HCV, hepatitis C virus; IVDU, intravenous drug use.
aMean (standard deviation).
bMedian (interquartile range).
| Characteristics . | Standard Monitoring n = 36 . | Minimal Monitoring n = 38 . |
|---|---|---|
| Male (n, %) | 23 (64%) | 22 (58%) |
| Agea | 52.0 (11.3) | 49.7 (10.0) |
| HCV genotype 1 | 24 (67%) | 26 (68%) |
| HCV genotype 3 | 12 (33%) | 12 (32%) |
| Treatment naive | 33 (92%) | 37 (97%) |
| Previous interferon-based treatment | 3 (8%) | 1 (3%) |
| Cirrhosis | 0 | 0 |
| Liver stiffness scorea | 6.2 (1.8) | 5.8 (1.7) |
| Obesity (BMI >30) | 2 (6%) | 2 (5%) |
| Depression/anxiety | 6 (17%) | 5 (13%) |
| Diabetes mellitus | 0 | 1 (3%) |
| Hazardous alcohol use | 1 (3%) | 2 (5%) |
| Current IVDU | 0 | 1 (3%) |
| Baseline ALTb | 56 (46–100) | 62 (46–78) |
| Baseline creatinineb | 75 (67–83) | 76 (67–81) |
| Baseline platelet countb | 221 (186–263) | 225 (201–268) |
| Characteristics . | Standard Monitoring n = 36 . | Minimal Monitoring n = 38 . |
|---|---|---|
| Male (n, %) | 23 (64%) | 22 (58%) |
| Agea | 52.0 (11.3) | 49.7 (10.0) |
| HCV genotype 1 | 24 (67%) | 26 (68%) |
| HCV genotype 3 | 12 (33%) | 12 (32%) |
| Treatment naive | 33 (92%) | 37 (97%) |
| Previous interferon-based treatment | 3 (8%) | 1 (3%) |
| Cirrhosis | 0 | 0 |
| Liver stiffness scorea | 6.2 (1.8) | 5.8 (1.7) |
| Obesity (BMI >30) | 2 (6%) | 2 (5%) |
| Depression/anxiety | 6 (17%) | 5 (13%) |
| Diabetes mellitus | 0 | 1 (3%) |
| Hazardous alcohol use | 1 (3%) | 2 (5%) |
| Current IVDU | 0 | 1 (3%) |
| Baseline ALTb | 56 (46–100) | 62 (46–78) |
| Baseline creatinineb | 75 (67–83) | 76 (67–81) |
| Baseline platelet countb | 221 (186–263) | 225 (201–268) |
Abbreviations: ALT, alanine aminotransferase; BMI, body mass index; HCV, hepatitis C virus; IVDU, intravenous drug use.
aMean (standard deviation).
bMedian (interquartile range).
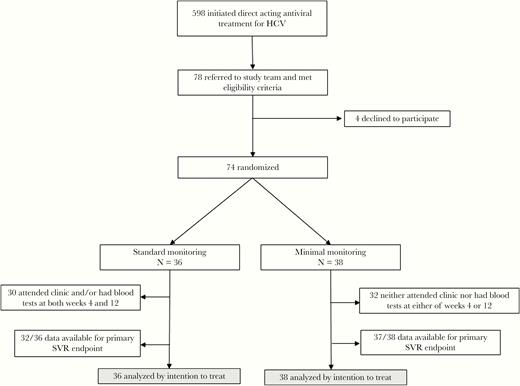
CONSORT diagram. HCV, hepatitis C virus; SVR, sustained virologic resposnse.
Of the 36 patients allocated to standard monitoring, only 30 had blood tests and/or face-to-face clinic visits at both weeks 4 and 12. Of the 38 assigned to minimal monitoring, 32 neither attended clinic nor had any blood tests at either of weeks 4 or 12 (Figure 1).
The coprimary outcome measures are reported in Table 3. When those with missing SVR data are assumed to be failures, there was no significant difference in SVR12 between the minimal monitoring group (97%; 95% confidence interval [CI], 86% to 100%) and the standard monitoring group (89%; 95% CI, 74% to 97%). When those with missing SVR data were disregarded, SVR12 was achieved in 100% of patients in both groups. There was also no significant difference in staff time or patient satisfaction in the 2 groups, although there was a nonsignificant trend towards less staff time in minimal monitoring group, with total staff time reduced by 17 minutes (52 versus 69 minutes, P = .08) and face-to-face time reduced by 15 minutes (25 versus 40 minutes, P = .005); total phone time was increased in the minimal monitoring group by 4.5 minutes (P = .007).
| Outcome measure . | Standard Monitoring N = 36 . | Minimal Monitoring n = 38 . | P Value . |
|---|---|---|---|
| SVR (missing assumed failure) | 32 (89%) | 37 (97%) | NS |
| SVR (no assumptions if missing) | 32/32 (100%) | 37/37 (100%) | NS |
| Total staff time spent (minutes, median IQR) | 69 (54–80) | 52 (40–75) | NS (.08) |
| Total face-to-face time (minutes, median IQR) | 40 (30–55) | 25 (15–30) | .005 |
| Total email time | 0 (IQR 0–0, total range 0–20) | 0 (0–0, total range 0–5) | NS |
| Total phone time | 7.5 (1–13.5) | 12 (9–20) | .007 |
| Total letter time | 10 (10–21.5) | 10 (10–24) | NS |
| Total other time | 0 (IQR 0–0, total range 0–15) | 0 (IQR 0-0, total range 0–50) | NS |
| Overall patient satisfaction score (mean [sd]) | 9.8 (0.6) | 9.6 (1.1) | NS |
| How satisfied were you with the level of care and support? | 9.6 (0.7) | 9.7 (1.0) | NS |
| Did staff meet your needs? | 9.6 (0.6) | 9.5 (1.2) | NS |
| How satisfied were you with the frequency of appointments? | 9.5 (0.8) | 9.5 (1.1) | NS |
| How anxious were you about side effects BEFORE treatment? | 5.3 (2.2) | 5.4 (2.3) | NS |
| How anxious were you about side effects DURING treatment? | 3.5 (1.6) | 3.6 (2.1) | NS |
| Outcome measure . | Standard Monitoring N = 36 . | Minimal Monitoring n = 38 . | P Value . |
|---|---|---|---|
| SVR (missing assumed failure) | 32 (89%) | 37 (97%) | NS |
| SVR (no assumptions if missing) | 32/32 (100%) | 37/37 (100%) | NS |
| Total staff time spent (minutes, median IQR) | 69 (54–80) | 52 (40–75) | NS (.08) |
| Total face-to-face time (minutes, median IQR) | 40 (30–55) | 25 (15–30) | .005 |
| Total email time | 0 (IQR 0–0, total range 0–20) | 0 (0–0, total range 0–5) | NS |
| Total phone time | 7.5 (1–13.5) | 12 (9–20) | .007 |
| Total letter time | 10 (10–21.5) | 10 (10–24) | NS |
| Total other time | 0 (IQR 0–0, total range 0–15) | 0 (IQR 0-0, total range 0–50) | NS |
| Overall patient satisfaction score (mean [sd]) | 9.8 (0.6) | 9.6 (1.1) | NS |
| How satisfied were you with the level of care and support? | 9.6 (0.7) | 9.7 (1.0) | NS |
| Did staff meet your needs? | 9.6 (0.6) | 9.5 (1.2) | NS |
| How satisfied were you with the frequency of appointments? | 9.5 (0.8) | 9.5 (1.1) | NS |
| How anxious were you about side effects BEFORE treatment? | 5.3 (2.2) | 5.4 (2.3) | NS |
| How anxious were you about side effects DURING treatment? | 3.5 (1.6) | 3.6 (2.1) | NS |
The co-primary outcome measures are shown in bold text. The components of the measures are shown in normal text.
Abbreviations: IQR, interquartile range; NS, not significant; sd, standard deviation; SVR, sustained virologic resposnse.
| Outcome measure . | Standard Monitoring N = 36 . | Minimal Monitoring n = 38 . | P Value . |
|---|---|---|---|
| SVR (missing assumed failure) | 32 (89%) | 37 (97%) | NS |
| SVR (no assumptions if missing) | 32/32 (100%) | 37/37 (100%) | NS |
| Total staff time spent (minutes, median IQR) | 69 (54–80) | 52 (40–75) | NS (.08) |
| Total face-to-face time (minutes, median IQR) | 40 (30–55) | 25 (15–30) | .005 |
| Total email time | 0 (IQR 0–0, total range 0–20) | 0 (0–0, total range 0–5) | NS |
| Total phone time | 7.5 (1–13.5) | 12 (9–20) | .007 |
| Total letter time | 10 (10–21.5) | 10 (10–24) | NS |
| Total other time | 0 (IQR 0–0, total range 0–15) | 0 (IQR 0-0, total range 0–50) | NS |
| Overall patient satisfaction score (mean [sd]) | 9.8 (0.6) | 9.6 (1.1) | NS |
| How satisfied were you with the level of care and support? | 9.6 (0.7) | 9.7 (1.0) | NS |
| Did staff meet your needs? | 9.6 (0.6) | 9.5 (1.2) | NS |
| How satisfied were you with the frequency of appointments? | 9.5 (0.8) | 9.5 (1.1) | NS |
| How anxious were you about side effects BEFORE treatment? | 5.3 (2.2) | 5.4 (2.3) | NS |
| How anxious were you about side effects DURING treatment? | 3.5 (1.6) | 3.6 (2.1) | NS |
| Outcome measure . | Standard Monitoring N = 36 . | Minimal Monitoring n = 38 . | P Value . |
|---|---|---|---|
| SVR (missing assumed failure) | 32 (89%) | 37 (97%) | NS |
| SVR (no assumptions if missing) | 32/32 (100%) | 37/37 (100%) | NS |
| Total staff time spent (minutes, median IQR) | 69 (54–80) | 52 (40–75) | NS (.08) |
| Total face-to-face time (minutes, median IQR) | 40 (30–55) | 25 (15–30) | .005 |
| Total email time | 0 (IQR 0–0, total range 0–20) | 0 (0–0, total range 0–5) | NS |
| Total phone time | 7.5 (1–13.5) | 12 (9–20) | .007 |
| Total letter time | 10 (10–21.5) | 10 (10–24) | NS |
| Total other time | 0 (IQR 0–0, total range 0–15) | 0 (IQR 0-0, total range 0–50) | NS |
| Overall patient satisfaction score (mean [sd]) | 9.8 (0.6) | 9.6 (1.1) | NS |
| How satisfied were you with the level of care and support? | 9.6 (0.7) | 9.7 (1.0) | NS |
| Did staff meet your needs? | 9.6 (0.6) | 9.5 (1.2) | NS |
| How satisfied were you with the frequency of appointments? | 9.5 (0.8) | 9.5 (1.1) | NS |
| How anxious were you about side effects BEFORE treatment? | 5.3 (2.2) | 5.4 (2.3) | NS |
| How anxious were you about side effects DURING treatment? | 3.5 (1.6) | 3.6 (2.1) | NS |
The co-primary outcome measures are shown in bold text. The components of the measures are shown in normal text.
Abbreviations: IQR, interquartile range; NS, not significant; sd, standard deviation; SVR, sustained virologic resposnse.
Secondary outcomes are reported in Table 4. There was no significant difference between groups for any of the following: total adverse events, serious adverse events, unplanned hospital or GP visits, additional investigations, or self-reported adherence. Although all participants in both groups reported > 90% adherence, 100% reported adherence was numerically less common in the minimal monitoring group (86% versus 97%, P = .12). The mean total cost of blood tests was significantly less in the minimal monitoring group ($123 versus $432, P < .001).
| Secondary outcome measure . | Standard Monitoring n = 36 . | Minimal Monitoring n = 38 . | P Value . |
|---|---|---|---|
| Any AEs | 16 (44%) | 18 (47%) | NS |
| SAEs | 0 | 0 | NS |
| Unplanned ED or GP visits | 4 (11%) | 6 (16%) | NS |
| Unplanned ED or GP visits attributable to study medication | 1a (3%) | 1b (3%) | NS |
| Unplanned hospital admissions | 0 | 0 | NS |
| Unplanned investigations | 1 (3%) | 0 | NS |
| Total cost of blood tests (mean per patient) | $431 | $123 | <.001 |
| 100% adherence | 30/31 (97%) | 31/36 (86%) | NS (P = .12) |
| ≥90% adherence | 31/31 (100%) | 36/36 (100%) | NS |
| Secondary outcome measure . | Standard Monitoring n = 36 . | Minimal Monitoring n = 38 . | P Value . |
|---|---|---|---|
| Any AEs | 16 (44%) | 18 (47%) | NS |
| SAEs | 0 | 0 | NS |
| Unplanned ED or GP visits | 4 (11%) | 6 (16%) | NS |
| Unplanned ED or GP visits attributable to study medication | 1a (3%) | 1b (3%) | NS |
| Unplanned hospital admissions | 0 | 0 | NS |
| Unplanned investigations | 1 (3%) | 0 | NS |
| Total cost of blood tests (mean per patient) | $431 | $123 | <.001 |
| 100% adherence | 30/31 (97%) | 31/36 (86%) | NS (P = .12) |
| ≥90% adherence | 31/31 (100%) | 36/36 (100%) | NS |
Abbreviations: AEs, adverse events; ED, emergency department; GP, general practitioner; NS, not significant; SAEs, serious AEs.
aMissed clinic visit so attended GP to get blood tests.
bAttended GP for nausea and vomiting.
| Secondary outcome measure . | Standard Monitoring n = 36 . | Minimal Monitoring n = 38 . | P Value . |
|---|---|---|---|
| Any AEs | 16 (44%) | 18 (47%) | NS |
| SAEs | 0 | 0 | NS |
| Unplanned ED or GP visits | 4 (11%) | 6 (16%) | NS |
| Unplanned ED or GP visits attributable to study medication | 1a (3%) | 1b (3%) | NS |
| Unplanned hospital admissions | 0 | 0 | NS |
| Unplanned investigations | 1 (3%) | 0 | NS |
| Total cost of blood tests (mean per patient) | $431 | $123 | <.001 |
| 100% adherence | 30/31 (97%) | 31/36 (86%) | NS (P = .12) |
| ≥90% adherence | 31/31 (100%) | 36/36 (100%) | NS |
| Secondary outcome measure . | Standard Monitoring n = 36 . | Minimal Monitoring n = 38 . | P Value . |
|---|---|---|---|
| Any AEs | 16 (44%) | 18 (47%) | NS |
| SAEs | 0 | 0 | NS |
| Unplanned ED or GP visits | 4 (11%) | 6 (16%) | NS |
| Unplanned ED or GP visits attributable to study medication | 1a (3%) | 1b (3%) | NS |
| Unplanned hospital admissions | 0 | 0 | NS |
| Unplanned investigations | 1 (3%) | 0 | NS |
| Total cost of blood tests (mean per patient) | $431 | $123 | <.001 |
| 100% adherence | 30/31 (97%) | 31/36 (86%) | NS (P = .12) |
| ≥90% adherence | 31/31 (100%) | 36/36 (100%) | NS |
Abbreviations: AEs, adverse events; ED, emergency department; GP, general practitioner; NS, not significant; SAEs, serious AEs.
aMissed clinic visit so attended GP to get blood tests.
bAttended GP for nausea and vomiting.
Discussion
Main Findings
In this pragmatic, randomized, controlled trial, a strategy of minimal monitoring lead to a cure rate that was no different to standard monitoring, with no more adverse events, less face-to-face staff time, and less expenditure on blood tests.
Comparison With the Literature
A recent retrospective study analyzed the value of routine blood test monitoring at treatment weeks 4 and 12 in 208 HCV patients treated with sofosbuvir-based regimens [8]. It found that (1) almost all patients achieve a negative HCV polymerase chain reaction by treatment week 4, (2) there was no correlation between on-treatment virological response and SVR, and (3) biochemical and hematological abnormalities were rare. The authors conclude that routine on-treatment monitoring is not warranted.
There have been no previously published randomized trials addressing this question. The SMART-C study, presented at EASL in 2019, is a multicenter, randomized, control trial enrolling noncirrhotic, treatment-naive HCV patients receiving glecaprevir/pibrentasvir for 8 weeks and comparing standard (clinic visits at week 4 and 8) versus simplified (phone contact at weeks 4 and 8) monitoring. Reported SVR12 rates were 92% in the simplified arm and 95% in the standard monitoring arm, but they did not reach the prespecified noninferiority margin despite randomizing 380 individuals. The authors concluded that a simplified monitoring schedule is feasible for those without adherence concerns, but that further studies with other regimens in different settings are required [9].
Despite no published randomized trials addressing this question, the Australasian consensus guidelines on HCV have recently changed to recommend no on-treatment monitoring with the exception of those receiving elbasvir plus grazoprevir (LFTs recommended at week 8) and the caveat that more intensive monitoring may be required in certain populations. The American Association for the Study of Liver Disease guidelines recommend blood tests at treatment week 4 including quantitative HCV viral load, UEC, and LFTs for all patients, as well as LFTs at 8 weeks for those on elbasvir/grazoprevir [10]. However, the EASL guidance (2018) suggests only baseline and SVR bloods in uncomplicated patients [11] as well as suggesting that if cost is an issue, SVR12 testing may be dispensable albeit with an moderate/weak evidence level.
Implications of Findings and Future Directions
The total staff time spent on patient support was less than expected in both the simplified and standard monitoring arms of this trial. This may have been due to the “practice drift” towards less intensive monitoring and support that was occurring as staff became more familiar and comfortable with DAA-based treatment regimens. There was reduced face-to-face time in the minimal monitoring group with a trend towards increased phone time, which could be important from a cost-effectiveness perspective. The fact that the difference in face-to-face time was so small between the 2 groups (the difference in the medians was 15 minutes despite 2 extra scheduled visits) implies that the week 4 and 12 visits may not be necessary because there is not much to discuss at these time points. A significant difference was clearly documented in the cost of pathology tests with the cost of monitoring for minimal group much cheaper, which is particularly relevant to scale-up and elimination agendas both within Australia and globally.
The trend towards poorer adherence in the minimal monitoring arm is of concern (86% versus 97% reported 100% adherence). This could potentially be explained by less direct engagement with hepatitis C nurses, but it may also be a chance finding. However, adherence of at least 80% of doses is likely sufficient with sofosbuvir-based therapy [12], and this did not differ between the 2 treatment arms. Innovative strategies to improve adherence (such as the use of apps or text messaging) may improve this and should be included in any future trials of minimal monitoring.
Whether or not a minimal monitoring strategy is safe and effective in less selected populations is unknown. Our eligibility criteria and those of SMART-C were both quite conservative. Now that these results are available, and clinicians are comfortable and familiar with the use and adverse effect profile of these agents, perhaps it is time to be bolder and examine broader populations. Those in whom this strategy could be tested include patients with compensated cirrhosis (Child-Pugh class A), those who are treatment experienced, and those with significant comorbidities including stable mental illness and/or current injection drug use. In such populations, pragmatic comparative effectiveness trials should be run before guidelines change.
Strengths and Limitations
This was a small trial, with 74 patients, originally intended to inform the design of a larger definitive trial, which would require over 800 patients. Despite this, the results are reassuring that minimal monitoring is safe: not only was it noninferior in terms of SVR, it was numerically superior. Due to evolution of clinical practice towards minimal monitoring, and then of national and international guidelines during the course of the trial, the larger definitive trial is no longer viable.
The population we studied was highly selected, with no cirrhotic and few treatment-experienced patients. Moreover, very few patients reported either hazardous alcohol use or current injection drug use. This limits generalizability, and the results should primarily be applied to uncomplicated treatment-naive patients.
Conclusions
A strategy of minimal monitoring appears to be safe and effective in noncirrhotic, treatment-naive patients receiving sofosbuvir-based therapy for chronic HCV infection. Although a larger trial is needed to confirm these pilot findings, one is unlikely to occur because standard practice has already evolved towards minimal monitoring.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Figure 1. Patient Satisfaction Questionnaire.
Author contributions. J. S. D. contributed to study conceptualization, design, implementation and coordination, recruitment, analysis of results, and first draft of manuscript. M. Y., C. M., J. T.-B., M. M., S. S., C. S., and T. J. contributed to study implementation and recruitment, review, and input into final manuscript. J. D. contributed to study design, implementation and coordination, analysis of results, review, and input into final manuscript.
Finnancial support. J. S. D. and J. D. received salary support from Australia’s National Health and Medical Research Council (Career Development Fellowship no. 1160331 [to J. D.]; Early Career Fellowship no. 1123427 [to J. D.]).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Acknowledgments
We thank Dr. Rob Pickles, Dr. Ella Meumann, Liz Ianna, and Karen Brown for assistance with patient recruitment and Drs. Steven Tong and Krispin Hajkowicz for input into the initial study design.





Comments