-
PDF
- Split View
-
Views
-
Cite
Cite
Jonathan W Uzorka, Jacco Wallinga, Lucia J M Kroft, Tom H M Ottenhoff, Sandra M Arend, Radiological Signs of Latent Tuberculosis on Chest Radiography: A Systematic Review and Meta-Analysis, Open Forum Infectious Diseases, Volume 6, Issue 7, July 2019, ofz313, https://doi.org/10.1093/ofid/ofz313
Close - Share Icon Share
Abstract
Current guidelines recommend screening for latent tuberculosis infection (LTBI) with a tuberculin skin test (TST) or interferon gamma release assay (IGRA), or both. Many also recommend chest radiography (CXR), although its added value is uncertain. This systematic review assessed the prevalence of abnormalities suggestive of LTBI on CXR (LTBI-CXR lesions) and evaluated the strength of the association.
We searched 4 databases up to September 2017 and systematically reviewed cross-sectional and cohort studies reporting LTBI-CXR lesions in individuals with a positive TST or IGRA, or both, result. Prevalence estimates were pooled using random effects models and odds ratios (ORs) were used to calculate risk estimates.
In the 26 included studies, the pooled proportion of individuals with LTBI having LTBI-CXR lesions was 0.15 (95% confidence interval [CI], 0.12–0.18]. In 16 studies that reported on individuals with LTBI and uninfected controls, LTBI-CXR lesions were associated with a positive TST result ≥ 5 mm or ≥ 10 mm (OR, 2.45; 95% CI, 1.00–5.99; and OR, 2.06; 95% CI, 1.38–3.09, respectively) and with a positive QuantiFERON result (OR, 1.99; 95% CI, 1.17–3.39) compared to CXR in uninfected controls. Although few studies reported specified lesions, calcified nodules were most frequently reported in individuals with LTBI (proportion, 0.07; 95% CI, 0.02–0.11).
Lesions on CXR suggestive of previous infection with Mycobacterium tuberculosis were significantly associated with positive tests for LTBI, although the sensitivity was only 15%. This finding may have added value when detection of past LTBI is important but immunodiagnostic tests may be unreliable.
INTRODUCTION
In individuals with latent tuberculosis (TB) infection (LTBI), the lifetime risk of developing active TB following initial infection with Mycobacterium tuberculosis (Mtb) is estimated to be around 10% [1]. This risk is strongly increased in recently infected individuals, in individuals with an impaired immune response due to HIV, or as a result of immunosuppressive drugs, such as tumor necrosis factor (TNF) antagonists or anti-rejection drugs [2–5]. Especially in these individuals at highest risk of reactivation, adequate LTBI screening and subsequent preventive treatment are key to prevent development of active disease [1, 3, 4]. In general, the screening includes a tuberculin skin test (TST) or interferon-gamma release assay (IGRA), or both, and often chest radiography (CXR). Although there are several ways to diagnose LTBI before the start of immunosuppressive therapy, a number of patients still develop active TB, often despite a negative TST/IGRA result [6, 7]. Interestingly, a recently published systematic review showed that the role of CXR is ambiguous in these different screening methods [8]. Some guidelines recommend performing CXR regardless of corresponding TST/IGRA result [3, 9–11]. Other guidelines state that performing CXR should be limited to those with certain risk factors for LTBI [12] or to individuals with a positive TST/IGRA result in order to exclude active TB [13]. Because screening for LTBI based on TST/IGRA results is suboptimal in immunosuppressed patients, who are at highest risk of reactivation TB, CXR can be of added value as it might reveal signs of prior TB infection in those with a false-negative TST/IGRA result [2, 14]. Such radiological findings are even associated with an increased risk of reactivation in immunocompetent individuals [2, 15], and they are, therefore, of special interest in groups with (future) impaired immunity.
We conducted the first systematic review and meta-analysis to evaluate the prevalence of lesions on CXR considered suggestive of prior TB infection in individuals with LTBI and to assess the strength of the association by comparing the frequency of such findings on CXR in those with LTBI and uninfected controls. The ultimate goal of this study was to evaluate the potential of CXR to increase the sensitivity of existing screening methods for LTBI in individuals eligible for immunosuppressive therapy.
METHODS
This systematic review and meta-analysis was conducted in accordance with PRISMA guidelines for reporting systematic reviews [16]. The protocol was registered prospectively (PROSPERO registration number 2018 CRD42018088641).
Eligibility Criteria
Eligible for inclusion were all studies reporting abnormalities on CXR suggestive of LTBI, further referred to as LTBI-CXR lesions, in correlation with immunological tests for infection with Mtb. LTBI-CXR lesions included calcified nodules, noncalcified nodules, pleural thickening, and fibrotic scarring. Case reports and case series were excluded. Individuals ≥18 years old in whom a TST/IGRA and a CXR was performed were included. Depending on the study protocols, a positive TST was defined as an induration size ≥ 5 mm or as ≥ 10 mm. If the cut-off TST induration size could not be retrieved, it was considered to be ≥ 10 mm. The 2 commercially available IGRA in the study period were the QuantiFERON-TB Gold in-tube (QFT) (Qiagen, Hilden, Germany) and T-SPOT.TB (T-SPOT) (Oxford Immunotec, Oxford, UK). QFT and T-SPOT were considered positive in case of an interferon-γ response ≥ 0.35 IU/mL and ≥ 8 spot forming units (SFU) (≥ 6 SFU in studies using the earlier T-SPOT cut-off value), respectively. The meta-analysis was limited to studies including both an Mtb-infected group and Mtb-uninfected control group. Primary outcome measured the following: (1) the proportion of individuals with LTBI having LTBI-CXR lesions (prevalence); (2) the presence of these lesions in individuals with LTBI compared to uninfected controls (OR); and (3) the prevalence of specified lesions, defined as (calcified) nodules, pleural thickening, and fibrotic scarring, in individuals with LTBI (and uninfected individuals if available). Secondary outcome measures were the influence of blinded assessment of the CXR by the radiologist on the effect size and an evaluation of radiological discrepancies between studies in high (≥ 40) and low TB-endemic areas (<40 cases of active TB/100 000 inhabitants per year).
Search Strategy
We conducted a literature search up to September 1, 2017, of PubMed/MEDLINE, Embase, Web of Science, and the Ccohrane Library databases. The search strategy is accessible through PROSPERO. No restrictions were made on publication date or publication status, and the languages were limited to English or Dutch. All identified records were screened on title and abstract. Eligibility assessment was performed by 2 independent reviewers (J.U. and S.A.), and disagreements between reviewers were discussed and resolved by consensus.
Data Collection
Data was extracted using a data collection form. We tested the form on 5 randomly-selected studies after which minor adjustments were made. Data were extracted by 1 author (J.U.) and checked by a second author (S.A.). Disagreements were discussed and resolved by consensus. Four authors of included studies were contacted for additional data, of whom 2 responded and 1 provided additional data. From each included study, data was collected on study characteristics, diagnostic methods, and radiological abnormalities in both Mtb-infected and Mtb-uninfected individuals, if applicable. The complete data collection form is provided in the online supplement (Supplementary Table S1).
Risk of Bias Assessment
The Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies by the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) was used for the assessment of bias [17]. We arbitrarily assigned each criterion 1 point. After scoring, a poor, fair, or good quality rating was defined by a score of ≤ 4, 5 or 6, and ≥ 7 points, respectively. Two reviewers (J.U. and S.A.) assessed quality independently, and disagreements were discussed and resolved by consensus. The risk of bias summary tables were made using Review Manager software, version 5.3 (The Nordic Cochrane Centre of The Cochrane Collaboration, Copenhagen, Denmark). Funnel plots were used to evaluate the risk of publication bias, and Egger’s test was performed to assess funnel plot asymmetry.
Statistical Analyses
Studies were classified by the study’s definition of LTBI (TST ≥ 5 mm, TST ≥ 10 mm, QFT positivity, and T-SPOT positivity), with several studies providing data on more than 1 of these criteria. The proportion of individuals with a positive test for LTBI and in whom LTBI-CXR lesions were found were pooled in diagnostic categories based on LTBI definition. The Freeman-Tukey double arcsine transformation method was used to stabilize the variances. Subsequently, risk estimates of lesions on CXR in individuals with a positive test for LTBI compared to test-negative controls were calculated using ORs. Pooled ORs were obtained by random-effects meta-analysis (Mantel-Haenszel method). To assess the sensitivity of outcome depending on the choice of meta-analysis method, we ran additional analyses using alternative methods, which yielded similar results. Statistical heterogeneity was assessed using the Cochran chi-square test, and the I2 statistic was used to evaluate the degree of variation among studies. Meta-regression analyses were performed to assess the potential influence of TB endemicity on the overall proportion with LTBI-CXR lesions, and subgroup meta-regression analyses were performed to explore the relation between blinding of the radiologist to TST/IGRA results and risk estimates (ORs). All analyses were performed using R, version 3.5.2.
RESULTS
Study Selection and Study Characteristics
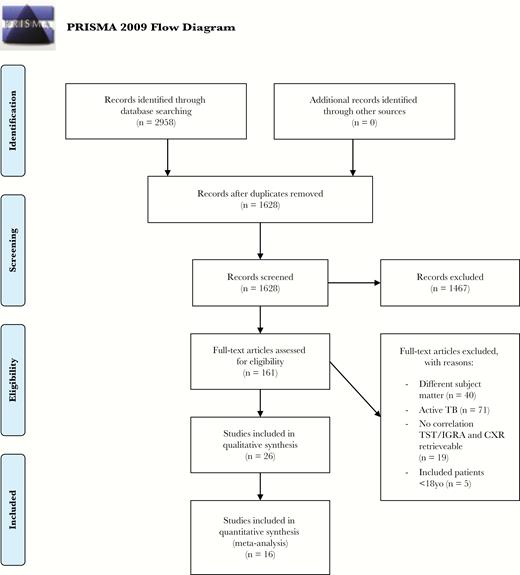
A flow chart of the study selection is shown in Figure 1. We identified 1628 studies by the database search after excluding duplicate publications. Of these, 1467 studies were excluded based on title and abstract. The remaining 161 studies were assessed for eligibility by reviewing the full text, of which 135 were excluded. Thus, 26 studies were included for data collection and quality assessment. Ten studies included only individuals with LTBI [18–27], while the remaining 16 studies included a control group of individuals with negative TST/IGRA results [28–43]. Of these 26 studies, 13 were cohort studies, 12 were cross-sectional studies, and 1 had a case control design (Table 1). The setting was most often occurred before anti-TNF screening (n = 6) or routine care (n = 5), and the majority of studies (n = 21) were performed in low TB-endemic countries.
| First Author, Publication Year [reference number] . | Country (TB- endemicity)a . | Setting . | Design . | Definition of Lesions Suggestive of Past TB . | Case Definition (Criterion for LTBI) . | N Subjects . | Ageb . | Sex (% male) . | Individuals with LTBI and Lesions on CXR N/N with LTBI (%) . | Uninfected Controls With Lesions on CXR N/N Controls (%) . |
|---|---|---|---|---|---|---|---|---|---|---|
| Studies with control group | ||||||||||
| Bonfiglioli, 2014 [28] | Brazil (high) | Anti-TNF screening | Cohort | Signs of previous TB (fibrotic lesions)/TB sequelae. | TST ≥ 5 mm | 202 | 52 ± 11 | 16.8 | 6/44 (13.6) | 8/158 (5.1) |
| Christopoulos, 2009 [29] | Greece (low) | Screening HD/PD | Cohort | Dense pulmonary nodules, with or without visible calcification, in the hilar area or upper lobes, smaller nodules with or without fibrotic scars in the upper lobes, accompanied with upper lobe volume loss bronchiectasis of the upper lobe and/or pleural scarring. | TST ≥ 10 mm | 272 | 25–77 | 71.0 | 27/62 (43.5) | 40/210 (19.0) |
| Costantino, 2013 [30] | France (low) | Anti-TNF screening | Cross-sectional | Suggestive of previous TB infection (pulmonary nodules, upper lobe bronchiectasis, apical pleural thickening, interstitial granulomatous calcifications, cavitations, and lymph node or pericardial calcifications). | TST ≥ 5 mm T-SPOT+ TST ≥ 5 mm and T-SPOT+ | 196 122 279 | 51.0 NR NR | 53.1 44.3 38.4 | 14/196 (7.1) 13/122 (10.7) 9/59 (15.3) | NA NA 2/220 (0.9) |
| Foster, 2016 [31] | Canada (low) | Screening HD/PD | Cohort | Evidence of previous TB infection (including infiltrates, tissue loss, and cavitations upper lobe). | TST ≥ 5 mm TST ≥ 10 mm | 476 465 | 62 ± 11 62 ± 11 | 49.1 49.1 | 14/62 (22.6) 12/43 (27.9) | 107/406 (25.4) 89/422 (21.1) |
| Jeong, 2012 [32] | South Korea (high) | Routine CXR | Case control | Old, healed TB (calcified nodular densities, fibrosis/nodular densities, basal pleural thickening ≥10mm, and bronchiectasis upper lobe). | TST ≥ 10 mm QFT+ | 271 319 | 18–88 18–88 | NR 58.6 | 89/131 (67.9) 150/228 (65.8) | 74/140 (52.9) 43/91 (47.3) |
| Joshi, 2007 [33] | India (high) | Screening HCW | Cross-sectional | Suggestive of inactive TB (fibrotic scar [with volume loss], [non-] calcified nodules [with volume loss], bronchiectasis, pleural thickening, diaphragmatic tenting, and blunt costophrenic angle. | TST ≥ 10 mm QFT+ TST ≥ 10 mm and QFT+ | 276 328 209 | 32 ± 12 NR NR | NR NR NR | 174/276 (63.0) 163/261 (62.5) 131/209 (62.7) | NA 43/67 (64.2) NA |
| Katsenos, 2011 [34] | Greece (low) | Contact investigation | Cross-sectional | Abnormal findings (fibrotic-appearing apical lesions). | TST ≥ 5 mm | 77 | 75 | 15.6 | 0/26 (0) | 6/51 (11.8) |
| Kim, 2010 [35] | South Korea (high) | Pre-transplant screening | Cohort | Abnormal findings suggestive of inactive TB. | TST ≥ 5 mm TST ≥ 10 mm T-SPOT+ | 209 209 184 | NR NR NR | NR NR NR | 5/47 (10.6) 3/21 (14.3) 5/65 (7.7) | 5/162 (3.1) 7/188 (3.7) 4/119 (3.4) |
| Kleinert, 2012 [36] | Germany (low) | Anti-TNF screening | Cross-sectional | Lesions suggestive of latent or prior TB. | TST ≥ 5 mm QFT+ T-SPOT+ TST ≥ 5 mm and IGRA+ | 1529 685 844 1368 | NR NR NR NR | 38.7 NR NR 37.9 | 15/173 (8.7) 3/50 (6.0) 15/70 (21.4) 13/66 (19.7) | 12/1356 (0.9) 7/635 (1.1) 2/777 (0.3) 7/1302 (0.5) |
| Roelsgaard, 1961 [37] | Kenya (high) | Population study | Cross-sectional | Infiltrate with cavity, infiltrate without cavity, calcified or fibrotic lesions, and pleural adhesions. Where more than 1 shadow was seen, the classification into type of lesion was determined by the most “severe“ finding. | TST ≥ 10 mm | 4946 | NR | NR | 231/2475 (9.3) | 82/2471 (3.3) |
| Seyhan, 2009 [38] | Turkey (low) | Screening HD/PD | Cross-sectional | TB scar lesions (dense pulmonary nodules with/without calcification in hilar area or upper lobes, and pleural thickening). | TST ≥ 10 mm QFT+ | 100 100 | 56 ± 15 56 ± 15 | 47.0 47.0 | 4/34 (11.8) 11/43 (25.6) | 12/66 (18.2) 5/57 (8.8) |
| Sichletidis, 2006 [39] | Greece (low) | Anti-TNF screening | Cohort | An abnormal CXR suggesting latent TB (apical fibrotic lesions and pleural calcification). | TST ≥ 10 mm | 613 | 49 ± 8 | 32.1 | 5/45 (11.1) | 0/568 (0) |
| Tafuri, 2011 [40] | Italy (low) | Immigrant screening | Cross-sectional | TB sequelae. | TST+ | 669 | 25 | NR | 99/554 (17.9) | 16/115 (13.9) |
| Triverio, 2009 [41] | Switzerland (low) | Immigrant screening | Cohort | Suggestive of prior TB. | TST ≥ 5 mm | 62 | 65 ± 15 | 74.2 | 2/12 (16.7) | 6/50 (12.0) |
| QFT+ | 57 | NR | NR | 3/13 (23.1) | 5/44 (11.4) | |||||
| T-SPOT+ | 55 | NR | NR | 1/18 (5.6) | 5/37 (13.5) | |||||
| Vassilopoulos, 2009 [42] | Greece (low) | Anti-TNF screening | Cohort | Suggestive of previous, inactive TB (calcified or noncalcified nodules or fibrotic scars). | TST ≥ 5 mm | 155 | 52 ± 16 | 41.9 | 9/58 (15.5) | 5/97 (5.2) |
| QFT+ | 155 | 52 ± 16 | 41.9% | 4/32 (12.5) | 10/123 (8.1) | |||||
| T-SPOT+ | 155 | 52 ± 16 | 41.9 | 4/39 (10.3) | 10/116 (8.6) | |||||
| Wauters, 2004 [43] | Belgium (low) | Screening HD/PD | Cross-sectional | TB lesions: dense pulmonary nodules with or without visible calcification in the hilar area or upper lobes or pleural scarring were scored as positive findings. | TST ≥ 10 mm | 224 | 68 ± 13 | 58.0 | 22/73 (30.1) | 34/151 (22.5) |
| Studies without control group | ||||||||||
| Bailey, 1977 [18] | United States (low) | Routine CXR | Cohort | Consistent with old TB. | TST+ | 1524 | NR | NR | 61/1524 (4.0) | NA |
| Eisenberg, 2009 [19] | United States (low) | Pre-employment | Cross-sectional | Abnormalities suggestive of chronic TB infection (calcified granulomas and lymph nodes, apical pleural thickening, fibrosis, and nodules). | TST ≥ 10 mm | 875 | 18–65 | 44.8 | 91/875 (10.4) | NA |
| Eisenberg, 2010 [20] | United States (low) | Pre-employment | Cross-sectional | Evidence of prior TB infection included apical or basal pleural thickening; fibrous scarring; calcified granulomas and/or, calcified lymph nodes; and noncalcified nodules. | TST ≥ 10 mm TST ≥ 10 mm and QFT+ | 2586 | 18–65 | 45.0 | 159/2586 (6.1) 8/135 (5.9) | NA NA |
| Eisenberg, 2017 [21] | United States (low) | Routine CXR | Cohort | Evidence of prior TB infection (apical pleural thickening, fibrous scarring, calcified granulomas/lymph nodes and noncalcified nodules). | TST ≥ 10 mm | 2518 | 18–93 | 38.9 | 196/2518 (7.8) | NA |
| Gershon, 2004 [22] | Canada (low) | TB clinic | Cohort | Abnormal findings consistent with previous TB. | TST ≥ 10 mm or TST ≥ 5 mm | 308 | NR | 56.8 | 77/308 (25.0) | NA |
| Gottridge, 1989 [23] | Unites States (low) | Screening HCW | Cohort | Apical pleural thickening, active parenchymal lesion, cavitating lesion, pleural effusion, fibrocalcific disease, unrelated abnormality, or other abnormal CXR findings consistent with previous TB. | TST ≥ 10 mm | 221 | 18–65 | 33.9 | 9/221 (4.1) | NA |
| Kunimoto, 2009 [24] | Canada (low) | Routine CXR | Cross-sectional | Findings suggestive of previous TB (TB scar). | TST ≥ 5 mm TST ≥ 5 mm and QFT+ | 1446 566 | NR 32 ± 16c | 37.5 41.7 | 304/1446 (21.0) 170/566 (30.0) | NA NA |
| Manadan, 2007 [25] | United States (low) | Anti-TNF screening | Cohort | Evidence of old granulomatous disease. | TST ≥ 10 mm | 43 | NR | NR | 10/43 (23.2) | NA |
| Meyer, 2003 [26] | United States (low) | Routine CXR | Cross-sectional | Lesions suggestive of tuberculosis infection abnormal, fibrosis, granuloma, consolidation or cavity, pleural disease, and calcified lymph nodes. | TST+ | 535 | 39 ± 15 | 48 | 75/535 (14.0) | NA |
| Nolan, 1988 [27] | United States (low) | Immigrant screening | Cohort | Abnormal CXRs consistent with inactive TB. | TST ≥ 10 mm | 3300 | NR | NR | 185/3300 (5.6) | NA |
| First Author, Publication Year [reference number] . | Country (TB- endemicity)a . | Setting . | Design . | Definition of Lesions Suggestive of Past TB . | Case Definition (Criterion for LTBI) . | N Subjects . | Ageb . | Sex (% male) . | Individuals with LTBI and Lesions on CXR N/N with LTBI (%) . | Uninfected Controls With Lesions on CXR N/N Controls (%) . |
|---|---|---|---|---|---|---|---|---|---|---|
| Studies with control group | ||||||||||
| Bonfiglioli, 2014 [28] | Brazil (high) | Anti-TNF screening | Cohort | Signs of previous TB (fibrotic lesions)/TB sequelae. | TST ≥ 5 mm | 202 | 52 ± 11 | 16.8 | 6/44 (13.6) | 8/158 (5.1) |
| Christopoulos, 2009 [29] | Greece (low) | Screening HD/PD | Cohort | Dense pulmonary nodules, with or without visible calcification, in the hilar area or upper lobes, smaller nodules with or without fibrotic scars in the upper lobes, accompanied with upper lobe volume loss bronchiectasis of the upper lobe and/or pleural scarring. | TST ≥ 10 mm | 272 | 25–77 | 71.0 | 27/62 (43.5) | 40/210 (19.0) |
| Costantino, 2013 [30] | France (low) | Anti-TNF screening | Cross-sectional | Suggestive of previous TB infection (pulmonary nodules, upper lobe bronchiectasis, apical pleural thickening, interstitial granulomatous calcifications, cavitations, and lymph node or pericardial calcifications). | TST ≥ 5 mm T-SPOT+ TST ≥ 5 mm and T-SPOT+ | 196 122 279 | 51.0 NR NR | 53.1 44.3 38.4 | 14/196 (7.1) 13/122 (10.7) 9/59 (15.3) | NA NA 2/220 (0.9) |
| Foster, 2016 [31] | Canada (low) | Screening HD/PD | Cohort | Evidence of previous TB infection (including infiltrates, tissue loss, and cavitations upper lobe). | TST ≥ 5 mm TST ≥ 10 mm | 476 465 | 62 ± 11 62 ± 11 | 49.1 49.1 | 14/62 (22.6) 12/43 (27.9) | 107/406 (25.4) 89/422 (21.1) |
| Jeong, 2012 [32] | South Korea (high) | Routine CXR | Case control | Old, healed TB (calcified nodular densities, fibrosis/nodular densities, basal pleural thickening ≥10mm, and bronchiectasis upper lobe). | TST ≥ 10 mm QFT+ | 271 319 | 18–88 18–88 | NR 58.6 | 89/131 (67.9) 150/228 (65.8) | 74/140 (52.9) 43/91 (47.3) |
| Joshi, 2007 [33] | India (high) | Screening HCW | Cross-sectional | Suggestive of inactive TB (fibrotic scar [with volume loss], [non-] calcified nodules [with volume loss], bronchiectasis, pleural thickening, diaphragmatic tenting, and blunt costophrenic angle. | TST ≥ 10 mm QFT+ TST ≥ 10 mm and QFT+ | 276 328 209 | 32 ± 12 NR NR | NR NR NR | 174/276 (63.0) 163/261 (62.5) 131/209 (62.7) | NA 43/67 (64.2) NA |
| Katsenos, 2011 [34] | Greece (low) | Contact investigation | Cross-sectional | Abnormal findings (fibrotic-appearing apical lesions). | TST ≥ 5 mm | 77 | 75 | 15.6 | 0/26 (0) | 6/51 (11.8) |
| Kim, 2010 [35] | South Korea (high) | Pre-transplant screening | Cohort | Abnormal findings suggestive of inactive TB. | TST ≥ 5 mm TST ≥ 10 mm T-SPOT+ | 209 209 184 | NR NR NR | NR NR NR | 5/47 (10.6) 3/21 (14.3) 5/65 (7.7) | 5/162 (3.1) 7/188 (3.7) 4/119 (3.4) |
| Kleinert, 2012 [36] | Germany (low) | Anti-TNF screening | Cross-sectional | Lesions suggestive of latent or prior TB. | TST ≥ 5 mm QFT+ T-SPOT+ TST ≥ 5 mm and IGRA+ | 1529 685 844 1368 | NR NR NR NR | 38.7 NR NR 37.9 | 15/173 (8.7) 3/50 (6.0) 15/70 (21.4) 13/66 (19.7) | 12/1356 (0.9) 7/635 (1.1) 2/777 (0.3) 7/1302 (0.5) |
| Roelsgaard, 1961 [37] | Kenya (high) | Population study | Cross-sectional | Infiltrate with cavity, infiltrate without cavity, calcified or fibrotic lesions, and pleural adhesions. Where more than 1 shadow was seen, the classification into type of lesion was determined by the most “severe“ finding. | TST ≥ 10 mm | 4946 | NR | NR | 231/2475 (9.3) | 82/2471 (3.3) |
| Seyhan, 2009 [38] | Turkey (low) | Screening HD/PD | Cross-sectional | TB scar lesions (dense pulmonary nodules with/without calcification in hilar area or upper lobes, and pleural thickening). | TST ≥ 10 mm QFT+ | 100 100 | 56 ± 15 56 ± 15 | 47.0 47.0 | 4/34 (11.8) 11/43 (25.6) | 12/66 (18.2) 5/57 (8.8) |
| Sichletidis, 2006 [39] | Greece (low) | Anti-TNF screening | Cohort | An abnormal CXR suggesting latent TB (apical fibrotic lesions and pleural calcification). | TST ≥ 10 mm | 613 | 49 ± 8 | 32.1 | 5/45 (11.1) | 0/568 (0) |
| Tafuri, 2011 [40] | Italy (low) | Immigrant screening | Cross-sectional | TB sequelae. | TST+ | 669 | 25 | NR | 99/554 (17.9) | 16/115 (13.9) |
| Triverio, 2009 [41] | Switzerland (low) | Immigrant screening | Cohort | Suggestive of prior TB. | TST ≥ 5 mm | 62 | 65 ± 15 | 74.2 | 2/12 (16.7) | 6/50 (12.0) |
| QFT+ | 57 | NR | NR | 3/13 (23.1) | 5/44 (11.4) | |||||
| T-SPOT+ | 55 | NR | NR | 1/18 (5.6) | 5/37 (13.5) | |||||
| Vassilopoulos, 2009 [42] | Greece (low) | Anti-TNF screening | Cohort | Suggestive of previous, inactive TB (calcified or noncalcified nodules or fibrotic scars). | TST ≥ 5 mm | 155 | 52 ± 16 | 41.9 | 9/58 (15.5) | 5/97 (5.2) |
| QFT+ | 155 | 52 ± 16 | 41.9% | 4/32 (12.5) | 10/123 (8.1) | |||||
| T-SPOT+ | 155 | 52 ± 16 | 41.9 | 4/39 (10.3) | 10/116 (8.6) | |||||
| Wauters, 2004 [43] | Belgium (low) | Screening HD/PD | Cross-sectional | TB lesions: dense pulmonary nodules with or without visible calcification in the hilar area or upper lobes or pleural scarring were scored as positive findings. | TST ≥ 10 mm | 224 | 68 ± 13 | 58.0 | 22/73 (30.1) | 34/151 (22.5) |
| Studies without control group | ||||||||||
| Bailey, 1977 [18] | United States (low) | Routine CXR | Cohort | Consistent with old TB. | TST+ | 1524 | NR | NR | 61/1524 (4.0) | NA |
| Eisenberg, 2009 [19] | United States (low) | Pre-employment | Cross-sectional | Abnormalities suggestive of chronic TB infection (calcified granulomas and lymph nodes, apical pleural thickening, fibrosis, and nodules). | TST ≥ 10 mm | 875 | 18–65 | 44.8 | 91/875 (10.4) | NA |
| Eisenberg, 2010 [20] | United States (low) | Pre-employment | Cross-sectional | Evidence of prior TB infection included apical or basal pleural thickening; fibrous scarring; calcified granulomas and/or, calcified lymph nodes; and noncalcified nodules. | TST ≥ 10 mm TST ≥ 10 mm and QFT+ | 2586 | 18–65 | 45.0 | 159/2586 (6.1) 8/135 (5.9) | NA NA |
| Eisenberg, 2017 [21] | United States (low) | Routine CXR | Cohort | Evidence of prior TB infection (apical pleural thickening, fibrous scarring, calcified granulomas/lymph nodes and noncalcified nodules). | TST ≥ 10 mm | 2518 | 18–93 | 38.9 | 196/2518 (7.8) | NA |
| Gershon, 2004 [22] | Canada (low) | TB clinic | Cohort | Abnormal findings consistent with previous TB. | TST ≥ 10 mm or TST ≥ 5 mm | 308 | NR | 56.8 | 77/308 (25.0) | NA |
| Gottridge, 1989 [23] | Unites States (low) | Screening HCW | Cohort | Apical pleural thickening, active parenchymal lesion, cavitating lesion, pleural effusion, fibrocalcific disease, unrelated abnormality, or other abnormal CXR findings consistent with previous TB. | TST ≥ 10 mm | 221 | 18–65 | 33.9 | 9/221 (4.1) | NA |
| Kunimoto, 2009 [24] | Canada (low) | Routine CXR | Cross-sectional | Findings suggestive of previous TB (TB scar). | TST ≥ 5 mm TST ≥ 5 mm and QFT+ | 1446 566 | NR 32 ± 16c | 37.5 41.7 | 304/1446 (21.0) 170/566 (30.0) | NA NA |
| Manadan, 2007 [25] | United States (low) | Anti-TNF screening | Cohort | Evidence of old granulomatous disease. | TST ≥ 10 mm | 43 | NR | NR | 10/43 (23.2) | NA |
| Meyer, 2003 [26] | United States (low) | Routine CXR | Cross-sectional | Lesions suggestive of tuberculosis infection abnormal, fibrosis, granuloma, consolidation or cavity, pleural disease, and calcified lymph nodes. | TST+ | 535 | 39 ± 15 | 48 | 75/535 (14.0) | NA |
| Nolan, 1988 [27] | United States (low) | Immigrant screening | Cohort | Abnormal CXRs consistent with inactive TB. | TST ≥ 10 mm | 3300 | NR | NR | 185/3300 (5.6) | NA |
Abbreviations: CXR, chest radiography; HCW, healthcare workers; HD/PD, hemodialysis/peritoneal dialysis; IGRA, interferon gamma release assay; LTBI, latent tuberculosis infection; NA, not applicable; NR, not reported; QFT, QuantiFERON Gold in-tube; TB, tuberculosis; TNF, tumor necrosis factor; T-SPOT, T-SPOT.TB; TST, tuberculin skin test.
a TB-endemicity was considered high in case of ≥40 cases of active TB/100 000 inhabitants per year.
b Mean (± standard deviation, if available) or range; median used only if mean or range were not described.
c Median.
| First Author, Publication Year [reference number] . | Country (TB- endemicity)a . | Setting . | Design . | Definition of Lesions Suggestive of Past TB . | Case Definition (Criterion for LTBI) . | N Subjects . | Ageb . | Sex (% male) . | Individuals with LTBI and Lesions on CXR N/N with LTBI (%) . | Uninfected Controls With Lesions on CXR N/N Controls (%) . |
|---|---|---|---|---|---|---|---|---|---|---|
| Studies with control group | ||||||||||
| Bonfiglioli, 2014 [28] | Brazil (high) | Anti-TNF screening | Cohort | Signs of previous TB (fibrotic lesions)/TB sequelae. | TST ≥ 5 mm | 202 | 52 ± 11 | 16.8 | 6/44 (13.6) | 8/158 (5.1) |
| Christopoulos, 2009 [29] | Greece (low) | Screening HD/PD | Cohort | Dense pulmonary nodules, with or without visible calcification, in the hilar area or upper lobes, smaller nodules with or without fibrotic scars in the upper lobes, accompanied with upper lobe volume loss bronchiectasis of the upper lobe and/or pleural scarring. | TST ≥ 10 mm | 272 | 25–77 | 71.0 | 27/62 (43.5) | 40/210 (19.0) |
| Costantino, 2013 [30] | France (low) | Anti-TNF screening | Cross-sectional | Suggestive of previous TB infection (pulmonary nodules, upper lobe bronchiectasis, apical pleural thickening, interstitial granulomatous calcifications, cavitations, and lymph node or pericardial calcifications). | TST ≥ 5 mm T-SPOT+ TST ≥ 5 mm and T-SPOT+ | 196 122 279 | 51.0 NR NR | 53.1 44.3 38.4 | 14/196 (7.1) 13/122 (10.7) 9/59 (15.3) | NA NA 2/220 (0.9) |
| Foster, 2016 [31] | Canada (low) | Screening HD/PD | Cohort | Evidence of previous TB infection (including infiltrates, tissue loss, and cavitations upper lobe). | TST ≥ 5 mm TST ≥ 10 mm | 476 465 | 62 ± 11 62 ± 11 | 49.1 49.1 | 14/62 (22.6) 12/43 (27.9) | 107/406 (25.4) 89/422 (21.1) |
| Jeong, 2012 [32] | South Korea (high) | Routine CXR | Case control | Old, healed TB (calcified nodular densities, fibrosis/nodular densities, basal pleural thickening ≥10mm, and bronchiectasis upper lobe). | TST ≥ 10 mm QFT+ | 271 319 | 18–88 18–88 | NR 58.6 | 89/131 (67.9) 150/228 (65.8) | 74/140 (52.9) 43/91 (47.3) |
| Joshi, 2007 [33] | India (high) | Screening HCW | Cross-sectional | Suggestive of inactive TB (fibrotic scar [with volume loss], [non-] calcified nodules [with volume loss], bronchiectasis, pleural thickening, diaphragmatic tenting, and blunt costophrenic angle. | TST ≥ 10 mm QFT+ TST ≥ 10 mm and QFT+ | 276 328 209 | 32 ± 12 NR NR | NR NR NR | 174/276 (63.0) 163/261 (62.5) 131/209 (62.7) | NA 43/67 (64.2) NA |
| Katsenos, 2011 [34] | Greece (low) | Contact investigation | Cross-sectional | Abnormal findings (fibrotic-appearing apical lesions). | TST ≥ 5 mm | 77 | 75 | 15.6 | 0/26 (0) | 6/51 (11.8) |
| Kim, 2010 [35] | South Korea (high) | Pre-transplant screening | Cohort | Abnormal findings suggestive of inactive TB. | TST ≥ 5 mm TST ≥ 10 mm T-SPOT+ | 209 209 184 | NR NR NR | NR NR NR | 5/47 (10.6) 3/21 (14.3) 5/65 (7.7) | 5/162 (3.1) 7/188 (3.7) 4/119 (3.4) |
| Kleinert, 2012 [36] | Germany (low) | Anti-TNF screening | Cross-sectional | Lesions suggestive of latent or prior TB. | TST ≥ 5 mm QFT+ T-SPOT+ TST ≥ 5 mm and IGRA+ | 1529 685 844 1368 | NR NR NR NR | 38.7 NR NR 37.9 | 15/173 (8.7) 3/50 (6.0) 15/70 (21.4) 13/66 (19.7) | 12/1356 (0.9) 7/635 (1.1) 2/777 (0.3) 7/1302 (0.5) |
| Roelsgaard, 1961 [37] | Kenya (high) | Population study | Cross-sectional | Infiltrate with cavity, infiltrate without cavity, calcified or fibrotic lesions, and pleural adhesions. Where more than 1 shadow was seen, the classification into type of lesion was determined by the most “severe“ finding. | TST ≥ 10 mm | 4946 | NR | NR | 231/2475 (9.3) | 82/2471 (3.3) |
| Seyhan, 2009 [38] | Turkey (low) | Screening HD/PD | Cross-sectional | TB scar lesions (dense pulmonary nodules with/without calcification in hilar area or upper lobes, and pleural thickening). | TST ≥ 10 mm QFT+ | 100 100 | 56 ± 15 56 ± 15 | 47.0 47.0 | 4/34 (11.8) 11/43 (25.6) | 12/66 (18.2) 5/57 (8.8) |
| Sichletidis, 2006 [39] | Greece (low) | Anti-TNF screening | Cohort | An abnormal CXR suggesting latent TB (apical fibrotic lesions and pleural calcification). | TST ≥ 10 mm | 613 | 49 ± 8 | 32.1 | 5/45 (11.1) | 0/568 (0) |
| Tafuri, 2011 [40] | Italy (low) | Immigrant screening | Cross-sectional | TB sequelae. | TST+ | 669 | 25 | NR | 99/554 (17.9) | 16/115 (13.9) |
| Triverio, 2009 [41] | Switzerland (low) | Immigrant screening | Cohort | Suggestive of prior TB. | TST ≥ 5 mm | 62 | 65 ± 15 | 74.2 | 2/12 (16.7) | 6/50 (12.0) |
| QFT+ | 57 | NR | NR | 3/13 (23.1) | 5/44 (11.4) | |||||
| T-SPOT+ | 55 | NR | NR | 1/18 (5.6) | 5/37 (13.5) | |||||
| Vassilopoulos, 2009 [42] | Greece (low) | Anti-TNF screening | Cohort | Suggestive of previous, inactive TB (calcified or noncalcified nodules or fibrotic scars). | TST ≥ 5 mm | 155 | 52 ± 16 | 41.9 | 9/58 (15.5) | 5/97 (5.2) |
| QFT+ | 155 | 52 ± 16 | 41.9% | 4/32 (12.5) | 10/123 (8.1) | |||||
| T-SPOT+ | 155 | 52 ± 16 | 41.9 | 4/39 (10.3) | 10/116 (8.6) | |||||
| Wauters, 2004 [43] | Belgium (low) | Screening HD/PD | Cross-sectional | TB lesions: dense pulmonary nodules with or without visible calcification in the hilar area or upper lobes or pleural scarring were scored as positive findings. | TST ≥ 10 mm | 224 | 68 ± 13 | 58.0 | 22/73 (30.1) | 34/151 (22.5) |
| Studies without control group | ||||||||||
| Bailey, 1977 [18] | United States (low) | Routine CXR | Cohort | Consistent with old TB. | TST+ | 1524 | NR | NR | 61/1524 (4.0) | NA |
| Eisenberg, 2009 [19] | United States (low) | Pre-employment | Cross-sectional | Abnormalities suggestive of chronic TB infection (calcified granulomas and lymph nodes, apical pleural thickening, fibrosis, and nodules). | TST ≥ 10 mm | 875 | 18–65 | 44.8 | 91/875 (10.4) | NA |
| Eisenberg, 2010 [20] | United States (low) | Pre-employment | Cross-sectional | Evidence of prior TB infection included apical or basal pleural thickening; fibrous scarring; calcified granulomas and/or, calcified lymph nodes; and noncalcified nodules. | TST ≥ 10 mm TST ≥ 10 mm and QFT+ | 2586 | 18–65 | 45.0 | 159/2586 (6.1) 8/135 (5.9) | NA NA |
| Eisenberg, 2017 [21] | United States (low) | Routine CXR | Cohort | Evidence of prior TB infection (apical pleural thickening, fibrous scarring, calcified granulomas/lymph nodes and noncalcified nodules). | TST ≥ 10 mm | 2518 | 18–93 | 38.9 | 196/2518 (7.8) | NA |
| Gershon, 2004 [22] | Canada (low) | TB clinic | Cohort | Abnormal findings consistent with previous TB. | TST ≥ 10 mm or TST ≥ 5 mm | 308 | NR | 56.8 | 77/308 (25.0) | NA |
| Gottridge, 1989 [23] | Unites States (low) | Screening HCW | Cohort | Apical pleural thickening, active parenchymal lesion, cavitating lesion, pleural effusion, fibrocalcific disease, unrelated abnormality, or other abnormal CXR findings consistent with previous TB. | TST ≥ 10 mm | 221 | 18–65 | 33.9 | 9/221 (4.1) | NA |
| Kunimoto, 2009 [24] | Canada (low) | Routine CXR | Cross-sectional | Findings suggestive of previous TB (TB scar). | TST ≥ 5 mm TST ≥ 5 mm and QFT+ | 1446 566 | NR 32 ± 16c | 37.5 41.7 | 304/1446 (21.0) 170/566 (30.0) | NA NA |
| Manadan, 2007 [25] | United States (low) | Anti-TNF screening | Cohort | Evidence of old granulomatous disease. | TST ≥ 10 mm | 43 | NR | NR | 10/43 (23.2) | NA |
| Meyer, 2003 [26] | United States (low) | Routine CXR | Cross-sectional | Lesions suggestive of tuberculosis infection abnormal, fibrosis, granuloma, consolidation or cavity, pleural disease, and calcified lymph nodes. | TST+ | 535 | 39 ± 15 | 48 | 75/535 (14.0) | NA |
| Nolan, 1988 [27] | United States (low) | Immigrant screening | Cohort | Abnormal CXRs consistent with inactive TB. | TST ≥ 10 mm | 3300 | NR | NR | 185/3300 (5.6) | NA |
| First Author, Publication Year [reference number] . | Country (TB- endemicity)a . | Setting . | Design . | Definition of Lesions Suggestive of Past TB . | Case Definition (Criterion for LTBI) . | N Subjects . | Ageb . | Sex (% male) . | Individuals with LTBI and Lesions on CXR N/N with LTBI (%) . | Uninfected Controls With Lesions on CXR N/N Controls (%) . |
|---|---|---|---|---|---|---|---|---|---|---|
| Studies with control group | ||||||||||
| Bonfiglioli, 2014 [28] | Brazil (high) | Anti-TNF screening | Cohort | Signs of previous TB (fibrotic lesions)/TB sequelae. | TST ≥ 5 mm | 202 | 52 ± 11 | 16.8 | 6/44 (13.6) | 8/158 (5.1) |
| Christopoulos, 2009 [29] | Greece (low) | Screening HD/PD | Cohort | Dense pulmonary nodules, with or without visible calcification, in the hilar area or upper lobes, smaller nodules with or without fibrotic scars in the upper lobes, accompanied with upper lobe volume loss bronchiectasis of the upper lobe and/or pleural scarring. | TST ≥ 10 mm | 272 | 25–77 | 71.0 | 27/62 (43.5) | 40/210 (19.0) |
| Costantino, 2013 [30] | France (low) | Anti-TNF screening | Cross-sectional | Suggestive of previous TB infection (pulmonary nodules, upper lobe bronchiectasis, apical pleural thickening, interstitial granulomatous calcifications, cavitations, and lymph node or pericardial calcifications). | TST ≥ 5 mm T-SPOT+ TST ≥ 5 mm and T-SPOT+ | 196 122 279 | 51.0 NR NR | 53.1 44.3 38.4 | 14/196 (7.1) 13/122 (10.7) 9/59 (15.3) | NA NA 2/220 (0.9) |
| Foster, 2016 [31] | Canada (low) | Screening HD/PD | Cohort | Evidence of previous TB infection (including infiltrates, tissue loss, and cavitations upper lobe). | TST ≥ 5 mm TST ≥ 10 mm | 476 465 | 62 ± 11 62 ± 11 | 49.1 49.1 | 14/62 (22.6) 12/43 (27.9) | 107/406 (25.4) 89/422 (21.1) |
| Jeong, 2012 [32] | South Korea (high) | Routine CXR | Case control | Old, healed TB (calcified nodular densities, fibrosis/nodular densities, basal pleural thickening ≥10mm, and bronchiectasis upper lobe). | TST ≥ 10 mm QFT+ | 271 319 | 18–88 18–88 | NR 58.6 | 89/131 (67.9) 150/228 (65.8) | 74/140 (52.9) 43/91 (47.3) |
| Joshi, 2007 [33] | India (high) | Screening HCW | Cross-sectional | Suggestive of inactive TB (fibrotic scar [with volume loss], [non-] calcified nodules [with volume loss], bronchiectasis, pleural thickening, diaphragmatic tenting, and blunt costophrenic angle. | TST ≥ 10 mm QFT+ TST ≥ 10 mm and QFT+ | 276 328 209 | 32 ± 12 NR NR | NR NR NR | 174/276 (63.0) 163/261 (62.5) 131/209 (62.7) | NA 43/67 (64.2) NA |
| Katsenos, 2011 [34] | Greece (low) | Contact investigation | Cross-sectional | Abnormal findings (fibrotic-appearing apical lesions). | TST ≥ 5 mm | 77 | 75 | 15.6 | 0/26 (0) | 6/51 (11.8) |
| Kim, 2010 [35] | South Korea (high) | Pre-transplant screening | Cohort | Abnormal findings suggestive of inactive TB. | TST ≥ 5 mm TST ≥ 10 mm T-SPOT+ | 209 209 184 | NR NR NR | NR NR NR | 5/47 (10.6) 3/21 (14.3) 5/65 (7.7) | 5/162 (3.1) 7/188 (3.7) 4/119 (3.4) |
| Kleinert, 2012 [36] | Germany (low) | Anti-TNF screening | Cross-sectional | Lesions suggestive of latent or prior TB. | TST ≥ 5 mm QFT+ T-SPOT+ TST ≥ 5 mm and IGRA+ | 1529 685 844 1368 | NR NR NR NR | 38.7 NR NR 37.9 | 15/173 (8.7) 3/50 (6.0) 15/70 (21.4) 13/66 (19.7) | 12/1356 (0.9) 7/635 (1.1) 2/777 (0.3) 7/1302 (0.5) |
| Roelsgaard, 1961 [37] | Kenya (high) | Population study | Cross-sectional | Infiltrate with cavity, infiltrate without cavity, calcified or fibrotic lesions, and pleural adhesions. Where more than 1 shadow was seen, the classification into type of lesion was determined by the most “severe“ finding. | TST ≥ 10 mm | 4946 | NR | NR | 231/2475 (9.3) | 82/2471 (3.3) |
| Seyhan, 2009 [38] | Turkey (low) | Screening HD/PD | Cross-sectional | TB scar lesions (dense pulmonary nodules with/without calcification in hilar area or upper lobes, and pleural thickening). | TST ≥ 10 mm QFT+ | 100 100 | 56 ± 15 56 ± 15 | 47.0 47.0 | 4/34 (11.8) 11/43 (25.6) | 12/66 (18.2) 5/57 (8.8) |
| Sichletidis, 2006 [39] | Greece (low) | Anti-TNF screening | Cohort | An abnormal CXR suggesting latent TB (apical fibrotic lesions and pleural calcification). | TST ≥ 10 mm | 613 | 49 ± 8 | 32.1 | 5/45 (11.1) | 0/568 (0) |
| Tafuri, 2011 [40] | Italy (low) | Immigrant screening | Cross-sectional | TB sequelae. | TST+ | 669 | 25 | NR | 99/554 (17.9) | 16/115 (13.9) |
| Triverio, 2009 [41] | Switzerland (low) | Immigrant screening | Cohort | Suggestive of prior TB. | TST ≥ 5 mm | 62 | 65 ± 15 | 74.2 | 2/12 (16.7) | 6/50 (12.0) |
| QFT+ | 57 | NR | NR | 3/13 (23.1) | 5/44 (11.4) | |||||
| T-SPOT+ | 55 | NR | NR | 1/18 (5.6) | 5/37 (13.5) | |||||
| Vassilopoulos, 2009 [42] | Greece (low) | Anti-TNF screening | Cohort | Suggestive of previous, inactive TB (calcified or noncalcified nodules or fibrotic scars). | TST ≥ 5 mm | 155 | 52 ± 16 | 41.9 | 9/58 (15.5) | 5/97 (5.2) |
| QFT+ | 155 | 52 ± 16 | 41.9% | 4/32 (12.5) | 10/123 (8.1) | |||||
| T-SPOT+ | 155 | 52 ± 16 | 41.9 | 4/39 (10.3) | 10/116 (8.6) | |||||
| Wauters, 2004 [43] | Belgium (low) | Screening HD/PD | Cross-sectional | TB lesions: dense pulmonary nodules with or without visible calcification in the hilar area or upper lobes or pleural scarring were scored as positive findings. | TST ≥ 10 mm | 224 | 68 ± 13 | 58.0 | 22/73 (30.1) | 34/151 (22.5) |
| Studies without control group | ||||||||||
| Bailey, 1977 [18] | United States (low) | Routine CXR | Cohort | Consistent with old TB. | TST+ | 1524 | NR | NR | 61/1524 (4.0) | NA |
| Eisenberg, 2009 [19] | United States (low) | Pre-employment | Cross-sectional | Abnormalities suggestive of chronic TB infection (calcified granulomas and lymph nodes, apical pleural thickening, fibrosis, and nodules). | TST ≥ 10 mm | 875 | 18–65 | 44.8 | 91/875 (10.4) | NA |
| Eisenberg, 2010 [20] | United States (low) | Pre-employment | Cross-sectional | Evidence of prior TB infection included apical or basal pleural thickening; fibrous scarring; calcified granulomas and/or, calcified lymph nodes; and noncalcified nodules. | TST ≥ 10 mm TST ≥ 10 mm and QFT+ | 2586 | 18–65 | 45.0 | 159/2586 (6.1) 8/135 (5.9) | NA NA |
| Eisenberg, 2017 [21] | United States (low) | Routine CXR | Cohort | Evidence of prior TB infection (apical pleural thickening, fibrous scarring, calcified granulomas/lymph nodes and noncalcified nodules). | TST ≥ 10 mm | 2518 | 18–93 | 38.9 | 196/2518 (7.8) | NA |
| Gershon, 2004 [22] | Canada (low) | TB clinic | Cohort | Abnormal findings consistent with previous TB. | TST ≥ 10 mm or TST ≥ 5 mm | 308 | NR | 56.8 | 77/308 (25.0) | NA |
| Gottridge, 1989 [23] | Unites States (low) | Screening HCW | Cohort | Apical pleural thickening, active parenchymal lesion, cavitating lesion, pleural effusion, fibrocalcific disease, unrelated abnormality, or other abnormal CXR findings consistent with previous TB. | TST ≥ 10 mm | 221 | 18–65 | 33.9 | 9/221 (4.1) | NA |
| Kunimoto, 2009 [24] | Canada (low) | Routine CXR | Cross-sectional | Findings suggestive of previous TB (TB scar). | TST ≥ 5 mm TST ≥ 5 mm and QFT+ | 1446 566 | NR 32 ± 16c | 37.5 41.7 | 304/1446 (21.0) 170/566 (30.0) | NA NA |
| Manadan, 2007 [25] | United States (low) | Anti-TNF screening | Cohort | Evidence of old granulomatous disease. | TST ≥ 10 mm | 43 | NR | NR | 10/43 (23.2) | NA |
| Meyer, 2003 [26] | United States (low) | Routine CXR | Cross-sectional | Lesions suggestive of tuberculosis infection abnormal, fibrosis, granuloma, consolidation or cavity, pleural disease, and calcified lymph nodes. | TST+ | 535 | 39 ± 15 | 48 | 75/535 (14.0) | NA |
| Nolan, 1988 [27] | United States (low) | Immigrant screening | Cohort | Abnormal CXRs consistent with inactive TB. | TST ≥ 10 mm | 3300 | NR | NR | 185/3300 (5.6) | NA |
Abbreviations: CXR, chest radiography; HCW, healthcare workers; HD/PD, hemodialysis/peritoneal dialysis; IGRA, interferon gamma release assay; LTBI, latent tuberculosis infection; NA, not applicable; NR, not reported; QFT, QuantiFERON Gold in-tube; TB, tuberculosis; TNF, tumor necrosis factor; T-SPOT, T-SPOT.TB; TST, tuberculin skin test.
a TB-endemicity was considered high in case of ≥40 cases of active TB/100 000 inhabitants per year.
b Mean (± standard deviation, if available) or range; median used only if mean or range were not described.
c Median.
CXR indicates chest radiography; IGRA, interferon-gamma release assay; TB, tuberculosis; TST, tuberculin skin test.
Risk of Bias Across Studies
Using the NIH's Quality Assessment Tool on all included studies [17], 7 studies were scored as poor [18, 22, 24, 25, 27, 29, 36], 14 as fair [19–21, 23, 26, 28, 30, 31, 33–35, 39, 40, 43], and 5 as good quality studies [32, 37, 38, 41, 42] (Supplementary Figures S1 and S2). Of the 16 studies included in the meta-analysis, 2 were scored as poor [29, 36], 9 as fair [28, 30, 31, 33–35, 39, 40, 43], and 5 as good quality studies [32, 37, 38, 41, 42]. Only 4 studies reported that the evaluating radiologist was blinded to TST/IGRA results [32, 37, 38, 43].
Proportion of Individuals With LTBI Showing LTBI-CXR Lesions
Prevalence data for LTBI-CXR lesions, often without specification of which lesions qualified as such, in individuals with a positive TST/IGRA result from 25 different studies, resulted in an overall proportion of 0.15 (95% CI, 0.12–0.18; Figure 2). One study was excluded from this analysis, because individuals with a TST ≥ 5 mm were not separated from those with a TST ≥ 10 mm [22]. In the latter study, the prevalence was 25%. In the different diagnostic categories based on LTBI definition, the pooled proportion of these lesions was highest in those with a positive QFT result (0.32; 95% CI, 0.13–0.54) and lowest in studies that defined LTBI by T-SPOT positivity (0.12; 95% CI, 0.07–0.17). Individuals in studies defining LTBI by a higher cut-off for TST positivity were associated with a slightly higher prevalence of lesions suggestive of past TB infection, although these estimates were associated with considerable heterogeneity. Meta-regression showed that low TB-endemicity was significantly associated with a lower proportion of Mtb-infected individuals with LTBI-CXR lesions (P < .01). An additional analysis was performed in which the studies with the highest and lowest value were omitted from the TST ≥ 10 mm and QFT-positive subgroup, which were Jeong et al [32] plus Gottridge et al [23] and Jeong et al [32] plus Kleinert et al [36], respectively. This resulted in a slightly lower proportion with LTBI-CXR lesions overall (0.14; 95% CI, 0.11–0.17), in the TST ≥ 10 mm subgroup (0.16; 95% CI, 0.11–0.21), and in the QFT-positive subgroup (0.31; 95% CI, 0.07–0.61) when compared to the analysis including all studies. Heterogeneity was somewhat reduced in the TST ≥ 10 mm subgroup (I2 = 94%, τ 2 = 0.084), but remained high in the QFT-positive subgroup (I2 = 98%, τ 2 = 0.013).
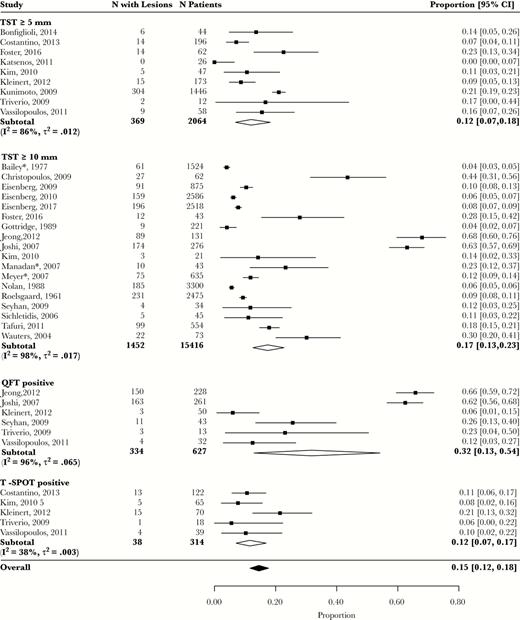
Proportion of Individuals With Positive Test for Latent Tuberculosis (TB) Infection in Whom Lesions Suggestive of Previous TB Infection Were Found on Chest Radiography.
Pooled proportion calculated by diagnostic category using the Freeman-Tukey double arcsine transformation method. QFT indicates QuantiFERON Gold in-tube; TST, tuberculin skin test; T-SPOT, T-SPOT.TB. Asterisk indicates that cut-off for a positive TST was not defined in this study, but it was considered to be 10 mm.
Association Between Positive TST/IGRA and LTBI-CXR Lesions, Compared With Uninfected Controls
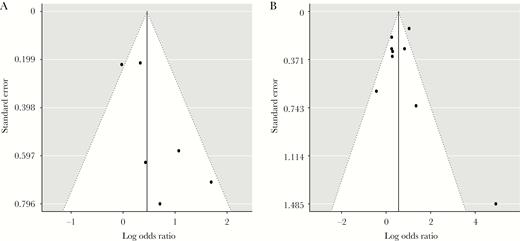
Fourteen studies provided data that enabled a calculation of the risk estimate for LTBI-CXR lesions in individuals with a positive TST (Figures 3A and 3B). Definitions for a positive TST included TST ≥ 5 mm or TST ≥ 10 mm. Latent tuberculosis infection defined as TST ≥ 5 mm or ≥ 10 mm was associated with LTBI-CXR lesions (OR, 2.45; 95% CI, 1.00–5.99 and OR, 2.06; 95% CI, 1.38–3.09, respectively). Heterogeneity was substantial across both categories. Five studies using TST ≥ 5 mm as criterion for LTBI reported that bacille Calmette-Guérin (BCG) vaccination rates were similar in individuals with LTBI and uninfected controls, except for 1 study in which uninfected controls were more often vaccinated by BCG [41]. The effect estimates of IGRA positivity on the risk of LTBI-CXR lesions were based on 7 studies (Figures 4A and 4B). QFT positivity was significantly associated with LTBI-CXR lesions (OR, 1.99; 95% CI, 1.17–3.39), whereas T-SPOT positivity was not, which may be related to the limited available data. Heterogeneity was moderate among studies using QFT positivity (I2 = 50%, P = .07), and considerable in those describing T-SPOT positivity (I2 = 89%, P < .01). In the meta-regression analysis, blinding of the radiologist for the TST/IGRA results did not affect the association between TST ≥ 10 mm or QFT positivity and LTBI-CXR lesions (P = .42 and P = .47, respectively). Funnel plots showed no evidence of asymmetry, regardless of the definition for LTBI (Figures 5A and 5B, Supplementary Figure S3). Egger’s test for asymmetry was not significant in studies defining LTBI by TST ≥ 5 mm, TST ≥ 10 mm, or QFT positivity (P = .72, P = .77 and P = .32, respectively). Thus, the risk of publication bias was low. For studies reporting on T-SPOT positivity, no funnel plot was constructed as the number of available studies was too low.
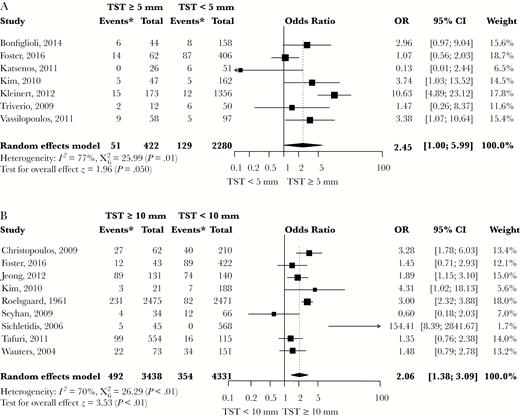
Meta-Analysis of Studies Showing the Crude Effect of a Positive TST Result on the Risk of Lesions Suggestive of Past TB Infection on Chest Radiography
(A) TST ≥ 5 mm versus TST < 5 mm; (B) TST ≥ 10 mm versus TST < 10 mm. CI indicates confidence interval; TST, tuberculin skin test. Asterisk indicates number of individuals with findings on chest radiography consistent with past tuberculosis infection.
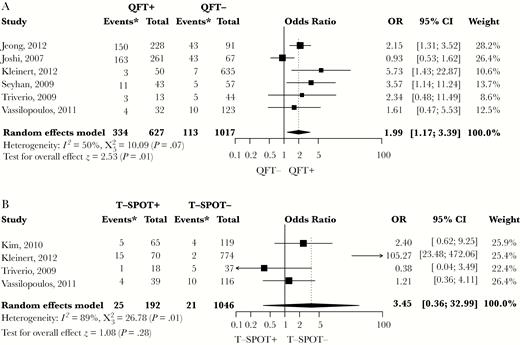
Meta-Analysis of Studies Showing the Crude Effect of a Positive Interferon-Gamma Release Assay Result on the Risk of Lesions Suggestive of Past Tuberculosis Infection on Chest Radiography
(A) QFT positive versus QFT negative; (B) T-SPOT positive versus T-SPOT negative. CI indicates confidence interval; IGRA, interferon-gamma release assay; QFT, QuantiFERON Gold in-tube; T-SPOT, T-SPOT.TB. Asterisk indicates number of individuals with findings on chest radiography consistent with past tuberculosis infection.
(A) Studies defining LTBI by QuantiFERON Gold in-tube positivity; (B) Studies defining latent tuberculosis infection (LTBI) by tuberculin skin test ≥ 10 mm.
Prespecified Radiological Findings
Nine studies reported LTBI-CXR lesions in more detail, as shown in Table 2. Presence of a calcified nodule was the most prevalent lesion observed among individuals with a positive test for LTBI, although still rare (proportion, 0.07; 95% CI, 0.02–0.15). Crude ORs for specified lesions on CXR were not estimated as only few studies reported on specified lesions in individuals without LTBI.
Pooled Proportions of Individuals With Latent Tuberculosis Infection and Specified Lesions on Chest Radiography
| Characteristics . | N Studies [reference number] . | N Individualsa . | (Pooled) Proportion of Individuals With Lesions on CXRb (95% CI) . | Heterogeneity . |
|---|---|---|---|---|
| Patients with LTBI | ||||
| Pleural thickening | 9 [19–21, 23, 26, 27, 33, 37, 39] | 12 883 | 0.02 (0.01; 0.03) | I2 = 95%, τ 2 = 0.004 |
| Calcified nodule | 7 [19–21, 23, 26, 33, 39] | 7108 | 0.07 (0.02; 0.15) | I2 = 99%, τ 2 = 0.029 |
| Noncalcified nodule | 6 [19–21, 23, 33, 39] | 6573 | 0.01 (0.00; 0.02) | I2 = 86%, τ 2 = 0.002 |
| Fibrotic scarring | 7 [19–21, 23, 26, 33, 39] | 7108 | 0.02 (0.01; 0.05) | I2 = 96%, τ 2 = 0.007 |
| Patients without LTBI | ||||
| Pleural thickening | 2 [37, 39] | 3039 | 0.01 (0.00; 0.04) | I2 = 96%, τ 2 = 0.007 |
| Calcified nodule | 1 [39] | 568 | 0 | NE |
| Noncalcified nodule | 1 [39] | 568 | 0 | NE |
| Fibrotic scarring | 1[39] | 568 | 0 | NE |
| Characteristics . | N Studies [reference number] . | N Individualsa . | (Pooled) Proportion of Individuals With Lesions on CXRb (95% CI) . | Heterogeneity . |
|---|---|---|---|---|
| Patients with LTBI | ||||
| Pleural thickening | 9 [19–21, 23, 26, 27, 33, 37, 39] | 12 883 | 0.02 (0.01; 0.03) | I2 = 95%, τ 2 = 0.004 |
| Calcified nodule | 7 [19–21, 23, 26, 33, 39] | 7108 | 0.07 (0.02; 0.15) | I2 = 99%, τ 2 = 0.029 |
| Noncalcified nodule | 6 [19–21, 23, 33, 39] | 6573 | 0.01 (0.00; 0.02) | I2 = 86%, τ 2 = 0.002 |
| Fibrotic scarring | 7 [19–21, 23, 26, 33, 39] | 7108 | 0.02 (0.01; 0.05) | I2 = 96%, τ 2 = 0.007 |
| Patients without LTBI | ||||
| Pleural thickening | 2 [37, 39] | 3039 | 0.01 (0.00; 0.04) | I2 = 96%, τ 2 = 0.007 |
| Calcified nodule | 1 [39] | 568 | 0 | NE |
| Noncalcified nodule | 1 [39] | 568 | 0 | NE |
| Fibrotic scarring | 1[39] | 568 | 0 | NE |
Abbreviations: CXR, chest radiography; LTBI, latent tuberculosis infection; NE, not estimated.
a Number of individuals with LTBI based on tuberculin skin test or interferon gamma release assay result.
b Only pooled if more than 1 study examined a certain characteristic.
Pooled Proportions of Individuals With Latent Tuberculosis Infection and Specified Lesions on Chest Radiography
| Characteristics . | N Studies [reference number] . | N Individualsa . | (Pooled) Proportion of Individuals With Lesions on CXRb (95% CI) . | Heterogeneity . |
|---|---|---|---|---|
| Patients with LTBI | ||||
| Pleural thickening | 9 [19–21, 23, 26, 27, 33, 37, 39] | 12 883 | 0.02 (0.01; 0.03) | I2 = 95%, τ 2 = 0.004 |
| Calcified nodule | 7 [19–21, 23, 26, 33, 39] | 7108 | 0.07 (0.02; 0.15) | I2 = 99%, τ 2 = 0.029 |
| Noncalcified nodule | 6 [19–21, 23, 33, 39] | 6573 | 0.01 (0.00; 0.02) | I2 = 86%, τ 2 = 0.002 |
| Fibrotic scarring | 7 [19–21, 23, 26, 33, 39] | 7108 | 0.02 (0.01; 0.05) | I2 = 96%, τ 2 = 0.007 |
| Patients without LTBI | ||||
| Pleural thickening | 2 [37, 39] | 3039 | 0.01 (0.00; 0.04) | I2 = 96%, τ 2 = 0.007 |
| Calcified nodule | 1 [39] | 568 | 0 | NE |
| Noncalcified nodule | 1 [39] | 568 | 0 | NE |
| Fibrotic scarring | 1[39] | 568 | 0 | NE |
| Characteristics . | N Studies [reference number] . | N Individualsa . | (Pooled) Proportion of Individuals With Lesions on CXRb (95% CI) . | Heterogeneity . |
|---|---|---|---|---|
| Patients with LTBI | ||||
| Pleural thickening | 9 [19–21, 23, 26, 27, 33, 37, 39] | 12 883 | 0.02 (0.01; 0.03) | I2 = 95%, τ 2 = 0.004 |
| Calcified nodule | 7 [19–21, 23, 26, 33, 39] | 7108 | 0.07 (0.02; 0.15) | I2 = 99%, τ 2 = 0.029 |
| Noncalcified nodule | 6 [19–21, 23, 33, 39] | 6573 | 0.01 (0.00; 0.02) | I2 = 86%, τ 2 = 0.002 |
| Fibrotic scarring | 7 [19–21, 23, 26, 33, 39] | 7108 | 0.02 (0.01; 0.05) | I2 = 96%, τ 2 = 0.007 |
| Patients without LTBI | ||||
| Pleural thickening | 2 [37, 39] | 3039 | 0.01 (0.00; 0.04) | I2 = 96%, τ 2 = 0.007 |
| Calcified nodule | 1 [39] | 568 | 0 | NE |
| Noncalcified nodule | 1 [39] | 568 | 0 | NE |
| Fibrotic scarring | 1[39] | 568 | 0 | NE |
Abbreviations: CXR, chest radiography; LTBI, latent tuberculosis infection; NE, not estimated.
a Number of individuals with LTBI based on tuberculin skin test or interferon gamma release assay result.
b Only pooled if more than 1 study examined a certain characteristic.
DISCUSSION
To the best of our knowledge, this study is the first to systematically assess the proportion of individuals with a positive immunodiagnostic test for LTBI having LTBI-CXR lesions. Pooled estimates from all included studies showed that approximately 15% of the individuals with LTBI had lesions on CXR that possibly reflect (containment of) prior Mtb-infection. In studies comparing individuals with LTBI and uninfected controls, a significant association was found between such lesions and corresponding TST/IGRA results. As only few studies reported on specified lesions, such as (calcified) nodules, fibrotic lesions, and thickening of the pleura, no risk effects could be calculated in that regard.
It is known that a (calcified) nodule and fibrotic scarring on CXR can result from the Mtb-host interaction during infection in the lung. After being phagocytosed by alveolar macrophages at the initial site of infection, Mtb-bacilli induce the recruitment of monocytes, neutrophils, B cells, and T cells, resulting in the formation of a granuloma [44]. The immune response usually contains this bacillary growth, eventually leading to the development of fibrosis in or around the granuloma [45, 46]. This fibrotic tissue sometimes becomes calcified and may be visible on CXR [44]. Of note, (calcified) nodules, fibrotic scarring, and pleural thickening on CXR are not pathognomonic and have an extensive differential diagnosis, including sarcoidosis and other granulomatous diseases, various other pulmonary infections, malignancies, and occupational lung diseases [46, 47].
In estimating the proportion of TST/IGRA positive individuals with LTBI-CXR lesions using a random-effects model, it was important to evaluate whether this pooled estimate was affected by extreme values. The additional analysis showed that omitting studies with extreme values affected the proportion only slightly. The assessment of the proportion also was performed in diagnostic categories in order to improve the precision of the estimation and to reduce heterogeneity. Although the CIs of estimates per category overlapped, the proportion was substantially higher in the QFT-positive group, mostly due to 2 studies reporting a remarkably high proportion of LTBI-CXR lesions among QFT-positive individuals [32, 33]. Both studies were conducted in a high TB-endemic area, and high TB-endemicity was significantly associated with a higher overall proportion of individuals with LTBI-CXR lesions in this review. Several other factors could have affected the accuracy of the estimation as well, including variations between populations and differences in study methods. The presence of lesions on CXR is influenced by the history of previous lung diseases, setting, and age of the screened population. For example, an older age is associated with more lesions on CXR. Both setting and age distribution varied among the included studies, but previous lung diseases could not be evaluated. In addition, most studies were not specifically aimed at examining radiological abnormalities which may have led to underreporting. Some relevant studies may have been missed due to language restrictions of the literature search. Finally, the definition of LTBI-CXR lesions varied among studies. Approximately half of the studies defined these lesions only very concisely or not at all, which could have resulted in observer bias.
The association between positive tests for LTBI and LTBI-CXR lesions, by comparison with uninfected controls, was examined in 4 diagnostic categories based on definition of a positive TST/IGRA test result. Interestingly, pooled ORs were comparable among these groups, ranging from 1.99 to 3.45. Unfortunately, this review did not include any studies that evaluated both sensitivity and specificity of particular radiological lesions for LTBI.
In this meta-analysis, the risk estimates compared to uninfected controls may have been affected by observer bias, because the evaluating radiologist was not blinded to TST/IGRA results in most included studies. Blinding is important as a radiologist can be more inclined to register minor lesions in individuals with a positive TST/IGRA result when compared to individuals with a known negative TST/IGRA result. Although this review showed that blinding did not significantly affect the effect size, this conclusion must be interpreted with some caution as the number of studies in which the radiologist was unaware of TST/IGRA result was small.
In clinical practice, it is often sufficient to diagnose or exclude recently acquired LTBI by immunological testing with TST/IGRA. In possibly recently infected individuals, such as contacts of a smear-positive TB index case, CXR is primarily performed to detect or exclude active TB and possible radiological signs of past TB infection are not contributive. In contrast, when the goal of CXR is detection of past infection with Mtb (eg, in the setting of screening before initiating immunosuppressive therapy), TST/IGRA sensitivity for LTBI is reduced and CXR lesions indicative of past LTBI may be the only positive finding and, thus, have therapeutic consequences. One retrospective cohort study demonstrated that individuals with LTBI-CXR lesions were more likely to develop posttransplant TB (2 of 33 individuals with vs 2 of 387 without lesions on CXR) [48]. Interestingly, both individuals with lesions had a negative TST during screening, illustrating the clinical relevance of CXR in pretransplant screening for LTBI. However, it remains to be determined which particular lesions are sufficiently specific for LTBI in order to justify preventive treatment. Therefore, it is important to carefully weigh the trade-off between the benefits of reducing the risk of progression to active TB and the harms of toxic side effects due to unjust preventive treatment. In this regard, in an assessment of CXR blinded to TST and IGRA results, 2 lesions were identified as highly specific for LTBI (ie, absent in most or all individuals with negative TST/IGRA), being a fibrotic scar ≥ 2 cm2 and a calcified nodule ≥ 1.5 mm [49]. However, further study is needed to corroborate this finding in various settings. Whereas the sensitivity of the CXR for past TB infection is limited, being around 15% in this review, the potential of computed tomography (CT) imaging in screening for LTBI was demonstrated by 2 previous studies, which showed that lesions suggestive of past TB infection on CT were in high concordance with positive IGRA results [50, 51]. However, CT is more expensive, has a higher radiation load, and might yield a higher number of false positive results compared to CXR, unless it can be made clear which type of lesions are sufficiently specific for past TB infection.
In conclusion, this systematic review and meta-analysis found that lesions on CXR suggestive of a previous TB infection—even though these were often poorly defined—were significantly associated with positive tests for LTBI. The findings of this study can contribute to evidence-based decision-making in the screening for LTBI in individuals at highest risk of developing active TB, although further study is needed to clarify which particular radiological findings are sufficiently specific for LTBI.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. This work was supported by the European Commission’s HORIZON2020’s research project, TBVAC2020 consortium, a project to diversify the current TB vaccine and biomarker pipeline (no. 643381 to T.H.M.O.). The text represents the authors’ views and does not necessarily represent a position of the Commission who will not be liable for the use made of such information.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
World Health Organization.





Comments